Abstract
Objectives:
To evaluate insomnia symptoms and the extent to which they are associated with clinical and demographic patient characteristics, daytime symptoms, and functional performance in patients with stable heart failure (HF).
Design:
Cross-sectional, observational.
Setting:
Five structured HF disease management programs in the Northeastern U.S.
Participants:
173 stable chronic HF patients
Interventions:
N/A
Measurements and Results:
Full polysomnography was obtained for one night in participants' homes. Participants completed the six-minute walk test, Medical Outcomes Study SF-36, Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, Multi-Dimensional Assessment of Fatigue Scale, Centers for the Epidemiological Studies of Depression Scale, and questionnaire items eliciting insomnia symptoms (self-reported difficulty initiating and maintaining sleep and waking too early in the morning). Over half of HF patients reported insomnia symptoms. These were associated with increased daytime symptoms (depression, fatigue), excessive daytime sleepiness, and functional performance in models that statistically controlled for clinical and demographic covariates. These relationships were not explained by sleep disordered breathing.
Conclusions:
Insomnia symptoms are common in patients with stable heart failure and are associated with daytime symptoms and decrements in functional performance.
Citation:
Redeker NS; Jeon S; Muench U; Campbell D; Walsleben J; Rapoport DM. Insomnia symptoms and daytime function in stable heart failure. SLEEP 2010;33(9):1210-1216.
Keywords: Depression, fatigue, heart failure, insomnia, quality of life, sleep
HEART FAILURE (HF) IS A DISABLING CHRONIC CONDITION THAT AFFLICTS OVER 5 MILLION AMERICANS1 AND IS ASSOCIATED WITH DECREMENTS IN functional performance, depression, excessive daytime sleepiness, and fatigue. As many as 73% of people with chronic heart failure (HF) report poor sleep quality,2–9 and many have poor sleep continuity.10–13 Insomnia symptoms are also common.7–9 Understanding the nature of insomnia symptoms and their associations with daytime symptoms and functional performance is necessary to guide sleep disorders treatment for HF patients.
People with HF report insomnia symptoms, including difficulty maintaining sleep (34% to 43% of sampled patients),7–9 falling asleep (23% to 47%),7–9 and waking too early in the morning (35% to 39%)7–9 in studies of HF patients awaiting transplant HF,8 hospitalized HF patients,9 and a small group of patients in a structured HF management program (n = 59).7 HF patients were consistently more likely to report difficulty initiating and maintaining sleep and to have objective evidence of prolonged sleep latency and poor sleep continuity than a comparison group recruited from the same community,7 and rates8,9 are higher than those reported by adults responding to the Sleep in America Poll.14 HF was associated with more than a two-fold increased risk of self-reported insomnia in a large community study.15 Although sleep disordered breathing (SDB) occurs in approximately 50% of HF patients10,11,16–26 and is often associated with difficulty maintaining sleep, few, if any studies have addressed the potential associations of SDB or polysomnographically recorded sleep characteristics with insomnia symptoms among HF patients.
Insomnia symptoms are associated with impaired functional performance, including decrements in exercise capacity,5 6-minute walk test distance (6MWT),27 and self-reported physical function,9,27,28 but others have not found relationships in people with HF.8,29 To our knowledge, the extent to which clinical and demographic variables contribute to the relationships between insomnia symptoms, daytime symptoms, and functional performance has not been addressed.
The objectives of this study were to examine the extent to which: (1) patients with stable HF report insomnia symptoms (Difficulty Initiating and Maintaining Sleep - DIMS); (2) demographic and clinical patient characteristics and SDB explain DIMS; and (3) self-reported DIMS is associated with daytime symptoms (depression, fatigue, excessive daytime sleepiness) and functional performance (6MWT and self-reported physical function), while accounting for SDB and potentially relevant clinical and demographic characteristics.
METHODS
The study was cross-sectional. The investigators obtained institutional review board approval from the university in which the first author was employed and all sites in which participants were recruited. Participants provided written informed consent. Details of the overall study design and methods were previously reported,30 but are summarized here for clarity.
Sample
We recruited patients with stable chronic HF from 5 HF disease management programs in the Northeastern United States. Cardiologists and nurse practitioners working in these programs referred potential participants who met the study criteria for participation in the study. The inclusion criteria included New York Heart Association Functional Classification (NYHA) II-IV HF and age ≥ 18 years of age. Exclusion criteria included pregnancy; alcohol and drug abuse; Parkinson's disease; obstructive valvular, hypertrophic, or surgically correctable heart disease; previously diagnosed sleep disorders; and renal failure requiring dialysis. Temporary exclusion criteria included unstable medical or psychiatric conditions, active titration of vasoactive medications, and hospitalization or emergency department visits within the past month.
Variables and Instruments
Functional performance
Functional performance is the “day to day corporeal activities people do in the normal course of their lives to meet basic needs, fulfill usual roles, and maintain health and wellbeing,”31 We evaluated its objective (six-minute walk test [6MWT]) and subjective (physical function scale of the Medical Outcomes Study SF-36) characteristics.
The 6MWT,32 a measure of distance walked in 6 minutes in the clinic setting, is correlated with oxygen consumption during treadmill testing,33 cycle ergometry, and self-reported functional status.32 It was conducted using standard methods.34
The Medical Outcomes Study SF36 V235,36 physical function sub-scale (PF )was used to elicit self-reported physical function. Cronbach's α of the PF subscale was 0.90 in the current study.
Daytime symptoms
The Multi-Dimensional Assessment of Fatigue Scale (MAF), developed from the Piper Fatigue Scale,37 was used to measure fatigue. For the present analysis, we computed a score for “sensory fatigue:” the average of the numeric rating scales (0-10) for degree, severity, and distress associated with fatigue. Cronbach's α was 0.91 for the sensory fatigue scale in the current study.
The Center for Epidemiological Studies Depression Scale (CESD)38,39 was used to measure depressive symptoms. The CESD has good reliability40 and adequate sensitivity and specificity.41 It had a Cronbach's α of 0.89 in the current study.42 A cut-off (CESD ≥ 16), suggestive of clinical depression, was used in the logistic analyses.
The Epworth Sleepiness Scale (ESS)43 was used to measure self-reported excessive daytime sleepiness.44 The ESS has well-documented reliability and validity in a variety of populations. Cronbach's α was 0.7842 in the data obtained in the current study. For the logistic analyses, we dichotomized the score (ESS ≥ 11), consistent with the literature.3
Insomnia symptoms
Insomnia symptoms (DIMS) were evaluated with questions from the Sleep Habits Questionnaire (SHQ) used in the Sleep Heart Health Study.45 The 3 items were rated on a 5-item scale to indicate the frequency with which respondents experienced difficulty initiating and maintaining sleep and awakening too early in the morning (DIMS). These symptoms were rated as “never” –“almost always.” Using the methods from the SHHS study,45 the presence of insomnia symptoms was defined as a response of often or almost always on one or more of these items. Cronbach's α was 0.83 for this scale.
Self-reported quantitative sleep characteristics
We evaluated respondents' perceptions of quantitative sleep characteristics (sleep duration, latency, time in bed) with items from the Pittsburgh Sleep Quality Index 46 and calculated sleep efficiency as follows: [sleep duration/time in bed] × 100.
Polysomnographic characteristics of sleep
Full unattended nocturnal polysomnography was conducted in participants' homes, with the Safiro (Compumedics, Inc.) sleep recorder for one night. Full details of this method were previously reported.30 Sleep data were scored with Rechtschaffen and Kales criteria.47 Respiratory events were defined according to standard criteria.48,42 In the current analysis, we utilized the respiratory disturbance index ([RDI] sum of apneas, hypopneas, and respiratory event related arousals)/hour of sleep as the primary measure of SDB. We also evaluated the percentage of sleep time during which participants had oxygen saturation ≤ 90% and calculated the arousal49,48 and periodic limb movement indices. The proportion of participants who had central vs. obstructive apnea were also calculated, using methods reported in our earlier paper.30
Participants were recruited at the time of a routine visit to the HF program. A research assistant explained the study, obtained informed consent, reviewed medical records, and performed the 6MWT. The study coordinator scheduled the polysomnography. Certified polysomnographic technicians made home visits to set up the PSG equipment and sensors. Unattended studies were recorded throughout the night, and the equipment was retrieved the following morning. Participants completed the study questionnaires while at home.
Data Analysis
Data were double-entered into an SPSS database, corrected for errors, and examined for the extent to which they met assumptions for parametric analysis. Descriptive and bivariate analyses were conducted to evaluate the associations between demographic and clinical patient characteristics, sleep disordered breathing, sleep characteristics, and DIMS.
Confirmatory factor analysis (CFA) was performed using M-plus version 5.2. (www.statmodel.com) to evaluate the effects of insomnia symptoms on the intercorrelated dependent variables (physical function, 6MWT, sensory fatigue, depressive symptoms, and excessive daytime sleepiness) using the latent variable model. We used CFA to test for latent variables including daytime symptoms (sensory fatigue, depression, excessive daytime sleepiness) and functional performance (6MWT and physical function).
We tested the effects of the insomnia symptoms and the demographic and clinical variables on the dependent variables and the interaction effects between insomnia symptoms (DIMS) and each of the clinical (NYHA classification, left ventricular ejection fraction, comorbidity, RDI) and demographic (age, gender) covariates. Goodness of fit was examined using the comparative fit index (CFI) and the root mean square error of approximation (RMSEA). The values of CFI > 0.9 and RMSEA < 0.08 indicate acceptable model fit.50,51
To confirm our findings from the CFA, we compared the least square means and the 95% confidence intervals of the original outcome variables (physical function, 6MWT, sleepiness, fatigue, and depression) from the generalized linear regression model after controlling for the same covariates used in the previous model. Additionally, we estimated the odds ratios (OR) for daytime sleepiness and depressive symptom, using clinical thresholds (ESS ≥ 11 and CES-D ≥ 16) from the logistic regression models.
RESULTS
Sample
We approached 324 patients, of whom 41 were ineligible upon further screening, 233 consented, and 35 declined to complete the PSG. Other reasons for incomplete data were death or deteriorating health (n = 4), intolerance of PSG monitoring, technical problems (n = 5), and loss to follow-up (n = 11). Full details have previously been published.30 Demographic and clinical patient characteristics and self-reported and PSG-measured sleep characteristics for the overall sample and for participants with and without insomnia symptoms are in Table 1. The sample consisted of 35% women and 35% (n = 61) minority group members, of whom the majority were black (n = 50). The age range of the sample was 21-28 years.
Table 1.
Comparison of participants with and without insomnia symptoms (DIMS) on demographic, clinical, and sleep variables
| Demographic Variables | Overall Sample (n = 173) | No DIMS (n = 84) M (SD)/N (%) | DIMS (n = 89) M (SD)/N (%) |
|---|---|---|---|
| Age (Years) | 60.3 (16.1) | 61.1 (16.6) | 59.6 (15.8) |
| Gender | |||
| Male | 113 (65.3%) | 60 (53.1%) | 53 (46.9%) |
| Female | 60 (34.7%) | 24 (40.0%) | 36 (60.0%) |
| Race | |||
| White | 110 (63.6%) | 57 (51.8%) | 53 (48.2%) |
| Minority | 61 (46.8%) | 26 (42.6%) | 35 (57.4%) |
| Clinical Variables | |||
| Left ventricular ejection fraction (%) | 32.6 (15.2) | 32.9 (15.6) | 32.4 (14.9) |
| NYHA classification | 2.5 (0.7) | 2.4 (0.6) | 2.5 (0.7) |
| Body mass index | 30.7 (8.0) | 30.6 (8.7) | 30.8 (7.4) |
| Charlson Comorbidity Index | 2.4 (1.5) | 2.3 (1.6) | 2.6 (1.5) |
| Clinical depression | 30 (17.3) | 11 (13.1) | 19 (21.3) |
| Self-Reported Sleep Characteristics | |||
| Time in bed (min) | 487.2 (99.6) | 494.2 (83.6) | 480.6 (112.8) |
| Sleep latency (min)*** | 29.2 (30.5) | 16.1 (12.1) | 41.6 (36.8) |
| Sleep duration (min)*** | 387.1 (100.5) | 428.0 (86.0) | 348.5 (98.2) |
| Sleep efficiency*** | 80.0 (17.7) | 86.6 (14.5) | 73.8 (18.3) |
| Polysomnographic Sleep Characteristics | |||
| Sleep duration (min)** | 323.7 (96.6) | 343.5 (85.2) | 304.9 (103.3) |
| Time in bed (min) ** | 423.5 (98.8) | 444.6 (84.4) | 403.7 (107.4) |
| Sleep efficiency* | 71.0 (16.3) | 73.7 (13.7) | 68.5 (18.1) |
| Sleep latency (min) | 30.2 (35.3) | 26.3 (37.2) | 33.8 (33.2) |
| Respiratory disturbance index (RDI) | 24.3 (19.0) | 26.5 (20.4) | 22.2 (17.4) |
| RDI ≥ 15 | 103 (60.1%) | 53 (63%) | 50 (48.1) |
| Percent time at oxygen saturation < 90% | 11.9 (18.8) | 13.0 (19.9) | 10.8 (17.9) |
| Predominant obstructive sleep apnea | 38 (22.6) | 21 (25.0) | 17 (20.2) |
| Predominant central sleep apnea | 16 (9.5) | 8 (9.5) | 8 (9.5) |
| EEG arousal index | 21.36 (10.49) | 22.26 (11.21) | 20.49 (9.74) |
| Periodic limb movement index > 5 | 42 (24.7) | 22 (26.2) | 20 (23.3) |
P < 0.001,
P < 0.01,
P < 0.05
Fifty-one percent of the sample reported difficulty initiating sleep, maintaining sleep, or waking too early in the morning often or almost always. The most common sleep symptom was trouble maintaining sleep (47%), followed by trouble falling asleep (42%), and waking too early in the morning (24%). Twenty-eight percent reported insufficient sleep often or almost always. As seen in Table 1, there were no statistically significant differences in insomnia symptoms based on age, race, body mass index, left ventricular ejection fraction, NYHA classification, comorbidity, history of clinical depression, RDI, predominant central or obstructive apnea, time at oxygen saturation < 90%, arousal index or periodic limb movement index > 5. Sixty percent of women compared with 47% of men reported insomnia symptoms (P = 0.102).
Self-reported, but not PSG-measured sleep latency, was significantly more prolonged in patients with insomnia symptoms, compared with those without. Patients with insomnia had significantly shorter sleep time and lower sleep efficiency measured by self-report and PSG. The insomnia-related differences in the self-reported sleep characteristics were larger than the insomnia-related differences in the PSG-measured sleep characteristics, with the exception of time in bed.
The latent variable “daytime symptoms” had high factor loadings on fatigue (0.724, SE = 0.056) and depression (0.716, SE = 0.057). The “functional performance” latent variable had factor loadings of 0.723 (SE = 0.057) and 0.651 (SE = 0 .060) on physical function and 6MWT. These two latent variables were highly correlated with one another (r = −0.631, SE = 0.198), but had lower correlations with excessive daytime sleepiness. Therefore, in subsequent analyses we separately considered the effects of insomnia symptoms on the latent variables daytime symptoms (sensory fatigue and depression), functional performance (self-reported physical function and 6MWT), and excessive daytime sleepiness.
We examined the effects of insomnia symptoms and the clinical and demographic covariates on the 2 latent variables and excessive daytime sleepiness (Table 2). The model was a good fit with the data as indicated by acceptable values of CFI and RMSEA. Participants with insomnia symptoms had significantly more daytime symptoms, more excessive daytime sleepiness, and lower functional performance.
Table 2.
Standardized coefficients of insomnia symptoms (DIMS), demographic, and clinical variables from the latent variable model
| Daytime Symptoms** |
Sleepiness |
Functional Performance** |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff. | SE | P | Coeff. | SE | P | Coeff. | SE | P | |
| DIMS (yes vs. no) | 0.98 | 0.29 | 0.001 | 0.18 | 0.07 | 0.013 | −0.45 | 0.13 | 0.001 |
| Demographic Variables | |||||||||
| Age | −0.25 | 0.13 | 0.058 | 0.07 | 0.10 | 0.519 | −0.28 | 0.10 | 0.006 |
| Gender (male vs. female) | −0.38 | 0.08 | < 0.001 | −0.03 | 0.08 | 0.669 | 0.46 | 0.07 | < 0.001 |
| Race (white vs. minority) | 0.13 | 0.09 | 0.158 | −0.10 | 0.09 | 0.257 | 0.20 | 0.08 | 0.020 |
| Clinical Variables | |||||||||
| Left ventricular ej. fraction % | −0.02 | 0.08 | 0.788 | 0.02 | 0.08 | 0.791 | −0.04 | 0.07 | 0.632 |
| NYHA classification | 0.19 | 0.08 | 0.022 | 0.08 | 0.08 | 0.323 | −0.40 | 0.08 | < 0.001 |
| Body mass index | −0.19 | 0.09 | 0.043 | 0.03 | 0.09 | 0.719 | < 0.01 | 0.08 | 0.994 |
| Charlson Comorbidity Index | 0.13 | 0.09 | 0.126 | 0.11 | 0.08 | 0.184 | −0.46 | 0.10 | < 0.001 |
| Respiratory disturbance index | 0.04 | 0.09 | 0.602 | 0.15 | 0.08 | 0.063 | −0.08 | 0.08 | 0.341 |
| %Time at O2 saturation < 90% | −0.06 | 0.08 | 0.420 | 0.06 | 0.08 | 0.462 | −0.08 | 0.07 | 0.266 |
| Interaction Effects | |||||||||
| Age × DIMS | −0.60 | 0.29 | 0.041 | - | - | - | - | - | - |
| Charlson Comorbidity Index × DIMS | - | - | - | - | - | - | 0.33 | 0.15 | 0.025 |
indicates effects on the latent variables; Goodness of fit Test: CFI = 0.970/RMSEA = 0.041
There were significant associations between several demographic and clinical variables and the dependent variables (Table 2). Age was associated with lower levels of daytime symptoms, but poorer functional performance. Female gender was significantly associated with higher levels of daytime symptoms, more daytime sleepiness, and poorer functional performance. Poorer NYHA classification was significantly associated with higher daytime symptoms and impairment of functional performance, while lower BMI was associated with lower levels of daytime symptoms, and comorbidity was associated with poorer functional performance. There were no statistically significant associations between the latent variables and RDI, oxygen desaturation, arousal index or periodic limb movements. There was a statistically nonsignificant trend (P = 0.064), suggesting that insomnia symptoms were associated with a lower RDI.
The means of the dependent variables, adjusted for relevant covariates in the regression analyses, are presented in Table 3. On average, patients who reported insomnia symptoms (DIMS) had CESD scores 6 points higher than those who did not have DIMS, and this level was 4 points higher than the level of CESD suggestive of clinical depression (CESD ≥ 16). Participants with DIMS were more likely to be depressed (OR = 5.09; 95% CI = 2.41 − 10.75), after adjusting for clinical and demographic covariates. DIMS was not significantly associated with the dichotomized excessive daytime sleepiness score (OR = 1.47; 95% CI = 0.71 −3.05). However, the least-square mean of the ESS score, adjusted for the covariates, was significantly greater (about 2 points higher) in patients who had DIMS compared to those who did not have DIMS.
Table 3.
Least square means for fatigue, depression, sleepiness, physical function, and six-minute walk after adjusting for covariates in the general linear regression model
| Symptoms | Insomnia Symptoms (DIMS) |
|
|---|---|---|
| Yes | No | |
| Mean (95% CI) | Mean (95% CI) | |
| Sensory fatigue | 6.10 (5.59, 6.61) | 4.88 (4.33, 5.43) |
| Depression | 20.66 (18.45, 22.87) | 13.84 (11.47, 16.21) |
| Sleepiness | 9.55 (8.60, 10.51) | 7.72 (6.68, 8.75) |
| Functional Performance | ||
| Physical function | 17.10 (16.16, 18.04) | 18.60 (17.60, 19.60) |
| Six-minute walk (feet) | 875.3 (791.0, 959.6) | 976.8 (882.9, 1070.7) |
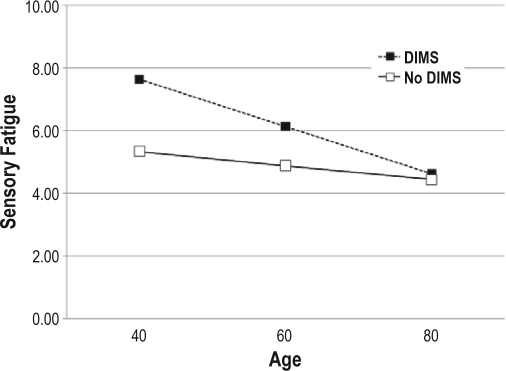
There was a statistically significant interaction effect between insomnia symptoms and age on daytime symptoms. Patients without insomnia symptoms reported fairly consistent levels of sensory fatigue across age levels, but age was inversely associated with fatigue in participants who had insomnia (Figure 1A). The change in fatigue for patients with DIMS was −0.07 (SE = 0.02) for each year of age. However, for those without DIMS it was −0.02 (SE = 0.02). There was a similar interaction effect between insomnia symptoms and age on depression (Figure 1B). The change in the CESD was −0.27 (SE = 0.08) for each year of age in patients with insomnia symptoms, while the change was only −0.10 (SE = 0.09) in those without.
Figure 1A.
Relationships between sensory fatigue and age in persons with insomnia symptoms and those without insomnia symptoms. Analysis is controlled for clinical and demographic covariates (gender, BMI, NYHA classification, left ventricular ejection fraction, comorbidity, respiratory disturbance index).
Figure 1B.
Relationships between CESD (depressive symptoms) and age in persons with insomnia symptoms and those without insomnia symptoms. Analysis is controlled for clinical and demographic covariates (gender, BMI, NYHA classification, left ventricular ejection fraction, comorbidity, respiratory disturbance index).
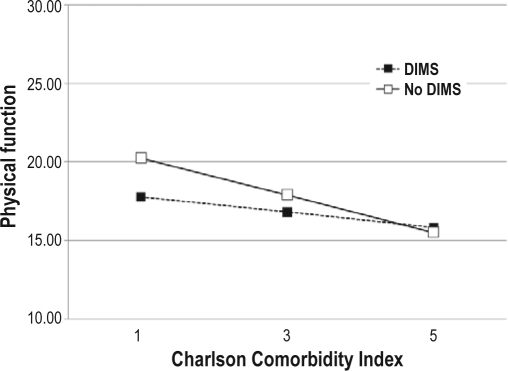
There was also a statistically significant interaction effect of insomnia symptoms and comorbidity on self-reported physical function. Comorbidity was significantly and negatively associated with physical function only in participants who did not report insomnia (Figure 2A). The change in physical function for patients with insomnia symptoms was −0.48 (SE = 0.34) for each one point increase in comorbidity, but the change in physical function for patients without insomnia symptoms was −1.19 (SE = 0.32). Similarly, the change in 6MWT distance for each one point increase in comorbidity was −17.3 feet (SE = 30.9) in patients with DIMS while the change was −74.7 feet in those without DIMS (Figure 2B). There were no other statistically significant interactions between DIMS and the clinical and demographic variables on the symptom or functional performance variables.
Figure 2A.
Relationships between self-reported physical function and comorbidity in persons with insomnia symptoms and those without insomnia symptoms. Analysis is controlled for clinical and demographic covariates (gender, age, BMI, NYHA classification, left ventricular ejection fraction, respiratory disturbance index).
Figure 2B.
Relationships between six-minute walk distance and comorbidity in persons with insomnia symptoms and those without insomnia symptoms. Analysis is controlled for clinical and demographic covariates (gender, age, BMI, NYHA classification, left ventricular ejection fraction, respiratory disturbance index).
DISCUSSION
Over half of patients with stable HF reported insomnia symptoms, with difficulty maintaining sleep the most common. Insomnia symptoms were more prevalent than in the Sleep Heart Health Study, a study of cardiovascular cohorts, in which 42.4% of women and 32.5% of men reported it, using the same items from the Sleep Habits Questionnaire.45
Insomnia symptoms were associated with polysomnographic and self-reported sleep characteristics, including sleep efficiency and duration and self-reported sleep latency. However, there were differences in magnitude between the self-report and objective variables. These discrepancies may be due to misperceptions about objective sleep characteristics, as is common in people with insomnia, or differences in the time-frames tapped by these measures (one night for PSGs and one month for the PSQI). The low PSG-recorded sleep efficiency may also reflect the intrusive nature of the sleep recordings.
We found that insomnia symptoms were not explained by SDB, EEG arousals, or periodic limb movements. The absence of a relationship between insomnia symptoms and SDB was consistent with past studies of older community residents.52,53 Our findings are also consistent with studies in which severity of HF (left ventricular ejection fraction, NYHA classification) did not explain insomnia symptoms.8,27
The large association between DIMS and depressive symptoms and the 100 foot difference in 6MWT distance, an objective measure of functional performance, between those with and without insomnia symptoms underscore the potential clinical significance of these findings. However, the clinical significance of the small incremental increase in sleepiness associated with DIMS is not clear. Although there has been little previous focus on the association between DIMS and excessive daytime sleepiness in HF, previous studies, including our work using the current sample,30 found no associations between SDB and ESS scores. Whether these findings are due to the lack of sensitivity of the ESS, a true lack of difference/association, or the alerting effects of increased sympathetic arousal associated with HF42 is not known. The extent to which insomnia symptoms are associated with objectively measured sleepiness in HF patients should be explored in a future study.
This study extended the findings of previous studies of HF populations5,8,27,29 by incorporating subjective and objective measures of daytime function and multi-dimensional measures of sleep. Unlike most previous studies, our sample was racially diverse, with 35% minority participants, the majority of whom were black. Recruitment of stable patients from structured HF disease management programs where treatment was somewhat standardized may have reduced the potential impact of acute illness on the sleep, symptom, and functional variables.
Use of confirmatory factor analysis permitted us to address overlaps between depressive symptoms and fatigue—problems that are both highly prevalent in this population and difficult to disentangle, given that fatigue is a somatic component of depression.54 Although there was no bivariate relationship between age and DIMS, the interaction effect between DIMS and age on fatigue and depression suggest that the impact of DIMS on these daytime symptoms is greater in young adults than older adults. Older adults had similar levels of fatigue and depression regardless of DIMS. The reasons for this are not clear, but older adults may become accustomed to insomnia symptoms, attribute them to aging, and/or underestimate them. It is also possible that the generally lower levels of fatigue and depression in the older adults are associated with adaptation of one's activities in response to long term experience with insomnia, fatigue, or depression.
The interaction effects between DIMS and comorbidity on self-reported and objective functional performance suggest that among people with DIMS, comorbidity has little effect on self-reported and objective functional performance. People who do not have DIMS have better functional performance at low levels of comorbidity than those with DIMS. However, this advantage does not exist at high levels of comorbidity. People with and without DIMS have similar levels of functional performance at higher levels of comorbidity.
Taken together, the interaction effects suggest that age and comorbidity may be important factors related to the impact of DIMS. These interactions and their implications for the diagnosis and treatment of insomnia should be examined in future studies.
Limitations
We did not utilize the full ICSD or DSM insomnia criteria that also include sleep-related dysfunction. Therefore, our data represent the proportion of the sample who reported selected insomnia symptoms rather than the proportion that have a clinical diagnosis of insomnia, and may overestimate the true prevalence of insomnia. The importance of these distinctions in obtaining accurate prevalence data was emphasized in a recent report.55 The rationale for this choice was the need to quantify the unique variance in symptoms and functional performance associated with insomnia symptoms. It would have been tautological to include sleep-related daytime dysfunction as a component of insomnia as an explanatory variable for daytime symptoms and functional performance. In addition, the nearly universal nature of daytime symptoms and functional performance deficits among HF patients (especially those with NYHA class II-IV HF that constituted the sample) may make it difficult for them to accurately attribute these experiences to insomnia.
A second limitation is inability to quantify the extent to which participants who reported insomnia symptoms might be appropriate candidates for insomnia treatment, as indicated by the presence of full insomnia criteria. Given the virtually universal nature of daytime dysfunction in this population and the dearth of studies of insomnia treatment among HF patients, there is a need to further identify HF patients who may benefit from behavioral or pharmacological treatment of insomnia or its symptoms.
Due to the cross-sectional nature of this study, it is possible that insomnia symptoms, daytime function, and functional performance decrements all reflect the underlying pathophysiology of HF. However, the absence of associations between insomnia and measures of severity of illness, such as left ventricular ejection fraction, comorbidity, and NYHA classification, conflict with this explanation. The chronic nature of HF, insomnia symptoms, and related problems, such as clinical depression, suggest the difficulty in disentangling the temporal relationships among these comorbid problems. For example, depression may be a symptom, cause of, or result of insomnia. Although insomnia symptoms were associated with depressive symptoms, there was no association with clinical depression, as recorded in the medical record.
Conclusions and Implications
Insomnia symptoms are common among stable HF patients and are associated with important quality of life concerns, including symptoms of depression, fatigue, excessive daytime sleepiness, and functional performance. Age and comorbidity appear to be important factors associated with the differential impact of DIMS on symptoms and functional performance. The presence of insomnia symptoms, despite the stable condition of the HF patients who received evidence-based HF disease management, suggests that HF disease management alone is not sufficient to ameliorate insomnia symptoms. Future studies are needed to further evaluate the temporal relationships and bio-behavioral mechanisms underlying the associations between insomnia and daytime function in HF patients and to identify HF patients who may benefit from pharmacological and/or behavioral insomnia treatment.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Walsleben has participated in speaking engagements for Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Our sincere acknowledgments for the assistance of Laura Adams, Robert Berkowitz, Lenore Blank, Nancy Bonnet, Ming-Guo Chen, George Evans, Ronald Freudenberger, Michelle Gilbert, Marybeth Gregory, Rakiel Kanayefska, Agha Khan, Syed Naqvi, Eileen Oates, Rubab Qureshi, Alison Rosen, Leslie Faith Morritt-Taub, Teresa Williams, and Mark J. Zucker.
This project was funded by NIH R01NR08022 (Redeker, PI).
REFERENCES
- 1.Thom T, Haase N, Rosamond W, et al. Heart Disease and Stroke Statistics--2006 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Friedman MM, King KB. Correlates of fatigue in older women with heart failure. Heart Lung. 1995;24:512–8. doi: 10.1016/s0147-9563(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 3.Grady KL, Jalowiec A, Grusk BB, White-Williams C, Robinson JA. Symptom distress in cardiac transplant candidates. Heart Lung. 1992;21:434–9. [PubMed] [Google Scholar]
- 4.Jaarsma T, Halfens R, Abu-Saad HH, Dracup K, Stappers J, van Ree J. Quality of life in older patients with systolic and diastolic heart failure. Eur J Heart Fail. 1999;1:151–60. doi: 10.1016/s1388-9842(99)00007-0. [DOI] [PubMed] [Google Scholar]
- 5.Mayou R, Blackwood R, Bryant B, Garnham I. Cardiac failure: Symptoms and functional status. J Psychosom Res. 1991;35:399–407. doi: 10.1016/0022-3999(91)90035-m. [DOI] [PubMed] [Google Scholar]
- 6.Principe-Rodriguez K, Strohl KP, Hadziefendic S, Pina IL. Sleep symptoms and clinical markers of illness in patients with heart failure. Sleep Breath. 2005;9:127–33. doi: 10.1007/s11325-005-0023-0. [DOI] [PubMed] [Google Scholar]
- 7.Redeker NS, Stein S. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart Lung. 2006;35:252–61. doi: 10.1016/j.hrtlng.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Erickson VS, Westlake CA, Dracup KA, Woo M, Hage A. Sleep disturbance symptoms in patients with heart failure. AACN Clin Iss. 2003;14:477–87. doi: 10.1097/00044067-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Brostrom A, Stromberg A, Dalstrom U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19:234–42. doi: 10.1097/00005082-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 11.Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–22. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 12.Chan J, Sanderson J, Chan W, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997;111:1488–93. doi: 10.1378/chest.111.6.1488. [DOI] [PubMed] [Google Scholar]
- 13.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 14.National Sleep Foundation. 2005 Sleep in American Poll. [Accessed November 21, 2006];2005 http://www.sleepfoundation.org/hottopics/index.php?secid=16&id=24. [Google Scholar]
- 15.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 16.Blackshear JL, Kaplan J, Thompson RC, Safford RE, Atkinson EJ. Nocturnal dyspnea and atrial fibrillation predict Cheyne-Stokes respirations in patients with congestive heart failure. Arch Intern Med. 1995;155:1297–302. [PubMed] [Google Scholar]
- 17.Javaheri S, Abraham WT, Brown C, Nishiyama H, Giesting R, Wagoner LE. Prevalence of obstructive sleep apnoea and periodic limb movement in 45 subjects with heart transplantation. Eur Heart J. 2004;25:260–6. doi: 10.1016/j.ehj.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Trupp RJ, Hardesty P, Osborne J, et al. Prevalence of sleep disordered breathing in a heart failure program. Congest Heart Fail. 2004;10:217–20. doi: 10.1111/j.1527-5299.2004.03557.x. [DOI] [PubMed] [Google Scholar]
- 19.Rao A, Georgiadou P, Francis DP, et al. Sleep-disordered breathing in a general heart failure population: relationships to neurohumoral activation and subjective symptoms. J Sleep Res. 2006;15:81–8. doi: 10.1111/j.1365-2869.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 20.Lanfranchi PA, Somers VK. Sleep-disordered breathing in heart failure: characteristics and implications. Resp Physiol Neurobiol. 2003;136:153–65. doi: 10.1016/s1569-9048(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 21.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106:21–8. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 23.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 24.Sin DD, Man GC, Jones RL. Central sleep apnea and heart failure. N Engl J Med. 2000;342:293–4. [PubMed] [Google Scholar]
- 25.Chatterjee K, Massie B. Systolic and diastolic heart failure: differences and similarities. J Card Fail. 2007;13:569–76. doi: 10.1016/j.cardfail.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Redeker N, Hilkert R. Sleep and quality of life in stable heart failure. J Card Fail. 2005;11:700–4. doi: 10.1016/j.cardfail.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Manocchia M, Keller S, Ware JE. Sleep problems, health-related quality of life, work functioning and health care utilization among the chronically ill. Qual Life Res. 2001;10:331–45. doi: 10.1023/a:1012299519637. [DOI] [PubMed] [Google Scholar]
- 29.Steptoe A, Mohabir A, Mahon NG, McKenna WJ. Health related quality of life and psychological wellbeing in patients with dilated cardiomyopathy. Heart. 2000;83:645–50. doi: 10.1136/heart.83.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redeker NS, Muensch U, Zucker MJ, et al. Sleep disordered breathing, symptoms, and functional performance in stable heart failure. Sleep. 2010;33:551–60. doi: 10.1093/sleep/33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leidy NK. Functional status and the forward progress of merry-go-rounds: Toward a coherent analytical framework. Nurs Res. 1994;43:196–202. [PubMed] [Google Scholar]
- 32.Guyatt GH, Thompson PJ, Berman LB, et al. How should we measure function in patients with chronic heart and lung disease? J Chron Dis. 1985;38:517–24. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 33.Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed) 1986;292:653–5. doi: 10.1136/bmj.292.6521.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyatt G. Use of the six-minute walk test as an outcome measure in clinical trials in chronic heart failure. Heart Fail. 1987;3:211–71. [Google Scholar]
- 35.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I.Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 36.Ware JE. Boston: Health Institute, New England Medical Center; 1993. SF-36 Health survey manual and interpretation guide. [Google Scholar]
- 37.Piper B, Lindsey A, Dodd M, Ferketich S, Paul S, Wellver S. The development of an instrument to measure the subjective dimensions of fatigue. In: Funk S, Tornquist E, Champagne M, Wiese R, editors. Key aspects of comfort: management of pain, fatigue, and nausea. New York: Springer; 1989. [Google Scholar]
- 38.Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clin Gerontol. 1986;5:119–36. [Google Scholar]
- 39.Devins G, Orme C. Center for Epidemiologic Studies Depression Scale. In: Keyser D, Sweetland R, editors. Test Critiques. Kansas City, MO: Westport Publishers; 1985. pp. 144–60. [Google Scholar]
- 40.Devins G, Orme C, Costello C. Measuring depressive symptoms in illness populations: Psychometric properties of the center for epidemiologic studies depression (CES-D) scale. Psychol Health. 1988;2:139–56. [Google Scholar]
- 41.Mulrow CD, Williams JW, Jr, Gerety MB, Ramirez G, Montiel OM, Kerber C. Case-finding instruments for depression in primary care settings. Ann Intern Med. 1995;122:913–21. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- 42.Redeker NS, Thomas RJ, Mietus JE, Chung-Kang P, Goldberger AL. Assessment of sleep quality in stable heart failure using the ECG-derived sleep spectrogram. submitted. [Google Scholar]
- 43.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 45.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 46.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 47.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/ Brain Research Institute, University of California; 1968. [Google Scholar]
- 48.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 49.American Academy of Sleep Medicine Task Force. EEG arousals: Scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 50.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–46. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 51.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen K, Long K, editors. Testing structural equation models. Newbury Park: Sage; 1993. pp. 136–62. [Google Scholar]
- 52.Johansson P, Alehagen U, Svanborg E, Dahlstrom U, Brostrom A. Sleep disordered breathing in an elderly community-living population: Relationship to cardiac function, insomnia symptoms and daytime sleepiness. Sleep Med. 2009;10:1005–11. doi: 10.1016/j.sleep.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Gooneratne NS, Gehrman PR, Nkwuo JE, et al. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 54.Redeker NS. Somatic symptoms explain depressive symptoms in heart failure patients vs. a comparison group. Circulation. 2005;112(17, Supp II) [Google Scholar]
- 55.Ohayon MM, Reynolds CF., 3rd Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD) Sleep Med. 2009;10:952–60. doi: 10.1016/j.sleep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]