Abstract
Study Objectives:
To investigate whether enhancement of slow wave sleep (SWS) with sodium oxybate reduces the impact of sleep deprivation.
Design:
Double-blind, parallel group, placebo-controlled design
Setting:
Sleep research laboratory
Participants:
Fifty-eight healthy adults (28 placebo, 30 sodium oxybate), ages 18-50 years.
Interventions:
A 5-day protocol included 2 screening/baseline nights and days, 2 sleep deprivation nights, each followed by a 3-h daytime (08:00-11:00) sleep opportunity and a recovery night. Sodium oxybate or placebo was administered prior to each daytime sleep period. Multiple sleep latency test (MSLT), psychomotor vigilance test (PVT), Karolinska Sleepiness Scale (KSS), and Profile of Mood States were administered during waking hours.
Measurements and Results:
During daytime sleep, the sodium oxybate group had more SWS, more EEG spectral power in the 1-9 Hz range, and less REM. Mean MSLT latency was longer for the sodium oxybate group on the night following the first daytime sleep period and on the day following the second day sleep period. Median PVT reaction time was faster in the sodium oxybate group following the second day sleep period. The change from baseline in SWS was positively correlated with the change in MSLT and KSS. During recovery sleep the sodium oxybate group had less TST, SWS, REM, and slow wave activity (SWA) than the placebo group.
Conclusions:
Pharmacological enhancement of SWS with sodium oxybate resulted in a reduced response to sleep loss on measures of alertness and attention. In addition, SWS enhancement during sleep restriction appears to result in a reduced homeostatic response to sleep loss.
Citation:
Walsh JK; Hall-Porter JM; Griffin KS; Dodson ER; Forst EH; Curry DT; Eisenstein RD; Schweitzer PK. Enhancing slow wave sleep with sodium oxybate reduces the behavioral and physiological impact of sleep loss. SLEEP 2010;33(9):1217-1225.
Keywords: Slow wave sleep, sleepiness, attention, sleep deprivation, sodium oxybate
SLOW WAVE SLEEP (SWS) HAS BEEN HYPOTHESIZED TO BE A STATE OF RELATIVELY HIGH NEURAL RECUPERATION FROM WAKEFULNESS.1,2 THIS HYPOTHESIS has been prompted by a number of observations, including: (a) enhanced SWS following sleep deprivation in proportion to the duration of prior wakefulness3; (b) reduced amounts of SWS during nocturnal sleep following afternoon/evening naps4; (c) a decline in SWS across a night of sleep5; and (d) increased SWS following nights of fragmented sleep.6 Within the two-process model of sleep regulation, heightened SWS has been viewed as reflecting Process S, the homeostatic component.7 Many authors have proposed that increased SWS represents ongoing cortical recovery from prior wakefulness activities and is a time of relatively heightened neurophysiologic restoration or recuperation.1,2,8
In a prior study involving selective SWS deprivation, there was a suggestion from post hoc analyses that SWS may play a role in preventing adverse effects of sleep loss.9 Additionally, we recently published the results of two investigations of pharmacologically enhanced SWS (with tiagabine or gaboxadol) during sleep restriction, which demonstrated evidence of preserved alertness or neurobehavioral performance despite sleep restriction.10,11 In the current study, we examined whether pharmacological enhancement of SWS with sodium oxybate reduces the impact of sleep deprivation upon sleepiness, attention, cognition, mood, and recovery sleep. Sodium oxybate has been demonstrated to increase SWS and/or slow wave activity (SWA) in a dose-related fashion in normal subjects12,13 and in patients with narcolepsy14,15 and fibromyalgia.16 Sodium oxybate is the sodium salt of γ-hydroxybutyrate, an endogenous fatty acid synthesized in the brain, which appears to exert most of its effects through GABAB receptors, although it binds to both GHB and GABAB receptors.17 Time to peak plasma concentration is 0.5-1.25 hours and the elimination half-life ranges from about 30 to 50 minutes.18
METHODS
Study Design
A randomized, double-blind, placebo-controlled, parallel groups design was used to compare sodium oxybate 3.5 g and placebo. The study design involved 5 consecutive nights and the intervening 4 days (see Figure 1). On Nights 1 and 2, subjects slept at night. On Nights 3 and 4, subjects remained awake all night but were permitted a 3-h sleep opportunity the next morning from 08:00 to 11:00. Night 5 served as a recovery night. Subjects received placebo or sodium oxybate 3.5 g in randomized, double-blind fashion 15 min prior to each of the two 3-h daytime sleep opportunities. This dose was chosen to maximize the effect on SWS during the 3-h sleep periods while limiting residual sedation and side effects. Van Cauter et al. demonstrated that 3.5 g of sodium oxybate increased SWS only during the first one-third of the night in healthy subjects.13 Group assignment was stratified to ensure approximate balance in age and sex distribution. Polysomnography (PSG) was conducted during scheduled sleep periods. Assessments of sleep propensity, performance, memory, and mood were conducted during waking hours. For each subject, the study period was a minimum of 8 days and a maximum of 22 days from initial screening to end-of-study visit. The protocol was approved by the Institutional Review Board of St. Luke's Hospital. All subjects signed informed consent and were compensated for participation. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. The study design and methods, data analyses, interpretation of study findings, and manuscript production were the sole responsibility of the authors. Jazz Pharmaceuticals supplied study drug and matching liquid placebo. This investigation was listed at www.clinicaltrials.gov.
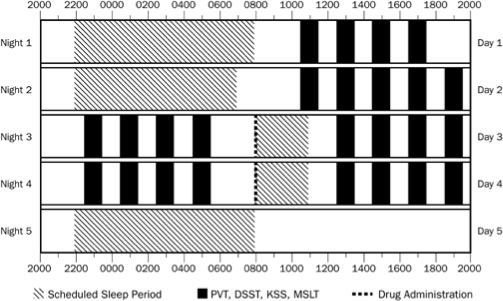
Figure 1.
Study schedule. Hatched bars indicate times of nighttime and daytime sleep periods. Black bars indicate test times for the PVT, DSST, KSS, and MSLT. The dashed line indicates time of administration of sodium oxybate or placebo prior to sleep periods on Day 3 and Day 4. For each test time, a 15-min PVT was administered, followed by DSST, KSS, and MSLT subtest. Exact times of administration of all study procedures are provided in the text under Methods.
Subject Recruitment and Selection
Subjects were recruited via media advertisements. A general description of the study was provided and preliminary screening was conducted by telephone. Interested and eligible persons were scheduled for a screening office visit during which a thorough explanation of the study was provided and subjects gave written informed consent. Clinical screening procedures included a sleep, psychiatric, and medical history; physical examination; electrocardiogram; Epworth Sleepiness Scale (a score ≥ 15 was exclusionary); clinical laboratory testing (hematology and chemistry); and urine screen for drugs of abuse. These procedures insured that subjects were free of chronic sleep disturbance, DSM-IV psychiatric diagnoses including substance abuse in the past 2 years, and current or recent medical illness. Females could not be pregnant (as verified by a serum pregnancy test) or lactating and had to confirm the use of adequate contraceptive procedures throughout the study. During the prior 2 months, the subjects must have reported a bedtime between 21:00 and 01:00 at least 5 nights per week and usual nightly sleep duration between 6.5 and 8.5 h. A body mass index of 19-34 kg/m2 (inclusive) was also required. Subjects could not work night or rotating shifts or have crossed more than 3 time zones in the prior month. Subjects were also excluded if they used any psychotropic medication or sedating or alerting over-the-counter drugs during the prior week or 5 half-lives (whichever was longer), consumed an average of > 300 mg caffeine or > 3 alcoholic beverages per day, or smoked > 5 cigarettes per day. Participation in a clinical research trial or blood donation within 30 days, prior use of sodium oxybate, history of a positive test for human immunodeficiency virus, hepatitis B surface antigen, and hepatitis C virus were additional exclusion criteria.
Subjects were excluded if, during the Night 1 PSG, the apnea-hypopnea index was > 10 per hour or the periodic limb movement arousal index was > 10 per hour. Subjects were also excluded if the mean latency on the multiple sleep latency test (MSLT) conducted following the Night 2 PSG was < 4 min.
Study Procedures
Each subject underwent a 5-night/ 4-day protocol (Figure 1). Nights 1 and 2 were screening/baseline nights. PSG was recorded for 10 h on Night 1 (22:00 to 08:00) and 9 h on Night 2 (23:00 to 07:00). The durations of these recordings were selected to minimize the impact of prior sleep history. During the day following these nights (Day 1 and Day 2), subjects underwent a battery of tests including MSLT, psychomotor vigilance test (PVT), digit symbol substitution test (DSST), Karolinska Sleepiness Scale (KSS), Profile of Mood States (POMS), and memory tests.
On Nights 3 and 4, subjects were kept awake from 22:00 until 08:00 and underwent MSLT, PVT, DSST, KSS, and POMS testing. On Days 3 and 4, subjects were randomized to receive sodium oxybate 3.5 g or matching placebo liquid at 07:45 and were then permitted a 3-h sleep opportunity from 08:00 to 11:00. MSLT, PVT, and other tests resumed following this sleep period each day. On Night 5, PSG recording was conducted from 22:00 to 08:00 to assess recovery sleep.
Subjective sleep measures were collected with post-sleep questionnaires. Changes in health status and concomitant medications were recorded throughout the study. Alcohol was prohibited beginning 48 h prior to laboratory Night 1 and for the duration of participation. Caffeine consumption and vigorous exercise were prohibited when subjects were in the sleep laboratory. Meals were provided at 08:00, 12:00, and 18:00 on Days 1 and 2; 02:00 on Nights 3 and 4; and 11:00 and 18:00 on Days 3 and 4. Snacks were offered at 15:30 on Days 1-4, and 22:00 and 05:30 on Nights 3 and 4. When drug was administered at 07:45 on Days 3 and 4, subjects had not eaten for > 2 hours. Subjects were continuously monitored by research study personnel to confirm adherence to study protocol requirements including avoidance of sleep during scheduled wake times. When not performing study-specific tasks, subjects were occupied with reading, television viewing, or other sedentary activities.
Within 7 days after completion of the laboratory procedures, subjects were seen for a brief physical examination and other safety assessments as determined by the study physician.
DEPENDENT MEASURES
Polysomnography (PSG)
Sleep was recorded with the Embletta system using the following recording montage: right and left electrooculogram, submental electromyogram (EMG), electrocardiogram (V5), and 6 EEG derivations (C3-A2, C4-A1, O1-A2, O2-A1, F3-A2, F4-A1). On Night 1, the recording also included nasal thermocouple and right and left anterior tibialis EMG. Sampling rate for all EEG signals was 200 Hz. At this rate, the Embletta recording system rejects frequencies > 90 Hz to avoid aliasing during spectral analysis. No additional filtering was applied during data acquisition. All PSGs were scored according to standard methods19 using the C3-A2 derivation, with filtering for EEG set at 0.3 to 35 Hz for visual scoring. Each subject's PSGs were scored by a single scorer.
EEG Spectral Power
Spectral analysis was accomplished using Vitascore 1.30 software (TEMEC Instruments B.V., Kerkrade, The Netherlands). Each PSG recording was converted to European Data Format and re-sampled to 256 Hz prior to analysis. No filtering was applied. Recordings containing artifact in more than 25% of epochs were excluded from analysis. Spectral analysis was conducted on either C3-A2 or C4-A1 during NREM epochs using a 4-second tapering window, overlapping 25%. Quarter-Hz bins were summed to obtain absolute power (μV2/Hz) in 1-Hz bins up to 31 Hz. SWA (power in the 0.75-4.5 Hz band) was also calculated. Since time in bed was only 3 hours on Days 3 and 4, spectral analysis was conducted for NREM during the first 3 hours of all 5 PSG recordings.
Post-sleep Questionnaire
Within 10 min after each PSG, subjects completed a questionnaire on which they reported estimates of sleep latency, sleep duration, number of awakenings, and wake after sleep onset. Sleep quality and refreshing nature of sleep were also rated on a 7-point categorical scale.
Multiple Sleep Latency Test (MSLT)
The MSLT evaluates sleep propensity by electrophysiologically measuring the latency to fall asleep at multiple times throughout the day. MSLT subtests were conducted at 2-h intervals from 11:00 to 17:00 on Day 1; 11:00 to 19:00 on Day 2; 13:00 to 19:00 on Days 3 and 4; and 23:00 to 05:00 on Nights 3 and 4. MSLTs were conducted using standard research procedures,20 and all MSLTs for a subject were scored by a single scorer.
Psychomotor Vigilance Test (PVT)
The PVT is a simple reaction time test which measures sustained attention and psychomotor function.21 The PVT was performed for 15 min at 2-h intervals from 10:30 to 16:30 on Day 1; 12:30 to 18:30 on Days 2, 3, and 4; and 22:30 to 04:30 on Nights 3 and 4. Dependent variables analyzed were median reaction time, number of lapses (reaction time > 500 msec), and mean of the slowest 10% reaction times.
Digit Symbol Substitution Test (DSST)
The DSST is a cognitive throughput task used to measure speed and accuracy by requiring participants to substitute symbols (of pre-designated digit-symbol pairs) with corresponding digits continuously for 1.5 min. The key that links symbols and digits is visually available throughout testing. The DSST was administered approximately 5 min after each PVT. Six different versions were used in counterbalanced fashion. The primary performance measure was the number of correct substitutions.
Profile of Mood States (POMS)
The POMS is a self-administered adjective rating scale that measures 6 dimensions of affect or mood: (1) tension-anxiety, (2) depression-dejection, (3) anger-hostility, (4) vigor-activity, (5) fatigue-inertia, and (6) confusion-bewilderment.22 Subjects rate how they feel “now” with respect to 65 adjectives on a 5-point scale (0 = not at all, 4 = extremely). The POMS was completed at 14:00 on Days 2, 3, and 4, and at 01:30 on Nights 3 and 4.
Karolinska Sleepiness Scale (KSS)
The KSS is a 9-point rating scale which provides a subjective measurement of sleepiness (1 = very alert, 9 = very sleepy).23 The KSS was completed approximately 2 min prior to each MSLT subtest.
Memory Tests
A word pair declarative memory test and a finger tapping procedural memory test were performed and will be reported in a subsequent publication.
Wisconsin Card Sorting Task (WCST)
The WCST assesses concept formation and abstraction ability by having the subject identify 3 predetermined criterion principles.24 This task is subject-paced, but stimuli are presented continuously until the principles have been identified. Typical task duration is 20-30 min. As duplicate forms were not available for this test, it was administered once to each subject at 03:30 on Night 4. Variables of interest included percent correct trials, number of trials to completion of the first criterion, total number of errors, and number of perseverative errors.
Statistical Analysis
For data with multiple time points, a linear mixed model (LMM; SYSTAT version 12) was used to evaluate group differences while controlling for sex, age, and baseline value (when available). Factors for day/night, time of day/night, and their interactions were included as appropriate. Alpha was set at 0.05. Two-sided tests were used. Significant group by day interactions were followed by group comparisons on mean values for each day. Significant group by time interactions were followed by group comparisons at the 4 test times. For data with multiple time points per day, planned comparisons were conducted between groups for mean data on Day 3 and Day 4. For spectral data, groups were compared via general linear model ANOVA on individual 1-Hz bins from 1 through 15 Hz and on SWA (0.75–4.5 Hz) using Night 2 power as a covariate.
Night 2 PSG values were used as baseline for analysis of Day 3 and Day 4 PSG. Similarly, Night 2 spectral power was used as baseline for analysis of Day 3 and Day 4 spectral data. For MSLT, PVT, KSS, DSST, and POMS, Day 2 data were used as baseline for evaluation of Day 3 and Day 4 data; while Night 3 data were used as baseline for evaluation of Night 4 data. Subjective assessments of sleep were analyzed without baseline.
To evaluate group differences in recovery sleep, general linear model (GLM) was applied to PSG data from Night 5 using Night 2 as baseline. Group differences were also assessed for Stage 3, Stage 4, SWS, and SWA, using data from the first 3 h of the recordings.
Spectral data were analyzed from the placebo group alone to evaluate changes during sleep loss and recovery sleep without the effect of sodium oxybate using LMM on log-transformed absolute power from the first 3 h of Night 2, Day 3, Day 4, and Night 5 recordings. A Night 2 versus Night 5 comparison was also conducted for the sodium oxybate group to determine if the drug affected recovery sleep differently than placebo. Significant F-ratios were followed by paired comparisons. As these analyses were considered exploratory, no correction for multiple comparisons was applied.
The dependent variables of the WCST (number of errors, number of perseverative errors, and number of trials to first criterion) are highly inter-correlated; therefore a multivariate analysis of variance (MANOVA) was employed to compare groups while controlling for age and sex. Errors, perseverative errors, and trials to first criterion were log-transformed prior to analysis to normalize distributions.
Mean values from statistical comparisons are presented in the text as least square means ± standard errors generated by the statistical models. Data in tables and figures are unadjusted means and standard deviations (unless otherwise stated) presented for the reader's convenience.
Mean sleep latency on the MSLT on Day 3 and Day 4 were considered co-primary dependent measures. A sample size of 30 per group provided 73% power to detect a 2.0-min difference between groups (88% power for a 2.5-min difference) on mean MSLT latency using a 2-sided test and assuming a pooled standard deviation of 3.0 min.
RESULTS
Subjects
One hundred twenty-four individuals gave written informed consent; 10 persons withdrew consent prior to randomization, and 50 individuals failed screening (29 MSLT, 5 BMI, 5 sleep history, 3 sleep apnea, 2 medical history, 4 positive alcohol/drug screen, 2 skin irritation). Sixty-four healthy male and female subjects ages 18-50 inclusive, were randomized to the 2 study groups; 57 completed the protocol and 7 terminated prematurely. The reasons for premature termination were nausea (4 sodium oxybate), menstrual cramps (1 sodium oxybate), and tiredness (1 sodium oxybate, 1 placebo). Data analyses examining effect of sodium oxybate on sleep, waking function, and recovery sleep were conducted on all subjects receiving randomized study drug who completed study assessments on Day 3 (N = 58). This included one sodium oxybate subject who withdrew from the study following Night 4. Data analyses also included 2 subjects whose preliminary MSLT scoring indicated they met the Day 2 MSLT inclusion criterion (mean latency > 4 min) but for whom final scoring showed latencies of 2.9 and 3.5 min. Table 1 presents demographic data on the data analysis sample. The placebo and sodium oxybate groups were similar in age, sex and racial distribution, body mass index, and number of years of education.
Table 1.
Demographic characteristics of study participants (data analysis set)
| Placebo | Sodium Oxybate | |
|---|---|---|
| Number of subjects | 28 | 30 |
| Females/Males | 17/11 | 19/11 |
| Caucasian/African American | 24/4 | 28/2 |
| Mean age in years (range; SD) | 27.1 (19-48; 8.8) | 27.1 (18-49; 9.2) |
| Mean years of education (SD) | 14.8 (1.7) | 14.9 (1.6) |
| Mean body mass index (SD) | 24.7 (3.4) | 24.9 (1.6) |
Polysomnography and Spectral Analysis
Unadjusted mean PSG data for the baseline night (Night 2), daytime sleep periods (Days 3 and 4), and the recovery night (Night 5) are shown in Table 2. The 2 groups were numerically similar at baseline for TST, minutes of each sleep stage, and all other PSG variables.
Table 2.
Observed mean (SD) polysomnography variables for sodium oxybate (SO) and placebo groups
| Night 2: Baseline |
Day 3 |
Day 4 |
Night 5: Recovery |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo | SO | Placebo | SO | Placebo | SO | Placebo | SO | |
| Total sleep time (min) | 478.6 (35.72) | 467.0 (47.51) | 171.7 (5.28) | 171.9 (8.33) | 172.4 (8.28) | 174.4 (5.23) | 550.5 (29.36) | 520.6 (55.25)‡ |
| Latency to persistent sleep (min) | 23.4 (16.81) | 32.1 (29.41) | 2.9 (3.28) | 2.2 (2.40) | 1.3 (1.13) | 2.5 (3.08) | 16.0 (17.26) | 28.2 (21.17)‡ |
| Stage 1 (min) | 65.4 (23.69) | 67.3 (20.29) | 16.2 (7.77) | 10.0 (7.97)‡‡ | 13.7 (7.53) | 11.6 (10.61) | 53.8 (18.06) | 61.8 (20.74) |
| Stage 2 (min) | 239.6 (32.62) | 239.3 (38.32) | 63.4 (16.13) | 62.5 (18.91) | 60.6 (19.05) | 66.0 (21.81) | 276.6 (37.08) | 281.8 (40.83) |
| Stage 3 (min) | 41.2 (15.75) | 37.7 (12.00) | 28.3 (13.34) | 33.5 (15.41) | 25.3 (12.31) | 34.7 (18.68)‡‡ | 51.0 (20.62) | 44.6 (17.10) |
| Stage 4 (min) | 34.1 (28.62) | 26.3 (24.86) | 32.6 (26.64) | 53.8 (26.22)‡‡ | 34.7 (25.61) | 50.5 (29.81)‡‡ | 35.0 (30.44) | 17.8 (20.24)‡ |
| SWS (min) | 75.3 (36.38) | 63.9 (26.81) | 60.9 (21.38) | 87.3 (25.76)‡‡ | 60.0 (25.07) | 85.2 (27.27)‡‡ | 86.0 (43.39) | 62.4 (29.48)‡ |
| REM (min) | 98.2 (23.65) | 96.5 (23.33) | 31.2 (14.39) | 12.2 (11.84)‡‡ | 38.0 (14.23) | 11.5 (11.23)‡‡ | 134.1 (22.58) | 114.6 (23.22)‡ |
| Wake after sleep onset (min) | 40.4 (36.05) | 43.5 (34.46) | 6.2 (3.88) | 6.5 (8.18) | 6.4 (8.10) | 3.9 (4.92) | 35.0 (29.25) | 53.8 (53.92) |
| Number of shifts to wake or Stage 1 | 52.0 (18.64) | 48.3 (15.42) | 13.0 (5.60) | 7.2 (6.29)‡‡ | 11.0 (7.70) | 7.2 (7.54) | 42.4 (14.16) | 46.5 (16.90) |
| Number of awakenings ≥ 30 sec | 33.7 (11.75) | 31.0 (10.49) | 7.1 (3.34) | 3.6 (3.21) | 6.3 (3.73) | 3.4 (3.43) | 27.8 (10.47) | 30.8 (11.84) |
| Number of awakenings ≥ 1 min | 9.7 (5.64) | 10.5 (5.66) | 2.1 (1.78) | 1.3 (1.42) | 1.5 (1.73) | 1.0 (1.22) | 6.9 (5.26) | 9.4 (6.06) |
| Latency to SWS (min) | 13.2 (4.58) | 15.0 (8.45) | 17.0 (14.97) | 20.1 (18.94) | 17.1 (12.01) | 14.4 (14.71) | 14.3 (5.23) | 37.5 (37.00) |
| REM Latency (min) | 68.3 (25.57) | 68.6 (22.49) | 45.9 (27.10) | 62.3 (63.09) | 36.3 (25.40) | 81.3 (61.52) | 62.8 (26.95) | 79.9 (47.51) |
P < 0.05, sodium oxybate significantly different from placebo;
P < 0.01, sodium oxybate significantly different from placebo
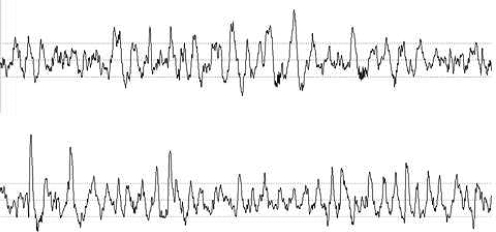
Averaged across Days 3 and 4, least square mean TST values were 171.8 ± 0.9 for placebo and 172.8 ± 0.9 min for sodium oxybate and did not differ significantly (P = 0.37). The sodium oxybate group displayed significantly more stage 3 (34.4 ± 2.3 vs. 25.8 ± 2.3 min, P = 0.01), stage 4 (54.3 ± 2.6 vs. 30.4 ± 2.7 min, P < 0.001), and SWS (88.2 ± 2.8 vs. 56.8 ± 2.9 min, P < 0.001) than did the placebo group. Figure 2 displays 30-sec EEG tracings from a representative subject with and without drug. Morphology of the waveform with drug was very similar to that with placebo. The placebo group showed more REM (34.9 ± 2.2 vs. 12.3 ± 2.2 min, P < 0.001) and slightly more stage 1 (15.3 ± 1.3 vs. 11.0 ± 1.2 min, P = 0.016) than the sodium oxybate group. There were no group differences in stage 2 sleep. The placebo group had slightly more shifts to wake or stage 1 (12 ± 1.0 vs. 7.7 ± 1.0, P = 0.002).
Figure 2.
A representative 30-sec EEG tracing during slow wave sleep for one subject on placebo (top) versus sodium oxybate (bottom). The placebo tracing was taken from Night 2; the sodium oxybate tracing was taken from Day 3.
Figure 3 shows NREM spectral data for Day 3 and Day 4 expressed as percent change from NREM during the first 3 h of baseline Night 2. Power density was higher for sodium oxybate than placebo on Day 3 and Day 4 for SWA and for individual 1-Hz bins ranging from 1 to 9 Hz (P < 0.02 for all), with the exception of the 2-Hz bin on Day 4. Power was lower for sodium oxybate in the 12-Hz and 13-Hz bins on Day 3 and the 12-Hz bin on Day 4 (P < 0.05 for all).
Figure 3.
NREM spectral power values for each 1-Hz bin for the sodium oxybate and placebo groups on Day 3 and Day 4, expressed as percent change from baseline Night 2 (first 3 h). Baseline is indicated as a horizontal black line at 0%. Standard error bars are shown. Horizontal lines at the bottom of the figure indicate significant differences between groups (analyses conducted up to 15 Hz). Solid lines indicate group differences on Day 3 PSG, and stippled lines indicate group differences on Day 4 PSG.
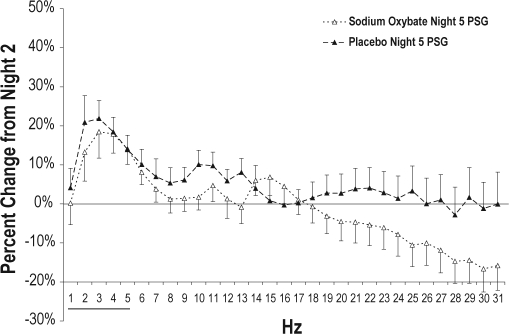
Recovery sleep on Night 5 differed significantly between groups, as can be seen in Table 2. The sodium oxybate group had less TST (521.9 ± 7.7 vs. 548.6 ± 7.7 min, P = 0.016), longer LPS (26.8 ± 3.57 vs. 16.6 ± 3.63 min, P = 0.05), less stage 4 (20.9 ± 2.85 vs. 32.8 ± 2.82 min, P = 0.004), less SWS (68.7 ± 3.8 vs. 81.4 ± 3.9 min, P = 0.023), less REM (114.4 ± 4.0 vs. 133.1 ± 4.0 min, P = 0.002), and a trend for more wake after sleep onset (53.7 ± 6.9 vs. 36.1 ± 7.0 min, P = 0.076). In addition, analysis of stage 3, stage 4, SWS, and SWA from the first 3 h of the recordings demonstrated less Stage 4 (30.5 ± 4.8 vs. 50.0 ± 4.8 min, P = 0.006), less SWS (41.2 ± 3.3 vs. 51.6 ± 3.3 min, P = 0.03), and less SWA (3940.4 ± 182.4 vs. 4520.2 ± 189.1 μV2/Hz; P = 0.04) for sodium oxybate than for placebo. Night 5 SWA was increased over Night 2 levels in the placebo group (4288.3 ± 2147.9 vs. 4746.1 ± 2495.3 μV2/Hz, P = 0.038) but were not significantly different in the sodium oxybate group (Night 2 = 3816.4 ± 2008.0 μV2/Hz, Night 5 = 3760.1 ± 1771.8 μV2/Hz, P = 0.71). Night 5 spectral power for sodium oxybate and placebo is displayed in Figure 4.
Figure 4.
NREM spectral power values for each 1-Hz bin for the sodium oxybate and placebo groups on Night 5 (first 3 h), expressed as percent change from baseline Night 2 (first 3 h). Baseline is indicated as a horizontal black line at 0%. Standard error bars are shown. The black horizontal line at the bottom of the figure indicates a significant difference between groups in SWA (0.75-4.5 Hz).
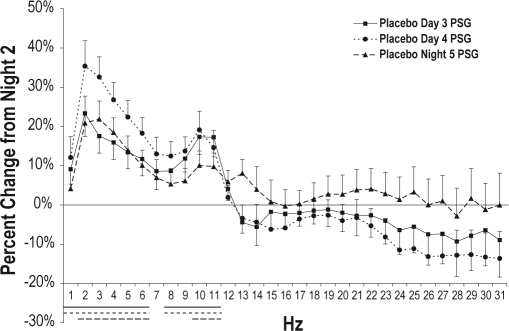
Spectral power in the placebo group was further analyzed to assess spectral power changes across sleep periods (Figure 5). Compared to baseline Night 2, SWA was increased on Day 3 (P < 0.001), Day 4 (P < 0.001), and Night 5 (P = 0.014). SWA decreased from Day 4 to Night 5 (P = 0.012). Examination of individual 1-Hz bins showed power was significantly increased, relative to Night 2, from 1 to 6 Hz and 8 to 11 Hz on both Day 3 and Day 4, and from 2 to 6 Hz and 10 to 11 Hz on Night 5 (P < 0.02 for all). Power from 2 to 5 Hz was higher on Day 4 than on Day 3 (P < 0.05 for all). Compared to Day 4, power on Night 5 was lower in the 2-, 3-, 5-, 6-, and 9-Hz bins (P < 0.03 for all) and higher in the 13-Hz bin (P = 0.008).
Figure 5.
NREM spectral power values for each 1-Hz bin for the placebo group on Day 3, Day 4, and Night 5 (first 3 h), expressed as percent change from baseline Night 2 (first 3 h). Baseline is indicated as a horizontal black line at 0%. Standard error bars are shown. Horizontal lines at the bottom of the figure indicate significant differences from paired comparisons on log-transformed data. Solid lines indicate differences between Night 2 and Day 3 PSG. Stippled lines indicate differences between Night 2 and Day 4 PSG. Dashed lines indicate differences between Night 2 and Night 5 PSG.
Post-sleep Questionnaire
There were no significant group differences on any subjective sleep estimates on Day 3, Day 4, or Night 5. Table 3 contains mean values for the subjective measures for each group.
Table 3.
Observed mean (SD) self-reported sleep variables for sodium oxybate (SO) and placebo groups
| Night 2 - Baseline |
Day 3 |
Day 4 |
Night 5 - Recovery |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo | SO | Placebo | SO | Placebo | SO | Placebo | SO | |
| Total sleep time (min) | 484.3 (52.01) | 487.2 (44.66) | 168.2 (34.54) | 167.5 (35.35) | 176.2 (70.89) | 167.8 (17.56) | 510.0 (98.54) | 488.4 (75.43) |
| Sleep latency (min) | 25.0 (27.51) | 32.8 (20.64) | 7.6 (5.90) | 7.5 (6.75) | 5.6 (5.82) | 7.3 (5.75) | 33.8 (70.13) | 22.9 (13.87) |
| Wake after sleep onset (min) | 14.7 (14.50) | 32.2 (34.67) | 2.9 (6.77) | 3.9 (6.81) | 3.7 (6.72) | 3.5 (6.73) | 12.4 (11.05) | 35.1 (53.67) |
| Number of awakenings | 2.3 (1.32) | 2.5 (1.83) | 0.6 (0.93) | 0.5 (0.64) | 0.8 (1.37) | 0.3 (0.49) | 3.3 (9.23) | 2.0 (1.50) |
| Sleep quality rating ▲ | 5.0 (0.90) | 5.0 (0.87) | 5.3 (0.88) | 5.7 (0.92) | 5.1 (0.91) | 5.3 (0.84) | 5.4 (1.09) | 5.5 (0.87) |
| Refreshing nature of sleep rating ▲ | 4.9 (0.99) | 5.1 (0.74) | 4.4 (1.28) | 4.7 (1.09) | 4.3 (1.04) | 4.8 (1.27) | 5.2 (1.08) | 5.3 (0.88) |
1 = extremely poor, 7 = excellent
Multiple Sleep Latency Test
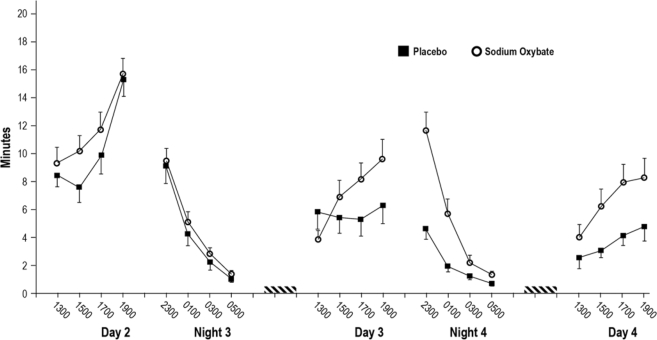
Figure 6 displays observed mean MSLT latencies by time of day for Days 2 (baseline), 3 and 4, and Nights 3 (baseline) and 4 for both study groups. Evaluation of MSLT data from Days 3 and 4 showed significant main effects for group (P = 0.035), time (P = 0.008), and study day (P = 0.004). There was also a group by day interaction (P = 0.04) and group by time interaction (P = 0.004). MSLT latency was longer for the sodium oxybate group than the placebo group on Day 4 (6.7 ± 0.88 vs. 3.3 ± 0.89 min, P = 0.017). Day 3 mean latencies were 7.1 ± 0.87 for sodium oxybate and 5.4 ± 0.89 for placebo (P = 0.2). Group differences increased across the day on both Day 3 and Day 4. MSLT latencies were longer for the sodium oxybate group at 17:00 (Day 3 and 4 mean: 7.8 ± 0.92 vs. 4.7 ± 0.93 min, P = 0.03) and 19:00 (Day 3 and 4 mean: 7.9 ± 0.94 vs. 4.5 ± 0.96 min, P = 0.017).
Figure 6.
Mean latency (and standard error) on the multiple sleep latency test at each test time for both study groups. Black squares indicate the placebo group, and open circles indicate the sodium oxybate group. The dashed bars indicate the 3-h sleep period on Day 3 and Day 4. Placebo or sodium oxybate was administered prior to both daytime sleep periods. Groups are significantly different on Night 4 (P < 0.001) and on Day 4 (P = 0.017).
On Night 4 there were significant main effects of group and time as well as a group by time interaction (P < 0.001 for all). Mean latency on Night 4 was 5.0 ± 0.47 min for the sodium oxybate group as compared to 2.1 ± 0.49 min for placebo. Paired comparisons to clarify the group by time interaction indicated group differences at the 23:00 and 01:00 time points (P < 0.001 for both).
Psychomotor Vigilance Test
On Days 3 and 4, all PVT measures showed significant day and time main effects (P < 0.006 for all). In general, performance was worse on Day 4 than on Day 3 and worse at the first test point on each day. Median reaction time also displayed a group by day interaction (P = 0.01), with faster reaction time on Day 4 in the sodium oxybate group (264.7 ± 4.7 msec) compared to placebo (280.0 ± 4.8 msec; P = 0.041. On Day 3, least square median reaction times were 259.7 ± 4.7 msec and 264.4 ± 4.8 msec for sodium oxybate and placebo, respectively. The mean number of lapses in the placebo group increased from 3.6 ± 0.9 on Day 3 to 6.2 ± 0.9 on Day 4, while in the sodium oxybate group lapses increased from 2.8 ± 0.9 on Day 3 to 4.0 ± 0.9 on Day 4. However, the group by day interaction for number of lapses was not significant (P = 0.078).
No group differences were noted on Night 4. Lapses and median reaction time showed worsening performance as the night progressed (main effect of time, P < 0.002 for all). In the placebo group lapses increased from 4.38 ± 1.4 at 22:30 to 8.73 ± 1.4 at 04:30, for a nightly average of 6.55 ± 4.0. In the sodium oxybate group, lapses similarly increased from 3.79 ± 1.4 to 8.2 ± 1.4, for an average of 6.46 ± 4.0.
Karolinska Sleepiness Scale
Groups did not differ on ratings of sleepiness on Days 3 and 4. Mean KSS scores were 5.8 ± 0.2 for sodium oxybate and 5.7 ± 0.2 for placebo (P = 0.60). Although there was a group by time interaction (P = 0.048), groups did not differ at any single time point. Sleepiness decreased significantly in both groups across the day (P < 0.001), ranging from 6.6 at 12:50 to 5.3 at 18:50.
On Night 4, the sodium oxybate group rated themselves as significantly less sleepy than the placebo group (7.0 ± 0.2 vs. 7.5 ± 0.2; P = 0.047). Both groups rated themselves increasingly sleepy as the night progressed, ranging from a mean of 5.8 at 22:50 to a mean of 8.3 at 04:50 (P < 0.001).
Digit Symbol Substitution Test and Wisconsin Card Sorting Task
Groups did not differ on DSST performance on Days 3 and 4. The mean number of correct substitutions on Days 3 and 4 for the sodium oxybate group was 80.5 ± 1.0 compared to 81.8 ± 1.1 for the placebo group (P = 0.34), and on Night 4 the comparable values were 76.3 ± 0.9 and 76.1 ± 1.0 (P = 0.86). Groups also did not differ on the DSST on Night 4. There was a trend for a group by time interaction on Night 4 (P = 0.056). However, groups did not differ at any individual time of night. No significant group differences were identified on the WCST based upon the results of the MANOVA (P = 0.113).
Profile of Mood States
Mixed model analysis revealed significant group differences on Day 3 and Day 4 (administration time 1400), with more negative mood for the sodium oxybate group. Specifically, tension-anxiety (P < 0.03), vigor-activity (P < 0.03), fatigue-inertia (P < 0.04), confusion-bewilderment (P < 0.02), and depression-dejection (P < 0.04) all were more negative with sodium oxybate. No significant differences between groups were found on Night 4 (administration time 01:30) for any POMS scale.
Adverse Events
Adverse events are reported for all 64 subjects who were randomized to drug or placebo. The most common adverse event was headache which did not appear to differ in frequency between the 2 study groups (16 sodium oxybate subjects, 12 placebo subjects). More subjects in the sodium oxybate group reported dizziness or lightheadedness (9 sodium oxybate, 0 placebo), nausea (11, 1), vomiting (4, 0), and enuresis (2, 0). The reports of dizziness, lightheadedness, nausea, or vomiting came from a total of 15 subjects (14 sodium oxybate, 1 placebo) as these events occurred in combination in most subjects. Seven subjects discontinued prematurely, 6 of whom were randomized to sodium oxybate (4 for dizziness, lightheadedness, nausea, and/or vomiting, 1 for menstrual cramps, 1 for tiredness) and one who was randomized to placebo (tiredness). Dizziness or lightheadedness, nausea, or vomiting were most likely to be reported on Day 3 around 11:00 when subjects awoke from the 3-h sleep period. In 4 subjects (3 of whom withdrew) symptom onset was between 09:00 and 10:00 (during the sleep period) on Day 3. Symptom duration appeared to be longer in the subjects who withdrew (> 4 h) compared to those who completed the study (generally 1-3 h).
Correlational Analyses
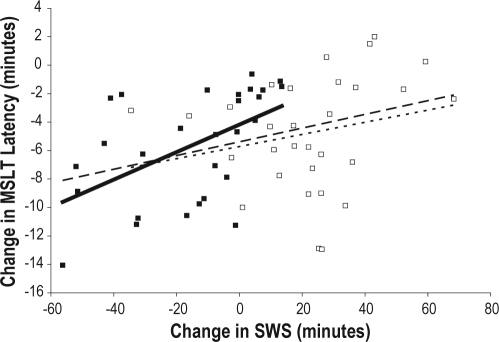
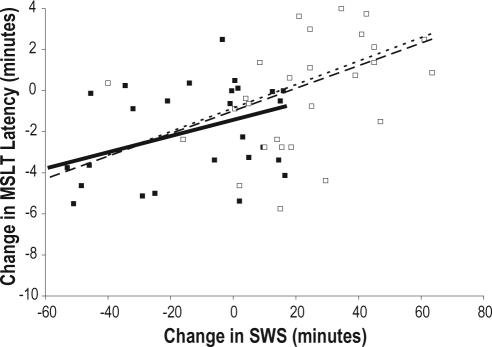
To examine the relationship between SWS and REM and outcome measures (MSLT, KSS, and PVT median reaction time), correlational analyses were conducted to determine if the change in minutes of sleep from baseline to sleep deprivation was related to the change in sleepiness/alertness as measured by the MSLT, KSS, and PVT. SWS and REM values from the entire recording on Night 2 were used as the best estimates of baseline sleep. The change in SWS (from Night 2 to the mean of Days 3 and 4) was positively correlated with the change in MSLT scores (from Day 2 to the mean of Days 3 and 4) for the entire study sample (r = 0.35, P = 0.008) and for the placebo group (r = 0.52, P = 0.004) but not for the sodium oxybate group (r = 0.23, P = 0.23; see Figure 7A). That is, an increase in SWS from baseline to sleep deprivation was associated with an increase in sleep latency (decreased sleepiness) on the MSLT. In addition, the change in SWS (from Night 2 to Day 3) was positively correlated with the change in MSLT (from Night 3 to Night 4) for all subjects (r = 0.52, P < 0.001) and for the sodium oxybate group (r = 0.43, P = 0.018), with a trend for the placebo group (r = 0.35, P = 0.07; see Figure 7B).
Figure 7A.
Regression lines showing the correlation between change in SWS (from Night 2 to the average of Days 3 and 4) with the change in MSLT latency (from Day 2 to the average of Day 3 and Day 4). The change in SWS is positively correlated with the change in MSLT for the entire study sample (dashed line, r = 0.35, P = 0.008) and for the placebo group (solid line, r = 0.52, P = 0.004) but not for the sodium oxybate group (stippled line, r = 0.23, P = 0.229).
Figure 7B.
Regression lines showing the correlation between change in SWS (from Night 2 to Day 3) with the change in MSLT latency (from Night 3 to Night 4). The change in SWS is positively correlated with the change in MSLT for the entire study sample (dashed line, r = 0.52, P < 0.001) and for the sodium oxybate group (stippled line, r = 0.43, P = 0.018) with a trend for the placebo group (solid line, r = 0.35, P = 0.07).
There were also significant associations between change in SWS (from Night 2 to the average of Days 3 and 4) and change in KSS scores (from Day 2 to the mean of Days 3 and 4) for all subjects (r = −0.26, P = 0.049), as well as subjects in the placebo group (r = −0.52, P = 0.004), but not in the sodium oxybate group (r = 0.16, ns). The change in SWS (from Night 2 to Day 3) was significantly correlated with the change in KSS (from Night 3 to Night 4) for all subjects (r = −0.37, P < 0.005), with trends for the individual groups (sodium oxybate, r = −0.34, P = 0.07; placebo, r = −0.36, P = 0.059).
Changes in SWS did not correlate with changes in PVT median reaction time. Nor were there significant correlations between changes in REM sleep and changes in MSLT, PVT, or KSS.
DISCUSSION
The findings of this study provide substantial evidence that following sleep loss, daytime sleep with sodium oxybate resulted in improved daytime alertness and attention relative to daytime sleep with placebo. The MSLT revealed group differences on Day 4 (and much of Day 3), as well as Night 4, indicating less sleepiness in the sodium oxybate group. There was a difference between groups on median PVT reaction time on Day 4, with the sodium oxybate group having shorter reaction times, as well as a trend toward fewer lapses. The KSS showed lower sleepiness ratings with sodium oxybate during Night 4. In addition, on Night 5 the placebo group had more TST, SWS, and SWA than the sodium oxybate group. The findings of a reduced impact of sleep loss on alertness and attention, together with a lower recovery sleep response on Night 5, suggests a reduced homeostatic sleep drive in the sodium oxybate group.
In support of that interpretation, the placebo group showed evidence of increasing homeostatic pressure during the sleep deprivation period. Compared to Night 2, SWA increased 19.4% on Day 3 and 26.5% on Day 4. On Night 5, SWA remained increased over baseline (by 13.9%), consistent with findings from studies on repeated partial sleep restriction.25,26 The reduction in SWA from Day 4 to Night 5 is likely because subjects had been awake only 11 hours prior to the Night 5 sleep period as compared to 21 hours prior to the Day 4 sleep period.27 In contrast, SWA on the recovery night was not significantly increased over baseline in the sodium oxybate group.
The magnitude of the difference in MSLT latencies between groups deserves emphasis. The group differences in mean MSLT exceeded 3 minutes on Day 4 and Night 4. In the late afternoon and early evening on Days 3 and 4, and early on Night 4, group differences were in the 3- to 7-min range. This compares to smaller MSLT differences in studies of sleepiness interventions which have been judged to be clinically relevant because they are accompanied by improvements in quality of life, patient sleepiness ratings, and ability to maintain wakefulness, or sustain attention. For example, modafinil 200 mg administered to narcolepsy or shift work sleep disorder patients produced significant increases in MSLT latencies with a magnitude between 1-2 minutes,28,29 and the mean in-crease in MSLT following treatment of sleep apnea with continuous positive airway pressure is approximately 1 minute.30
The DSST and WCST did not reveal any group differences. Similar apparent inconsistencies have been found in our prior studies of slow wave sleep enhancing drugs.10,11 That is, some dependent measures indicate a beneficial effect of the drug versus placebo, whereas other dependent measures show no difference. The POMS results seem to stand in contradiction to other findings, in that mood was more negative on Days 3 and 4 in the sodium oxybate condition. On the other hand, there were no POMS differences between groups on Night 4. It is possible that timing of the mood assessment is important. Daytime POMS was administered about 6 h post-dosing when sodium oxybate may still have been active, directly affecting mood. Nighttime POMS assessments were made about 17 h post-dosing and did not show group differences on any scale. Inspection of the MSLT and KSS data demonstrate that sleepiness levels about 5 h post-dosing are more comparable between groups than at later times, and in one case the sodium oxybate group is numerically sleepier than the placebo group (MSLT at 13:00 on Day 3). This suggests that direct drug effects of sodium oxybate 3.5 g may last 5-6 h and the beneficial effects are not manifest until the drug has been metabolized.
The primary difference between the daytime sleep of the two study groups was the significantly greater amount of SWS and spectral power in the 1-9 Hz range, along with reduced REM in the sodium oxybate group on Days 3 and 4. Importantly, TST was nearly identical for the two groups, and measures of sleep fragmentation showed differences that would not be expected to significantly affect sleepiness or performance. For example the mean number of ≥ 30-sec awakenings differed by about 1 per hour, and the number of shifts to stage 1 or wake differed by about 1.5 per hour. Thus, we believe that the greater degree of alertness and sustained attention, as well as the difference in recovery sleep, are associated with higher levels of SWS. The correlational analyses between minutes of SWS and MSLT sleep latency, demonstrating that increased SWS is associated with decreased sleepiness, provide additional support for this interpretation. An association between increased SWS and improved MSLT and KSS scores was noted for the entire study sample and in some cases for individual groups.
In conclusion, the results reported herein, in conjunction with prior studies from our laboratory,10,11 are consistent with the interpretation that pharmacologically increased SWS represents a heightening of at least some of the normal physiological processes of NREM sleep.
DISCLOSURE STATEMENT
This research was funded by Jazz Pharmaceuticals. Dr. Walsh has provided consulting services to Pfizer, Sanofi-Aventis, Cephalon, Schering-Plough/Organon, Neurocrine, Takeda America, Actelion, Sepracor, Jazz, Respironics, Transcept, Neurogen, GlaxoSmithKline, Somaxon, Eli Lilly, Evotec, Merck, Kingsdown, Vanda, Ventus, and Somnus. The authors' institution has received research funding from Pfizer, Merck & Co., Somaxon, Evotec, Actelion, Vanda, Neurogen, Sanofi-Aventis, Ventus, Respironics, and Jazz Pharmaceuticals.
ACKNOWLEDGMENTS
The authors appreciate the dedication of the research and technical staff who contributed to this research.
REFERENCES
- 1.Horne J. Human slow wave sleep: a review and appraisal of recent findings, with implications for sleep functions, and psychiatric illness. Experientia. 1992;48:941–54. doi: 10.1007/BF01919141. [DOI] [PubMed] [Google Scholar]
- 2.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 3.Dijk DJ, Brunner DP, Beersma DGM, Borbely AA. Electroencephalo-gram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 4.Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and sim-ulations. Am J Physiol. 1996;271:R501–10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 5.Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: Effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10:364–73. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- 7.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 8.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Walsh JK, Hartman PG, Schweitzer PK. Slow wave sleep deprivation and waking function. J Sleep Res. 1994;3:16–25. doi: 10.1111/j.1365-2869.1994.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh JK, Randazzo AC, Stone K, et al. Tiagabine is associated with sustained attention during sleep restriction: evidence for the value of slow wave sleep enhancement? Sleep. 2006;29:433–43. [PubMed] [Google Scholar]
- 11.Walsh JK, Snyder E, Hall J, et al. Slow wave sleep enhancement with gaboxadol reduces daytime sleepiness during sleep restriction. Sleep. 2008;31:659–72. doi: 10.1093/sleep/31.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapierre O, Montplaisir J, Lamarre M, Bedard MA. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: further considerations on REM sleep-triggering mechanisms. Sleep. 1990;13:24–30. doi: 10.1093/sleep/13.1.24. [DOI] [PubMed] [Google Scholar]
- 13.Van Cauter E, Plat L, Scharf MB, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. J Clin Invest. 1997;100:745–53. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–34. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- 15.Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:391–7. [PubMed] [Google Scholar]
- 16.Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatol. 2003;30:1070–4. [PubMed] [Google Scholar]
- 17.Crunelli V, Emri Z, Leresche N. Unravelling the brain targets of gamma-hydroxybutyric acid. Curr Opin Pharmacol. 2006;6:44–52. doi: 10.1016/j.coph.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgen L, Lane E, Lai A. Xyrem (sodium oxybate): a study of dose proportionality in healthy human subjects. J Clin Pharmacol. 2000;40:1053. [Google Scholar]
- 19.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Insti-tute; 1968. [Google Scholar]
- 20.Mitler MM, Carskadon MA, Hirshkowitz M. Evaluating sleepiness. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 1417–23. [Google Scholar]
- 21.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. [Google Scholar]
- 22.McNair DM, Lorr M, Droppleman L. Profile of mood states. Boston University School of Medicine; 1981. [Google Scholar]
- 23.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 24.Heaton RK, Chelune GJ, Talley JL, Kay G, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 25.Brunner DP, Dijk DJ, Borbely A. Repeated partial sleep deprivation progressively changes the EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 26.Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–22. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijk DJ, Brunner DP, Beersma DGM, Borbely AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 28.Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353:476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 29.US Modafinil in Narcolepsy Multicenter Study Group. Random-ized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology. 2000;54:1166–75. doi: 10.1212/wnl.54.5.1166. [DOI] [PubMed] [Google Scholar]
- 30.Patel SR, White DP, Malhotra A, Stancina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]