Abstract
Study Objectives:
The aim of this study was to evaluate sleep patterns during the course of the disease in (NZB/NZW)F1 mice, an experimental model of systemic lupus erythematosus (SLE).
Design:
Female mice were implanted with electrodes for chronic recording of sleep-wake cycles during the entire experimental phase (9, 19, and 29 weeks of age). The disease course was also assessed. At each time-point, blood samples were collected from the orbital plexus to evaluate serum antinuclear antibodies (ANA), which are important serologic parameters of disease evolution. Pain perception was also evaluated.
Measurements and Results:
During the dark phase, (NZB/NZW)F1 mice aged 19 weeks spent more time in sleep, and, as a consequence, the total waking time was lower when compared with earlier periods. An augmented number of sleep-stage transitions and microarousals were observed at the 29th week of life in both light and dark phases. At this same time-point, the mice showed lower pain thresholds than they had at 9 weeks of life. The disease status was confirmed; the entire group of mice at 29 weeks of life showed positive ANA with high titer levels.
Conclusions:
The sleep-recording data showed that, during the progress and severe phases of the disease (19 and 29 wks of age, respectively), sleep architecture is altered. According to these results, increased sleep fragmentation, disease activity, and pain sensitivity are features observed in these mice, similar to symptoms of SLE.
Citation:
Palma BD; Tufik S. Increased disease activity is associated with altered sleep architecture in an experimental model of systemic lupus erythematosus. SLEEP 2010;33(9):1244-1248.
Keywords: Antinuclear antibody, lupus, (NZB/NZW)F1 mice, pain, sleep recording
SLEEP IS VITAL TO HEALTH AND QUALITY OF LIFE, WHEREAS POOR SLEEP IS ASSOCIATED WITH ADVERSE HEALTH CONSEQUENCES. THIS IS AN IMPORTANT issue in its own right because sleep deprivation is becoming a more frequent and more accepted occurrence in modern society. In addition, sleep disorders have been observed in a number of chronic inflammatory conditions such as autoimmune diseases. Some of the clinical states associated with sleep disturbances include rheumatoid arthritis, fibromyalgia, and systemic lupus erythematosus (SLE).1 Nevertheless, it is unclear whether the sleep disruption that is observed in patients with these diseases is a result of factors associated with the disease—such as pain, depression, and stress2—or the result of dysregulation of the relationship between sleep and the immune system.
Sleep disturbances are often reported by patients with SLE, and this may be associated with fatigue,3 a symptom that affects up to 80% of patients with SLE.4 According to Valencia-Flores and colleagues,5 these patients are sleepier during the day by virtue of sleep fragmentation due to more arousals and sleep-stage transitions (SST). In addition, the disease is exacerbated by sleep disruption, resulting in decreased sleep efficiency and less delta sleep. Additional polysomnographic findings associated with disease activity include an increased percentage of stage 1 sleep and alpha intrusions into non-rapid eye movement sleep. Furthermore, sleep apnea, periodic leg movements during sleep and narcolepsy have been associated with SLE.6
Much of our current knowledge of the pathogenesis of SLE is based on inference from a murine model, the New Zealand Black/New Zealand White (NZB/NZW)F1 hybrid strain. These mice spontaneously develop an autoimmune disease that closely resembles the immunologic and clinical characteristics of human SLE, such as high titer levels of antinuclear antibodies (ANA) associated with the development of rapidly progressive and lethal glomerulonephritis. Similar to its characteristics in humans, the disease is most common in female mice.
However, there is no evidence about sleep patterns in this experimental model of SLE. Recent data from our laboratory indicate the importance of adequate sleep in (NZB/NZW)F1 mice. We observed that mice submitted to sleep deprivation exhibited an earlier onset of the disease, as reflected by the increased ANA titer levels.7
The purpose of the present study was, therefore, to evaluate the sleep patterns in a spontaneous experimental model of SLE, which offers a great opportunity to study the interaction between sleep and autoimmune disease. The disease course in (NZB/NZW)F1 mice was also assessed by following the development of ANA and pain sensitivity. A characterization of the sleep architecture that is associated with the onset, progress, and severe phases of this disease is presented in this article.
MATERIALS AND METHODS
Mice
NZB female and NZW male mice were bred and raised in the animal facility of the Department of Psychobiology. After being weaned, (NZB/NZW)F1 mice were housed in groups of 6 in plastic cages filled with hardwood bedding and provided with water and rodent chow ad libitum. Because murine lupus is more prevalent in females, only this sex was used in this study. Experiments were conducted at 21°C ± 2°C under a 12:12-hour light-dark cycle (lights on at 07:00) with animals having free access to food and tap water throughout the study. All procedures were carried out in accordance with the National Institute of Health guidelines on animal care and were approved by the Ethical Committee of UNIFESP (CEP #1259/06).
Surgical Implantation of Electrodes
At 7 weeks of age, 14 animals were implanted with electrodes (nickel-chrome wire) for chronic recording of electrocorticographic and electromyographic activities, as has been described previously.8 Briefly, anesthesia was induced by intraperitoneal injection of ketamine (70-80 mg/kg) and xylazine (9 mg/kg), the animal was attached to a stereotaxic apparatus, and the electrodes were inserted. Two pairs of bipolar electrodes were implanted ipsilaterally in a corticofrontal derivation (2.0 mm lateral to Bregma and 1.0 mm rostral; 2.0 mm lateral to Bregma and 3.0 mm caudal to Bregma) for electrocorticographic recording, and electromyographic electrodes were inserted into the dorsal neck muscles. These points were established according to the coordinates of Franklin and Paxinos.9 All electrodes were anchored to the skull with acrylic cement. At the end of the surgical procedure, each animal received an intramuscular injection of 0.5 mL/kg of antibiotic (Pentabiotic, Wyeth, São Paulo, Brazil), and all appropriate antiseptic care was taken to avoid posterior infection. After surgery, animals were housed in individual cages and maintained in a room (under the same temperature and lighting conditions as previously described) adjacent to the polysomnography room, where the animal remained for a period of at least 7 days to allow for recovery from the surgery.
Blood sampling procedures and ANA determination
One day prior to the recording procedure, the mice were rapidly anesthetized (isoflurane vapors) and bled from the orbital venous plexus with the use of plain capillary tubes.10 After being centrifuged, the serum was separated and stored at −20°C until analysis.
Determination of ANA
ANA was determined by a standard indirect immunofluorescence technique using HEp-2 cells as the substrate (Virgo, Hemagen Diagnostics, Columbia, MD). Indirect immunofluorescence on HEp-2 cells remains the method of choice for ANA detection.11 The manufacturer's protocol was followed. The flourescein-isothiocynate–conjugated rabbit antimouse immunoglobulin was kindly donated by Biolab (Biolabs, Inc., Lawrenceville, GA). A positive and negative control were included with each assay. The sera were considered positive for the presence of ANA at a starting dilution of 1:50. All positive sera were tested for antibodies to double-stranded DNA (anti-dsDNA) by IIF on Crithidia lucilliae as a substrate (DTS) following the same procedures used for ANA detection.
Recording Procedures
Individual mouse cages were housed inside a Faraday chamber. After an adaptation period of 3 days to the recording cables, mice were recorded using the Somnologica software (EMBLA Medical digital polygraph, Reykjavik, Iceland). The polygraphic sleep monitoring was initiated at 10:00, and the recording sessions were carried out over 2 days. Sleep recording was monitored during light and dark phases lasting 12 hours each (food and water were available ad libitum). Polygraphic recordings were divided into 10-second epochs and visually scored blindly as wakefulness, slow wave sleep (SWS), and paradoxical sleep paradoxical sleep in accordance with standard criteria.12
The sleep parameters considered included the following: total sleep time (TST: sum of all sleep periods during the recording); total alertness time (sum of all alertness periods during the recording); sleep efficiency (percentage of TST during the recording time); total slow-wave sleep (SWS: sum of all SWS periods during the recording); total paradoxical sleep (sum of all paradoxical sleep periods during the recording); and microarousals (events at least 15 second long with abrupt modification of baseline electrocorticographic frequency accompanied by high-amplitude electromyographic activity followed by SWS).
Nociceptive Test
Immediately after the end of the first sleep recording (at 9 weeks of age), the animals were placed on a hot plate for evaluation of pain sensitivity. The hot-plate test was used to assess the pain sensitivity according to the method described by O'Callaghan and Holtzman.13 The animals were individually placed on the hot plate (Ugo Basile, Biological Research Apparatus Company, Comerio, Italy) heated at 55°C. The reaction time was defined as the latency in seconds for licking of the hind paw. The animal was removed from the hot plate immediately after measurement. If no response occurred within 90 seconds, the mouse was removed from the hot plate.
After the hot-plate test, the animals were placed back in their home cages, and their sleep patterns, pain sensitivity, and ANA titer levels were evaluated again at 19 and 29 weeks of age according to the same procedure as described (see Figure 1).
Figure 1.
Experimental procedures
Statistical Analysis
A 2-way repeated-measure analysis of variance (ANOVA) was carried out for the analysis of sleep pattern and pain sensitivity. Posthoc comparisons were performed using the Tukey test. Data are expressed as means ± SE. A P value ≤ 0.05 was considered to be statistically significant.
RESULTS
Sleep Parameters
Light phase
The ANOVA for each sleep parameter showed a significant time-point effect of SSTs (F2,12 = 3.90, P ≤ 0.04) and microarousals (F2,12 = 6.51, P ≤ 0.01). Posthoc analyses indicated that more SSTs (P ≤ 0.04) and microarousals (P ≤ 0.04) were observed at the 29th week of life, compared with previous time-points. No significant differences were observed in the other sleep parameters (Table 1).
Table 1.
Sleep parameters in NZB/NZWF1 mice at different time-points during the light period
| Age, wks |
|||
|---|---|---|---|
| Sleep parameter | 9 | 19 | 29 |
| TST, min | 393.3 ± 45.1 | 412.3 ± 80.1 | 390.4 ± 23.9 |
| SWS, min | 339.7 ± 39.9 | 321.7 ± 44.7 | 324.5 ± 23.7 |
| PS, min | 61.4 ± 5.5 | 68.3 ± 5.9 | 66 ± 3.3 |
| SE, % | 55.5 ± 7.7 | 55.1 ± 4.2 | 54 ± 5.8 |
| TWT, min | 322.3 ± 35.8 | 322.8 ± 24.6 | 333.2 ± 22.3 |
| SST, no. | 373.5 ± 67 | 336.4 ± 92.3 | 442.4 ± 22.3a |
| Arousals, no. | 146.1 ± 29.9 | 125.2 ± 41.2 | 177.9 ± 13.5a |
Data are shown as mean ± SE. NZB/NZW refers to a cross between New Zealand Black male and New Zealand White female mice; TST, total sleep time; SWS, slow-wave sleep; PS, paradoxical sleep; SE, sleep efficiency; TWT, total wake time; SST, sleep-stage transitions.
Indicates difference from 19 wks of age;
*P < 0.05.
Dark phase
ANOVA revealed significant time-point effects in all sleep parameters (Table 2).
Table 2.
Sleep parameters in NZB/NZWF1 mice at different time-points during the dark period
| Age, wks |
|||
|---|---|---|---|
| Sleep parameter | 9 | 19 | 29 |
| TST, min | 326 ± 44.4 | 391 ± 32.5a | 373.3 ± 27.1 |
| SWS, min | 272.7 ± 36.1 | 325.3 ± 29a | 309.7 ± 27.5 |
| PS, min | 53.2 ± 8.8 | 65.6 ± 5.5a | 63.6 ± 3.1a |
| SE, % | 45.3 ± 6.2 | 54.3 ± 4.5a | 51.8 ± 3.7 |
| TWT, min | 394 ± 44.4 | 329 ± 32.5a | 346.6 ± 27.1 |
| SST, no. | 278.5 ± 53.4 | 327.5 ± 96.3 | 420.4 ± 26b |
| Arousals, no. | 110.2 ±20.1 | 125.1 ±46.8 | 170.4 ± 15b |
Data are shown as mean ± SE. NZB/NZW refers to a cross between New Zealand Black male and New Zealand White female mice; TST, total sleep time; SWS, slow-wave sleep; PS, paradoxical sleep; SE, sleep efficiency; TWT, total wake time; SST, sleep-stage transitions.
Indicates statistical difference from 9 wks of age at P < 0.05.
Indicates statistical difference from 9 and 19 wks of age at P < 0.05.
TST and total wake time
The main effect of time was detected for TST and total wake time (F2,12 = 6.48, P ≤ 0.01 and F2,12 = 6.48, P ≤ 0.01, respectively). Mice 19 weeks of age spent more time in sleep in comparison with the previous time-point (P ≤ 0.01). As a consequence, the TWT was lower in mice 19 weeks of age, when compared with the earlier period. (P ≤ 0.01)
Sleep efficiency
Significant differences were revealed (F2,12 = 6.47, P ≤ 0.01), indicating that the increase of sleep efficiency was more pronounced at 19 weeks of age than at 9 weeks of age (P ≤ 0.01).
Slow-wave sleep
Analyses of SWS indicated a significant time-point effect (F2,12 = 5.07, P ≤ 0.02]. Increased SWS was observed at 19 weeks of life, when compared to the previous time-point (P ≤ 0.02).
Paradoxical sleep
PS was significantly different among the time-points (F2,12 = 11.86, P ≤ 0.001). The posthoc analysis showed that the total time of PS was higher at the 19th (P ≤ 0.001) and 29th (P ≤ 0.006) weeks of life, when compared with the previous time-point.
SST and microarousal
The analyses of SSTs (F2,12 = 16.23, P ≤ 0.0003) and microarousals (F2,12 = 12.51, P ≤ 0.001] indicated significant differences. Posthoc analyses indicated that an increased number of SSTs (P ≤ 0.0004) and microarousals (P ≤ 0.001) were observed at the 29th week of life, compared with previous time-points.
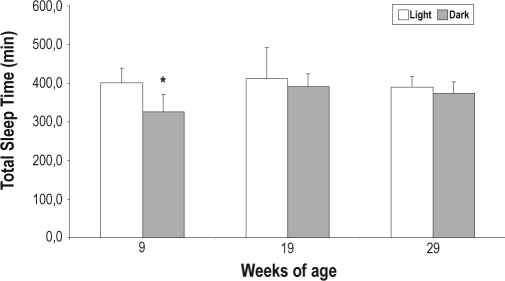
To determine the TST spent during the light phase and during the dark phase across the evolution of the disease, ANOVA was conducted within each time-point (9, 19, and 29 weeks of life). Analysis of TST (F5,30 = 3.84, P ≤ 0.008] showed a significant time-point effect. Posthoc analyses indicated that, at 9 weeks of life, as expected, the duration of TST during the light phase was higher than during the dark phase (P ≤ 0.02). However, at 19 and 29 weeks of life, TST during the light phase was not significantly different from the TST during the dark phase (Figure 2).
Figure 2.
Total sleep time in different time-points during the light and dark phase. Values are reported as mean ± SE *P ≤ 0.02, different from respective light phase.
Pain Perception
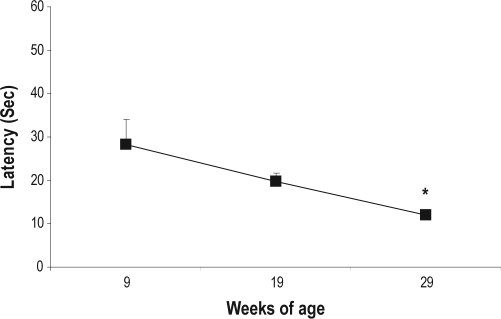
ANOVA of data from the hot-plate test revealed significant effects of the time-point (F2,26 = 4.87; P ≤ 0.01). Mice at 29 weeks of life showed lower pain thresholds, compared to thresholds at 9 weeks of life (P ≤ 0.01) (Figure 3).
Figure 3.
Hot-plate latencies at different time-points. Values are reported as mean ± SE. *P ≤ 0.01, different from 9 weeks of age.
DISCUSSION
This study shows that sleep patterns in an experimental model of SLE are significantly altered during the course of the disease. In comparison with (NZB/NZW)F1 mice aged 9 weeks (an age at which they are healthy), mice at 19 and 29 weeks of age exhibited altered sleep architecture. In fact, these time-points represent the evolution of the disease, inasmuch as ANA is related to the different phases of the disease (onset, progression, and severe phases) (Table 3). In the current study, we observed that, at 9 weeks of life, only 2 animals exhibited positive ANA with a low titer level, and an increasingly positive ANA case was detected at 19 weeks of life but with the same titer level as at the previous time-point. However, at 29 weeks of life, which was the severe phase of the disease, the highest titer was 1:1000, and the ANA tests were positive for all animals. Similar to the results of other studies,14,15 the onset and evolution of the disease was based on the presence and increasing titer of ANA, which is one the most important markers of disease activity. Beside this, only the mice of 29 weeks of age exhibited positive antibodies to anti-dsDNA (Table 3) that are central to the development of glomerulonephritis, a major manifestation in SLE.16
Table 3.
Characteristics of 14 mice with positive ANA tests
| Age, wks |
|||
|---|---|---|---|
| 9 | 19 | 29 | |
| Positive ANA test, no. (%) | 2 (14.2) | 7 (50) | 14 (100) |
| Highest ANA titer level | 100 | 100 | 1000 |
| Positive anti-dsDNA test, no. (%) | 0 | 0 | 8 (57.1) |
ANA refers to antinuclear antibodies; anti-dsDNA, double-stranded DNA
The data available from objective reports of sleep (polysomnography evaluation) in patients with SLE are not extensive; however, the results suggest that sleep disturbance is very common in this population.1 Subjective reports have also shown that sleep disturbance is prevalent among patients with SLE.17,18
The current study provides the first characterization of sleep architecture in an experimental model of lupus; thus, comparisons with other experimental results are limited. Our results are in line with portions of studies conducted in human beings using polysomnographic data, which most accurately assess sleep disturbances. Valencia-Flores et al.5 reported that patients with SLE exhibit sleep fragmentation, with more arousals and SST than in the control group. Similarly, Iaboni et al.19 reported high arousal frequencies. Our data show that, during both light and dark periods (the rest and activity periods of rodents, respectively), mice 29 weeks of age (severe phase of the disease) showed a fragmented sleep pattern with more SSTs and microarousals. In addition, we observed that mice at 19 weeks of age (time of progression of the disease) exhibited alterations in several sleep parameters during the dark phase, such as increases in sleep efficiency, TST, amount of SWS and paradoxical sleep. And as a consequence, a reduction in total wake time was detected. These data are consistent with a report that found that the TST in patients with SLE is higher than in the general population.20 In addition, Valencia-Flores et al.5 observed that patients with SLE are sleepier during the day (the correspondent night phase in rodents), probably by virtue of sleep fragmentation, which is associated with increased disease activity.
It was interesting to observe that, in the 19th week of life, during the dark phase, significant increases in TST, SWS, and paradoxical sleep were detected, thus raising sleep efficiency. Although the animals at 19 and 29 weeks of life did not show significant reductions in sleep-time parameters during the light phase, we believe that such increases in sleep duration in the dark phase may be due to a compensation phenomenon induced by sleep loss caused by excessive awakenings and SSTs. This is supported by our data that show TST spent in the 19th and 29th weeks of life during the light phase is equal to that of the dark phase, whereas animals in the ninth week of life spend more time sleeping in the light phase than in the dark phase, which corroborates the literature.
In human beings, chronically painful conditions are frequently associated with sleep disturbances.21 Animal studies also support the evidence that pain may cause disturbed sleep, such as markedly increased sleep fragmentation, reduced sleep efficiency, and less SWS.22–25 On the other hand, nonrestorative sleep, sleep deprivation, or both, have been considered to produce a hyperalgesic condition in clinical as well as animal studies.26–29 Therefore, in this context, the relationship between pain sensitivity and sleep quality appears to be reciprocal. Based on our data, it is possible to speculate that the sleep fragmentation observed in (NZB/NZW)F1 mice at 29 weeks of age is attributable to pain, inasmuch as a significantly reduced pain threshold was detected in these mice at this time-point. Sleep fragmentation appears to be an important marker in sleep architecture, mainly in chronically painful conditions. The disease activity in patients with SLE is associated with increases in sleep fragmentation, which, in turn, may induce sleep deprivation.5 Prior experimental research also indicates that fragmentation of sleep may affect sleep homeostasis, and, as a consequence, it may play an important role in nociception.22,25
Based on the interrelationships between pain sensitivity and altered sleep patterns, it is reasonable to assume that cytokines are a potential mediator of the sleep disturbances and bodily discomfort observed in chronic inflammatory conditions such as SLE. Increased serum levels of inflammatory cytokines have been associated with different SLE human phenotypes.30 Also, the involvement of inflammatory cytokines such as interleukin-1, interleukin-6 and tumor necrosis factor-α in the pathogenesis of murine lupus has been documented.31–33 Consistent with the bidirectional communications between sleep and the immune system, there is convincing evidence that inflammatory cytokines, in particular interleukin-1β and tumor necrosis factor-α, are involved in physiologic and pathologic sleep regulation.34 Thus, it is tempting to speculate that the sleep disruption observed in autoimmune disorders is more likely a result of dysregulation of the relationship between the sleep and immune system than merely a discomfort induced by the disease symptoms. It is also possible that sleep disturbances contribute directly to the pathogenesis of the disease. Specifically, previous data have shown that sleep disturbances are capable of accelerating the onset of lupus in (NZB/NZW)F1 mice, considering ANA production as the endpoint.7 This result emphasizes the importance of adequate sleep in SLE in which chronic sleep disturbances are reported.
In summary, the current study adds further evidence that, as in patients with SLE, the sleep pattern in (NZB/NZW)F1 mice is completely altered during the course of the disease. Sleep fragmentation and increased microarousals are associated with the development and severe phases of the disease during both the light and dark periods. Characterization of the sleep pattern in this model offers a great opportunity to study the importance of sleep quality in SLE and the relationship between sleep and the immune system in the autoimmune disease process.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Waldemarks Leite and Tomé Pimentel dos Anjos for their expert technical assistance. Research support was provided by FAPESP /CEPID (No. 06/57158-1 and 98/14303-3) and by the AFIP.
REFERENCES
- 1.Abad VC, Sarinas PS, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008;12:211–28. doi: 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:385–96. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- 3.Tench CM, McCurdie I, White PD, D'Cruz DP. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology. 2000;39:1249–54. doi: 10.1093/rheumatology/39.11.1249. [DOI] [PubMed] [Google Scholar]
- 4.Krupp LB, LaRocca NG, Muir J, Steinberg AD. A study of fatigue in systemic lupus erythematosus. J Rheumatol. 1990;17:1450–2. [PubMed] [Google Scholar]
- 5.Valencia-Flores M, Resendiz M, Castano VA, et al. Objective and subjective sleep disturbances in patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:2189–93. doi: 10.1002/1529-0131(199910)42:10<2189::AID-ANR21>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Iaboni A, Gladman DD, Urowitz MB, Moldofsky H. Disordered sleep, sleepiness, and depression in chronically tired patients with systemic lupus erythematosis. Sleep. 2004;27:A327–8. [Google Scholar]
- 7.Palma BD, Gabriel A, Jr, Colugnati FA, Tufik S. Effects of sleep deprivation on the development of autoimmune disease in an experimental model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1527–32. doi: 10.1152/ajpregu.00186.2006. [DOI] [PubMed] [Google Scholar]
- 8.Veasey SC, Valladares O, Fenik P, et al. An automated system for recording and analysis of sleep in mice. Sleep. 2000;23:1025–40. [PubMed] [Google Scholar]
- 9.Franklin KB, Paxinos G, editors. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- 10.Riley V. Adaptation of orbital technic to rapid serial blood studies. Proc Soc Exp Biol Med. 1960;104:751–4. doi: 10.3181/00379727-104-25975. [DOI] [PubMed] [Google Scholar]
- 11.Sinico RA, Bollini B, Sabadini E, Di Toma L, Radice A. The use of laboratory tests in diagnosis and monitoring of systemic lupus erythematosus. J Nephrol. 2002;15:S20–S7. [PubMed] [Google Scholar]
- 12.Timo-Iaria C, Negrao N, Schmidek WR, Rocha TL, Hoshino K. Phases and stages of sleep in the rat. Physiol Behav. 1970;5:402–7. doi: 10.1016/0031-9384(70)90162-9. [DOI] [PubMed] [Google Scholar]
- 13.O'Callaghan JP, Holtzman SG. Quantification of the analgesic activity of narcotic antagonists by a modified hot-plate procedure. J Pharmacol Exp Ther. 1975;192:497–505. [PubMed] [Google Scholar]
- 14.Chida Y, Sudo N, Kubo C. Social isolation stress exacerbates autoimmune disease in MRL/lpr mice. J Neuroimmunol. 2005;158:138–44. doi: 10.1016/j.jneuroim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Lechner O, Hu Y, Jafarian-Tehrani M, et al. Disturbed immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in murine lupus. Brain Behav Immun. 1996;10:337–50. doi: 10.1006/brbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 16.Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol. 2009;20:696–704. doi: 10.1681/ASN.2008010112. [DOI] [PubMed] [Google Scholar]
- 17.Costa DD, Bernatsky S, Dritsa M, et al. Determinants of sleep quality in women with systemic lupus erythematosus. Arthritis Rheum. 2005;53:272–8. doi: 10.1002/art.21069. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood KM, Lederman L, Lindner HD. Self-reported sleep in systemic lupus erythematosus. Clin Rheumatol. 2008;27:1147–51. doi: 10.1007/s10067-008-0884-2. [DOI] [PubMed] [Google Scholar]
- 19.Iaboni A, Ibanez D, Gladman DD, Urowitz MB, Moldofsky H. Fatigue in systemic lupus erythematosus: contributions of disordered sleep, sleepiness, and depression. Rheumatology. 2006;33:2453–7. [PubMed] [Google Scholar]
- 20.McKinley PS, Ouellette SC, Winkel GH. The contributions of disease activity, sleep patterns, and depression to fatigue in systemic lupus erythematosus. Arthritis Rheum. 1995;38:826–34. doi: 10.1002/art.1780380617. [DOI] [PubMed] [Google Scholar]
- 21.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56:51–7. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- 22.Landis CA, Robinson CR, Levine JD. Sleep fragmentation in the arthritic rat. Pain. 1988;34:93–9. doi: 10.1016/0304-3959(88)90186-8. [DOI] [PubMed] [Google Scholar]
- 23.Schutz TC, Andersen ML, Tufik S. Influence of temporomandibular joint pain on sleep patterns: role of nitric oxide. J Dent Res. 2004;83:693–7. doi: 10.1177/154405910408300907. [DOI] [PubMed] [Google Scholar]
- 24.Guevara-López U, Ayala-Guerrero F, Covarrubias-Gómez A, López-Muñoz FJ, Torres-González R. Effect of acute gouty arthritis on sleep patterns: a preclinical study. Eur J Pain. 2009;13:146–53. doi: 10.1016/j.ejpain.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Silva A, Andersen ML, Tufik S. Sleep pattern in an experimental model of osteoarthritis. Pain. 2008;140:446–55. doi: 10.1016/j.pain.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. Rheumatology. 1999;26:1586–92. [PubMed] [Google Scholar]
- 27.Hakki Onen S, Alloui A, Jourdan D, Eschalier A, Dubray C. Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Res. 2001;900:261–7. doi: 10.1016/s0006-8993(01)02320-4. [DOI] [PubMed] [Google Scholar]
- 28.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 29.Nascimento DC, Andersen ML, Hipolide DC, Nobrega JN, Tufik S. Pain hypersensitivity induced by paradoxical sleep deprivation is not due to altered binding to brain mu-opioid receptors. Behav Brain Res. 2007;178:216–20. doi: 10.1016/j.bbr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Dean GS, Tyrrell-Price J, Crawley E, Isenberg DA. Cytokines and systemic lupus erythematosus. Ann Rheum Dis. 2000;59:243–51. doi: 10.1136/ard.59.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in (NZB/NZW)F1 mice. Clin Exp Immunol. 1998;112:397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voronov E, Dayan M, Zinger H, et al. E. IL-1 beta-deficient mice are resistant to induction of experimental SLE. Eur Cytokine Netw. 2006;17:109–16. [PubMed] [Google Scholar]
- 33.Aringer M, Smolen JS. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. 2008;10:202. doi: 10.1186/ar2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–16. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]