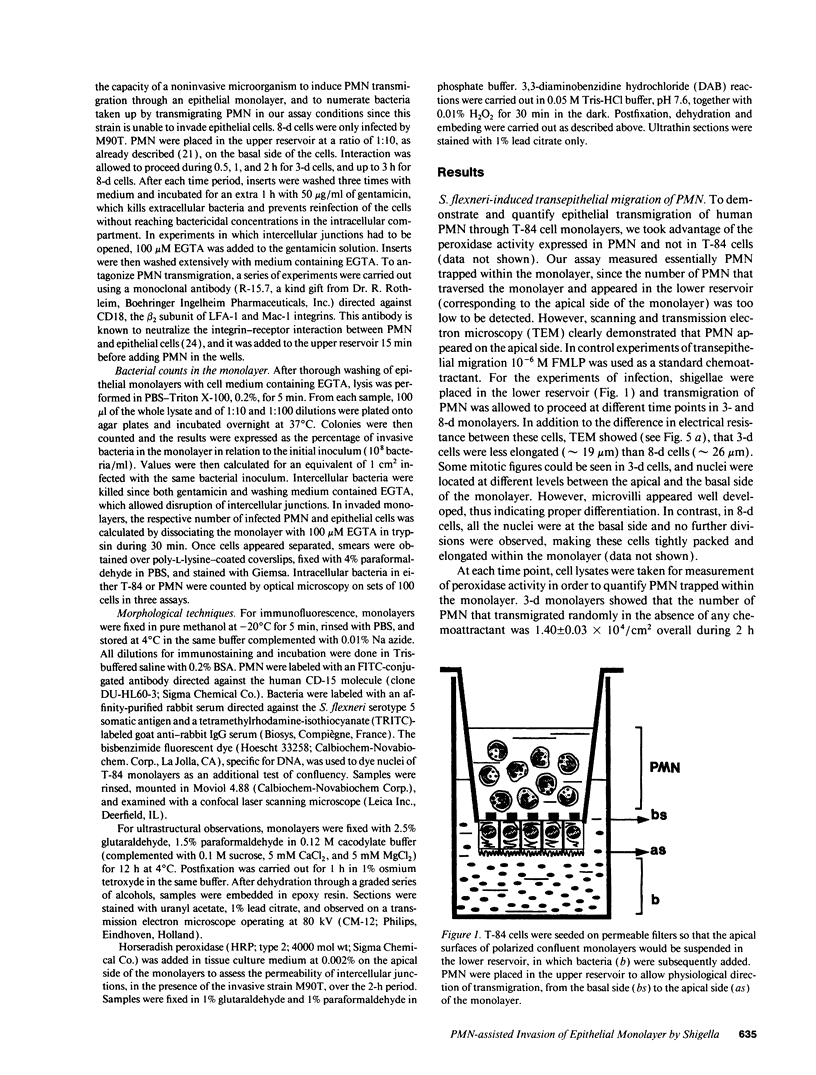
Abstract
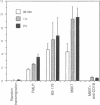
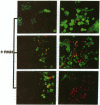
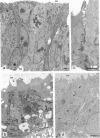
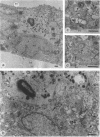
In vivo and in vitro, Shigella flexneri, an invasive pathogen of the human colon, cannot invade epithelial cells through their apical pole. To identify ways by which it may reach the cellular basolateral domain in order to invade, we have established an assay using the human colonic T-84 cell line grown on permeable filters. Human PMN were added to the basal pole of the cells, and invasive shigellae to their apical pole. Apical addition of bacteria induced strong transmigration of PMN, reaching a maximum after 1 h of incubation. Transmigration depended on a receptor-specific interaction since it was inhibited by an anti-CD18 monoclonal antibody that antagonizes binding of MAC1 on its putative epithelial cell receptor. After 1 h of PMN transmigration, shigellae started to invade the monolayer in areas of intense PMN infiltration. Invasion was clearly dependant on PMN transmigration since it was also inhibited by addition of an anti-CD18 monoclonal antibody. This in vitro assay is consistent with in vivo observations showing early PMN efflux within colonic crypts in the course of shigellosis. PMN transmigration may therefore allow invasion in the colon by opening the paracellular pathway to invasive microorganisms.
Full text
PDF

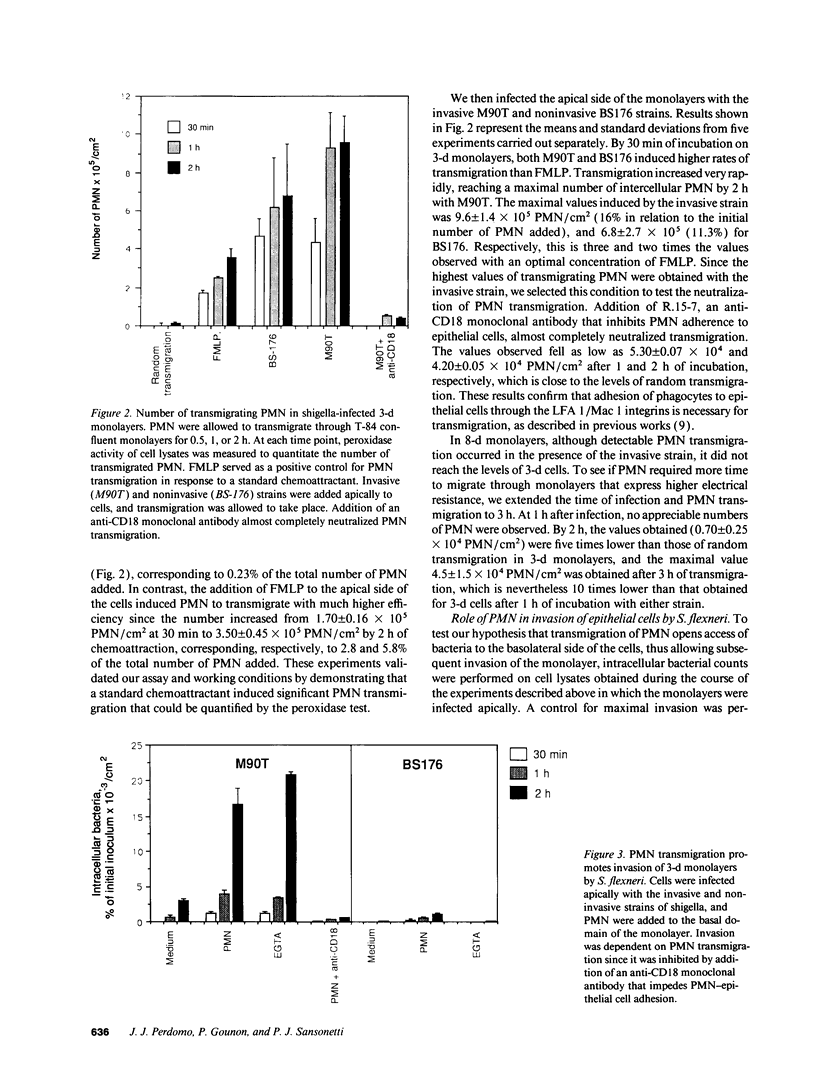

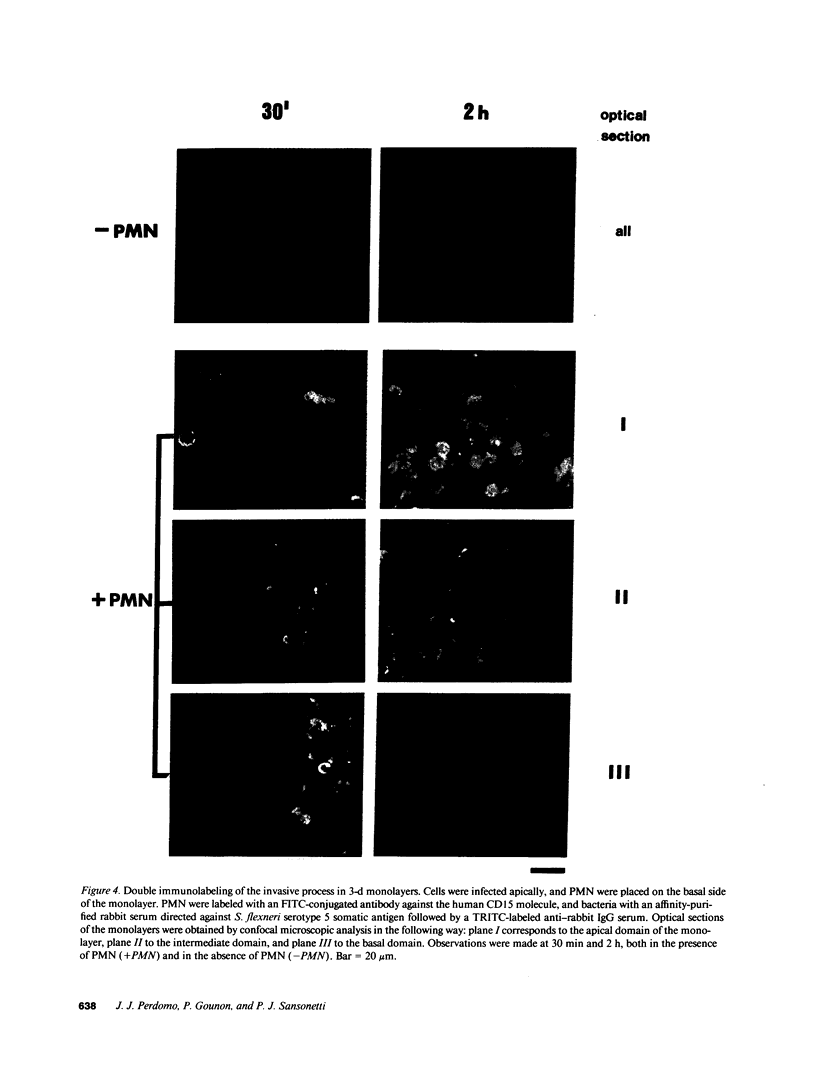
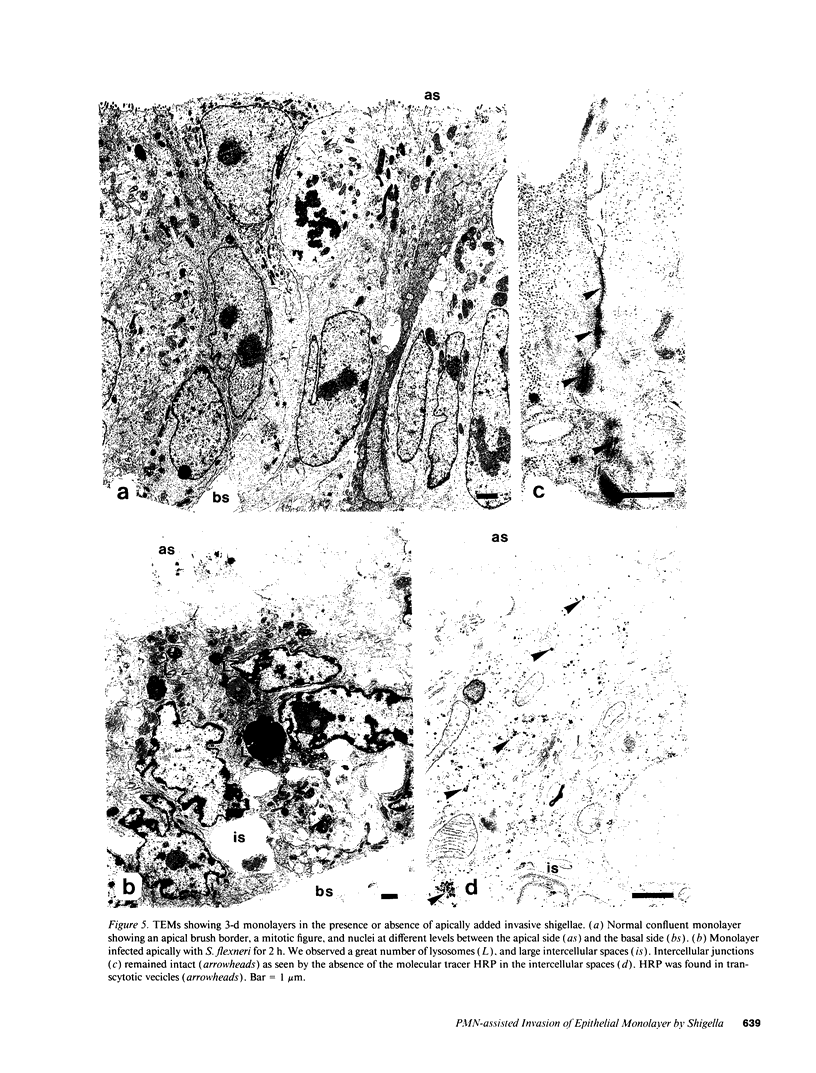
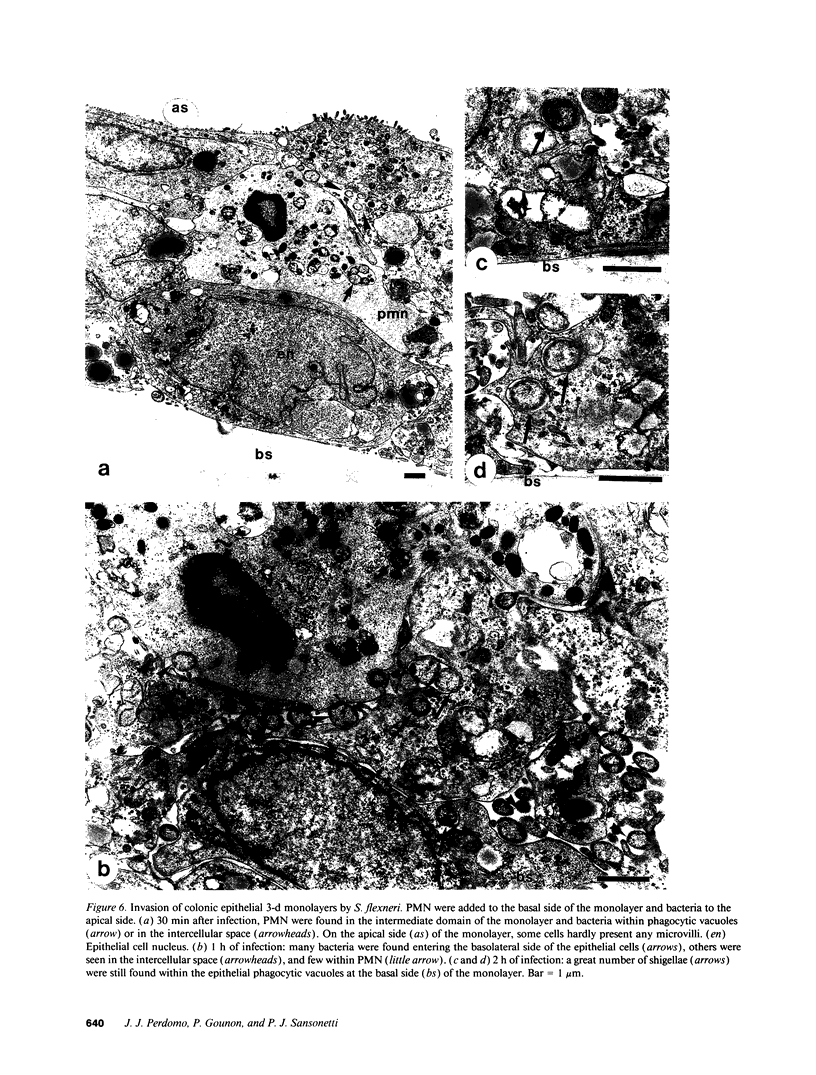



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale T. B., Abbas M. K. Comparison of leukotriene B4-induced neutrophil migration through different cellular barriers. Am J Physiol. 1990 Apr;258(4 Pt 1):C639–C647. doi: 10.1152/ajpcell.1990.258.4.C639. [DOI] [PubMed] [Google Scholar]
- Chadwick V. S., Mellor D. M., Myers D. B., Selden A. C., Keshavarzian A., Broom M. F., Hobson C. H. Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scand J Gastroenterol. 1988 Jan;23(1):121–128. doi: 10.3109/00365528809093861. [DOI] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer E. B., Milks L. C., Ojakian G. K. Transepithelial migration of human neutrophils: an in vitro model system. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4069–4073. doi: 10.1073/pnas.77.7.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Evans C. W., Taylor J. E., Walker J. D., Simmons N. L. Transepithelial chemotaxis of rat peritoneal exudate cells. Br J Exp Pathol. 1983 Dec;64(6):644–654. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Brock A. F., Schafer A. I. Leukotriene B4 stimulates polymorphonuclear leukocyte adhesion to cultured vascular endothelial cells. J Clin Invest. 1984 Oct;74(4):1552–1555. doi: 10.1172/JCI111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989 Apr;108(4):1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grützkau A., Hanski C., Hahn H., Riecken E. O. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990 Sep;31(9):1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker P. C., McKay J. S., Turnberg L. A. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980 Sep;79(3):508–511. [PubMed] [Google Scholar]
- Hawker P. C., McKay J. S., Turnberg L. A. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980 Sep;79(3):508–511. [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse H., Avrameas S. A method for the quantification of a colored or fluorescent signal in enzyme immunoassays by photodensitometry. J Immunol Methods. 1987 Oct 23;103(1):9–14. doi: 10.1016/0022-1759(87)90235-3. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol. 1985 Dec;101(6):2124–2133. doi: 10.1083/jcb.101.6.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Stafford J., Dharmsathaphorn K., Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987 May;92(5 Pt 1):1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- Mata L. J., Cáceres A., Tores M. F. Epidemic shiga dysentery in Central America. Lancet. 1971 Mar 20;1(7699):600–600. doi: 10.1016/s0140-6736(71)91202-5. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S314–S318. doi: 10.1093/clinids/13.supplement_4.s314. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Ultrastructural pathology of the rectal mucosa in Shigella dysentery. Am J Pathol. 1986 Apr;123(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- Mathan V. I., Bhat P., Kapadia C. R., Ponniah J., Baker S. J. Epidemic dysentery caused by the Shiga bacillus in a southern Indian village. J Diarrhoeal Dis Res. 1984 Mar;2(1):27–32. [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Cramer E. B. Differences in the ability of neutrophils and monocytes to traverse epithelial occluding junctions. J Leukoc Biol. 1988 Dec;44(6):485–492. doi: 10.1002/jlb.44.6.485. [DOI] [PubMed] [Google Scholar]
- Milks L. C., Brontoli M. J., Cramer E. B. Epithelial permeability and the transepithelial migration of human neutrophils. J Cell Biol. 1983 May;96(5):1241–1247. doi: 10.1083/jcb.96.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milks L. C., Conyers G. P., Cramer E. B. The effect of neutrophil migration on epithelial permeability. J Cell Biol. 1986 Dec;103(6 Pt 2):2729–2738. doi: 10.1083/jcb.103.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Vasselon T., Hellio R., Lesourd M., Sansonetti P. J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992 Jan;60(1):237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Masui H. Hormonal control of human colon carcinoma cell growth in serum-free medium. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3464–3468. doi: 10.1073/pnas.77.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987 Oct;80(4):1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. The selective and superoxide-independent disruption of intestinal epithelial tight junctions during leukocyte transmigration. Lab Invest. 1988 Oct;59(4):531–537. [PubMed] [Google Scholar]
- Neutra M. R., Kraehenbuhl J. P. Transepithelial transport and mucosal defence I: the role of M cells. Trends Cell Biol. 1992 May;2(5):134–138. doi: 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- Parkos C. A., Colgan S. P., Delp C., Arnaout M. A., Madara J. L. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol. 1992 May;117(4):757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Delp C., Arnaout M. A., Madara J. L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991 Nov;88(5):1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman M. M., Khan M. M., Aziz K. M., Islam M. S., Kibriya A. K. An outbreak of dysentery caused by Shigella dysenteriae type 1 on a coral island in the Bay of Bengal. J Infect Dis. 1975 Jul;132(1):15–19. doi: 10.1093/infdis/132.1.15. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Arondel J., Fontaine A., d'Hauteville H., Bernardini M. L. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine. 1991 Jun;9(6):416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- Struelens M. J., Patte D., Kabir I., Salam A., Nath S. K., Butler T. Shigella septicemia: prevalence, presentation, risk factors, and outcome. J Infect Dis. 1985 Oct;152(4):784–790. doi: 10.1093/infdis/152.4.784. [DOI] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Prevost M. C., Hellio R., Sansonetti P. J. Stress fiber-based movement of Shigella flexneri within cells. Infect Immun. 1991 May;59(5):1723–1732. doi: 10.1128/iai.59.5.1723-1732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis T. S., Starkey W. G., Stephen J., Haddon S. J., Osborne M. P., Candy D. C. The nature and role of mucosal damage in relation to Salmonella typhimurium-induced fluid secretion in the rabbit ileum. J Med Microbiol. 1986 Aug;22(1):39–49. doi: 10.1099/00222615-22-1-39. [DOI] [PubMed] [Google Scholar]
- Wassef J. S., Keren D. F., Mailloux J. L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989 Mar;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]