Abstract
Study Objectives:
We hypothesized that the facial phenotype is closely linked to upper airway anatomy. The aim of this study was to investigate the relationship between surface facial dimensions and upper airway structures using magnetic resonance imaging (MRI) in subjects with obstructive sleep apnea (OSA).
Design:
Cohort study.
Setting:
Sleep investigation unit.
Patients:
Sixty-nine patients (apnea-hypopnea index ≥ 10/h) underwent MRI as part of a study of upper airway anatomy in oral appliance therapy.
Interventions:
Measurements of a range of surface facial dimensions and upper airway soft tissue volumes were performed on the MR images using image-analysis software. Pearson correlation analyses were performed.
Measurements and Results:
Significant correlations were identified between a number of surface facial dimensions and neck circumference. Significant positive correlations were demonstrated between surface facial dimensions (including facial widths, facial heights, nose width, interocular and intercanthal widths) and upper airway structures. The strongest associations were between the tongue volume and the midface width (r = 0.70, P < 0.001), and lower-face width (r = 0.60, P < 0.001). Surface facial dimensions in combination were also strong determinants for tongue volume (r2 = 0.69). Correlations between surface soft tissue thickness and upper airway soft tissue volumes occurred at the level of the midface but not at the level of the lower face.
Conclusions:
This study demonstrates that there is a relationship between surface facial dimensions and upper airway structures in subjects with OSA. These findings support the potential role of surface facial measurements in anatomic phenotyping for OSA.
Citation:
Lee RWW; Sutherland K; Chan ASL; Zeng B; Grunstein RR; Darendeliler MA; Schwab RJ; Cistulli PA. Relationship between surface facial dimensions and upper airway structures in obstructive sleep apnea. SLEEP 2010;33(9):1249-1254.
Keywords: Obstructive sleep apnea, facial phenotype, upper airway structures, magnetic resonance imaging
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY ANATOMIC AND FUNCTIONAL ABNORMALITIES OF THE UPPER AIRWAY RESULTING IN A compromised airway space and an increase in upper airway collapsibility during sleep.1,2 Although obesity is considered to be the major attributing risk factor for OSA,3 craniofacial morphology is increasingly recognized as an important interacting factor in OSA pathogenesis.4–6 Craniofacial differences between subjects with OSA and control subjects have mainly been examined using cephalometry, and, thus, the emphasis has been on bony structures in these investigations.7–10 Although imaging studies using magnetic resonance imaging (MRI) have revealed new insights into the upper airway soft tissue structures in OSA,11 the surface tissues beyond the craniofacial skeleton have not been examined.
We have recently demonstrated that surface facial measurements obtained on digital photographs are different between subjects with and without OSA.12 In particular, the width of the face was significantly greater in subjects with OSA. This simple measurement was also the most important determinant of the presence of OSA, among other known anthropometric and craniofacial risk factors.13 Given the limitations of the existing upper airway and craniofacial imaging techniques, the use of a surface facial metric would potentially be a very useful approach for anatomic phenotyping in OSA. However, the anatomic basis for such a relationship between surface facial measurements and OSA is currently unknown. It is probable that the surface facial phenotype reflects the underlying bony framework, but, in addition, we hypothesized that surface facial dimensions capture phenotypic information that also relates to obesity and upper airway anatomy, both of which are important risk factors for OSA. In particular, we hypothesized that the face width, which is the most important photographic predictor for OSA, relates strongly to the size of upper airway structures. Hence, the aim of this study was to investigate the relationship between surface facial dimensions and upper airway structures using MRI during wakefulness in subjects with OSA as a way of validating the relevance of craniofacial photography as a phenotyping strategy in OSA.
MATERIALS AND METHODS
Subjects
Subjects included in this study had upper airway MRI performed as part of a study examining upper airway anatomy in the prediction of oral-appliance treatment outcome.14,15 Consecutive patients with a diagnosis of OSA (apnea-hypopnea index [AHI] ≥ 10 events/h) and at least 2 of the following symptoms—daytime sleepiness, snoring, witnessed apneas, or fragmented sleep—were recruited for the treatment with a mandibular advancement splint (MAS). Exclusion criteria were related to the MAS treatment (insufficient teeth to permit splint retention, periodontal disease, exaggerated gag reflex). Anthropometry (height, weight, and neck circumference) was obtained the night before the MRI. The study was approved by the institutional ethics committee, and written informed consent was obtained from all patients.
Magnetic Resonance Imaging
Spin-echo MRI of the upper airway was performed during wakefulness using a Philips INTERA 1.5T MRI scanner (Philips Electronics, Netherlands). With the aid of a gantry beam, the patient's head was positioned with the Frankfort plane perpendicular to horizontal. Foam pads were used to secure the head in this position. Images were acquired with a receive-only neck coil. Throughout the scan, patients were asked to breathe normally through their nose and to refrain from swallowing. Patients were also instructed to keep their mouth closed and to maintain a relaxed bite, with the tongue touching the front teeth.
An initial sagittal scan was performed to confirm head position. Contiguous T1-weighted spin-echo images were acquired through the long axis of the airway, centered around the midsagittal plane (50 slices, 1.25 mm thickness, 272 × 512 matrix). Axial scans of the upper airway (50 slices, 3 mm thickness, 224 × 512 matrix) were then acquired from above the level of the nasopharynx, to below the level of the vocal cords. The acquisitions were stored in DICOM format.
Surface facial measurements
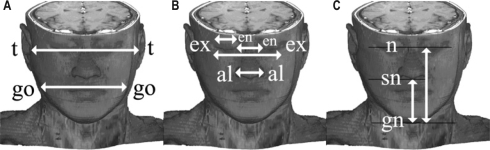
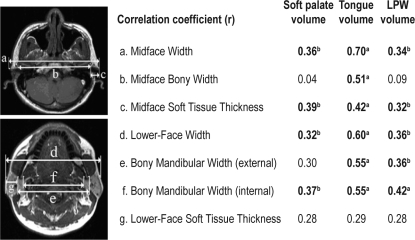
The axial MR image stacks were examined using image analysis software (Image J v1.36, NIH, Bethesda, MD). Surface facial dimensions obtained are illustrated in Figure 1. These measurements were obtained by examining the axial MR images and identification of the 8 surface landmarks of interest (tragion, gonion, exocanthion, endocanthion, alare, nasion, subnasion, gnathion). These were a subgroup of landmarks that can be obtained on craniofacial photographic analysis.12,13 The surface landmark coordinates (x, y, z) were then captured, and facial dimensions between each pair of landmark were calculated using these 3-dimensional coordinates. Facial ratios were also determined (bony facial ratio = bony face height [n-mentum]/bony face width [see Table 4]; surface facial ratio = face height [n-gn]/midface width [t-t]).16 Measurements of the bony and soft tissue subdivisions were obtained at the levels of midface width (defined by the landmark tragion) and lower-face width (defined by the landmark gonion) (Table 4).
Figure 1.
Three-dimensional magnetic resonance imaging reconstructions of the head and neck: surface facial dimensions. A. midface width (t-t), lower-face width (go-go); B. eye width (ex-en); interocular width (ex-ex), intercanthal width (en-en), nose width (al-al); C. face height (n-gn); lower face height (sn-gn). Landmarks: t-tragion; go-gonion; ex-exocanthion; en-endocanthion; al-alare; n-nasion; sn-subnasion; gn-gnathion.
Table 4.
Correlations between subdivisions of midface width and lower-face width and upper airway structures on MRI
MRI refers to magnetic resonance imaging; LPW, lateral pharyngeal wall. aP < 0.001; bP < 0.01.
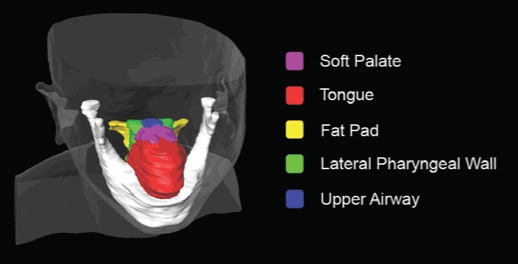
Upper airway volumetric measurements
Measurements obtained included volumes of the soft palate, tongue, parapharyngeal fat pads, lateral pharyngeal walls, and total airway (Figure 2) in accordance with previously described and validated technique11,17 using image analysis software (Amira v4.1, Mercury Computer Systems Inc.).
Figure 2.
Upper airway soft tissue volumetric measurements on magnetic resonance imaging
Polysomnography
Baseline diagnostic polysomnography was performed in accordance with previous studies and recommendations.18,19 Sleep staging was determined using standardized definitions.20 Apnea was defined as complete airflow cessation for at least 10 seconds with oxygen desaturation of at least 3% and/or associated with an arousal. Hypopnea was defined as a reduction in amplitude of airflow or thoracoabdominal wall movement greater than 50% of the baseline measurement for more than 10 seconds with an accompanying oxygen desaturation of at least 3%, and/or associated with arousals. AHI was calculated as the total number of apneas and hypopneas per hour of sleep. Polysomnography scoring was performed by experienced accredited sleep technologists.
Statistical Analysis
Statistical analysis was performed with SPSS (v13.0 for Windows, SPSS Inc., Chicago, IL). Pearson correlation analysis was performed to examine the relationships among surface facial dimensions, upper airway structures, and the AHI (natural log-transformed). A partial correlation procedure was used to examine linear relationships while controlling for the effect of another variable (BMI or height). To allow for multiple comparisons for correlation analyses, a Bonferroni correction was used. Accordingly, a P value less than 0.001 was considered statistically significant and a P value less than 0.01 was considered marginally significant.21 Univariate (BMI and neck circumference) and multivariate forward regression analysis (MRI surface facial measurements) were used to determine the independent predictors for tongue volume.
RESULTS
Subject Characteristics
Sixty-nine subjects were included in this study (Table 1). Forty-seven (68%) were men. The mean age and BMI were 50.4 ± 10.2 years and 29.6 ± 5.0 kg/m2, respectively. The mean AHI was 27.0 ± 14.7 events per hour, with a range from 10.3 to 75.7 events per hour.
Table 1.
Subject characteristics
| Subjects | n = 69 |
|---|---|
| Men | 47 (68.1) |
| Age, y | 50.4 ± 10.2 |
| Weight, kg | 85.5 ± 15.8 |
| Height, cm | 171.9 ± 7.7 |
| BMI, kg/m2 | 29.6 ± 5.0 |
| Neck circumference, cm | 39.9 ± 3.6 |
| AHI, events/h | 27.0 ± 14.7 (10.3-75.7) |
Data are shown as mean ± SD (range) or number (%).
BMI refers to body mass index; AHI, apnea-hypopnea index.
Surface Facial Dimensions and Upper Airway Structures
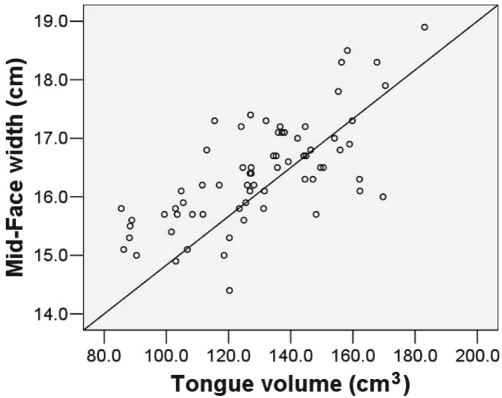
Significant correlations were identified between anthropometric measures of obesity and a number of surface facial dimensions (Table 2). In particular, the neck circumference had a strong positive relationship with both the midface width (r = 0.81, P < 0.001) and lower-face width (r = 0.78, P < 0.001). Significant positive correlations were also demonstrated between a number of surface facial dimensions and upper airway structures (Table 2). The strongest associations were between the tongue volume and the midface width (r = 0.70, P < 0.001) (Figure 3), and lower-face width (r = 0.60, P < 0.001). When controlled for BMI, both of these measurements remained moderately correlated with the tongue volume (r = 0.66, P < 0.001 and r = 0.53, P < 0.001, respectively). However, there were no correlations between these surface measurements and tongue volume when controlled for neck circumference.
Table 2.
Correlations between surface facial dimensions and upper airway structures on MRI
| Correlation Coefficient (r) | BMI | NC | Soft Palate Volume | Tongue Volume | Fat Pad Volume | LPW Volume | Airway Volume |
|---|---|---|---|---|---|---|---|
| BMI | - | 0.58a | 0.23 | 0.34b | 0.11 | 0.19 | 0.02 |
| NC | 0.58a | - | 0.50a | 0.76a | 0.06 | 0.47a | 0.26 |
| Midface Width | 0.59a | 0.81a | 0.36b | 0.70a | 0.10 | 0.34b | 0.28 |
| Lower-Face Width | 0.67a | 0.78a | 0.32b | 0.60a | 0.08 | 0.36b | 0.09 |
| Eye Widthc | −0.16 | 0.13 | 0.06 | 0.20 | 0.22 | 0.42 | −0.33 |
| Interocular Widthc | −0.05 | 0.46b | 0.23 | 0.49b | 0.34 | 0.41 | −0.01 |
| Intercanthal Widthc | 0.27 | 0.53b | 0.38 | 0.49b | 0.08 | 0.28 | 0.59a |
| Nose Width | 0.15 | 0.48a | 0.11 | 0.57a | 0.03 | 0.38 | −0.02 |
| Face Heightd | 0.24 | 0.30 | 0.44a | 0.43b | 0.24 | 0.38b | 0.37b |
| Lower Face Height | 0.28 | 0.35b | 0.42a | 0.39b | 0.06 | 0.40b | 0.19 |
| Bony Facial Ratiod | 0.17 | 0.13 | 0.44a | 0.12 | 0.07 | 0.40b | 0.14 |
| Surface Facial Ratiod | −0.21 | −0.32 | 0.27 | −0.11 | 0.10 | 0.16 | 0.21 |
MRI refers to magnetic resonance imaging; BMI, body mass index; NC, neck circumference; LPW, lateral pharyngeal wall; Bony facial ratio, bony face height/bony face width; surface facial ratio, face height/midface width.
P < 0.001;
P < 0.01;
Data available in 33 subjects where images were inclusive of the orbits;
Data available in 59 subjects where images were inclusive of the landmark nasion.
Figure 3.
Linear relationship between midface width and tongue volume on magnetic resonance imaging. r = 0.70; P < 0.001
Interocular width (r = 0.49, P < 0.01), intercanthal width (r = 0.49, P < 0.01), nose width (r = 0.57, P < 0.001), and facial heights (r = 0.43, P < 0.01) demonstrated moderate positive correlations with the tongue volume (Table 2). Intercanthal width (r = 0.59, P < 0.001) and face height (r = 0.37, P < 0.01) also had positive correlations with total airway volume (Table 2). When controlled for the subjects' height, a positive trend remained between total airway volume and intercanthal width (r = 0.42, P = 0.02) and face height (r = 0.39, P = 0.03), but these did not reach the a priori level for statistical significance. Surface facial ratio did not correlate with any of the upper airway structures, whereas the bony facial ratio correlated positively with the volume of soft palate (r = 0.44, P < 0.001) and the lateral pharyngeal wall (r = 0.40, P < 0.01) (Table 2).
The best explanatory surface facial variables for tongue volume were the combination of midface width, interocular width, and intercanthal width (r2 = 0.69, P < 0.001). This combination had a higher variance for tongue volume compared to BMI (r2 = 0.11, P = 0.005) or neck circumference (r2 = 0.58, P < 0.001) (Table 3).
Table 3.
Univariate and multivariate regression analysis for the determinants of tongue volume
| Explanatory Variables for Tongue Volume | r2 | |
|---|---|---|
| Anthropometry | BMI | 0.11, P = 0.005 |
| Neck Circumference | 0.58, P < 0.001 | |
| Surface facial dimensionsa | Combination of midface width, interocular width and intercanthal width | 0.69, P < 0.001 |
MRI refers to magnetic resonance imaging; BMI, body mass index.
Regression model variables (standardized coefficient β, P-value): midface width (0.505, P = 0.001); interocular width (0.299, P = 0.025); intercanthal width (0.253, P = 0.038).
Midface Width and Lower-Face Width Subdivisions
The midface-width measurement was composed of the midface bony width and the midface soft tissue thickness (Table 4). The thickness of midface soft tissue correlated with BMI (r = 0.68, P < 0.001), tongue (r = 0.42, P < 0.001), soft palate (r = 0.39, P < 0.01) and lateral pharyngeal wall volumes (r = 0.32, P < 0.01). The midface bony width also correlated with the tongue volume (r = 0.51, P < 0.001). However, the strongest relationship remained between the total midface width and tongue volume (r = 0.70, P < 0.001).
The lower-face width was composed of the bony mandibular width and the lower-face soft tissue thickness (Table 4). In contrast with the midface width subdivisions, there were no significant correlations between upper airway structures and soft tissue thickness at the level of the lower face. The latter also had a weaker correlation with BMI (r = 0.40, P = 0.001), compared with the midface soft tissue thickness.
Relationship with OSA Severity
There were no significant correlations between OSA severity and any of the anthropometric and MRI volumetric measurements. However, the surface facial measurements of midface width (r = 0.25, P < 0.05) and lower-face width (r = 0.27, P < 0.05) demonstrated a weak correlation with OSA severity, but these did not reach statistical significance. Facial ratios did not correlate with OSA severity.
DISCUSSION
This study confirms our hypothesis that there is a relationship between surface facial dimensions and upper airway structures in subjects with OSA using MRI during wakefulness. In particular, the strongest correlations were demonstrated between the volume of the tongue and the widths of the midface and lower face. Significant relationships between some surface facial measurements and anthropometrics of obesity were also demonstrated. Surface facial dimensions in combination were strong determinants for tongue volume.
Overall, these results support the notion that facial phenotype is closely linked with upper airway anatomy. Facial phenotype is determined collectively by the size and morphology of skeletal, muscular, and adipose tissues, together with surface facial features. Although these structures may have unique genetic determinants,22,23 shared embryologic origin of these components and upper airway structures are also well described.24 For example, the anterior two thirds of the tongue, muscles of mastication, maxilla, zygomatic bone, and mandible all originate from the first pharyngeal arch. The posterior third of the tongue is formed predominantly from the second and third pharyngeal arches; the second arch also gives rise to the muscles of facial expression. It is possible through these common embryologic links that surface facial dimensions and upper airway soft tissues remain closely associated in adults.
Although a cause-and-effect relationship between facial and upper airway structures cannot be determined in this study, a number of other hypotheses for such an association have been suggested. Changes to the craniofacial skeleton may occur secondary to soft-tissue stretching25 or soft tissue-induced osteogenic reaction and growth.26 Specifically, tongue size has previously been shown to correlate positively with mandibular and dental arch sizes27,28; conversely, surgical tongue volume reduction has been shown to slow skeletal growth and dental arch expansion.29 Thus, although it is possible that the tongue may grow and adapt to existing oral bony morphology, upper airway soft tissues could also influence the size of the bony maxillary and mandibular compartments.
We did not identify significant linear relationships between OSA severity and obesity or the upper airway structure volumes in this cohort of patients. However, the midface-width surface measurement was associated with the AHI, but the relationship was weak. Previous imaging studies have demonstrated the crucial role of upper airway structures in the pathogenesis of OSA.11,23,30 Specifically, the size or volume of the tongue has been shown to be larger in patients with OSA compared with control subjects. However, the linear relationship between size of upper airway structures and OSA severity was not assessed in these studies. Overall, the lack of correlation in our study suggests that the relationship may not be linear with absolute size but, rather, that the relative size of these soft tissue structures in relationship to the bony enclosure may be a more important determinant of OSA.30 The pathophysiology of OSA relates to the balance and interaction between bony and soft tissues surrounding the upper airway.4,6 Although this study may suggest that bony and soft tissue structures are closely related in size, it is the discrepant growth of these structures that ultimately determines the degree of airway compromise. The influence for such disparity in bony and soft tissue growth during craniofacial development is unknown.
Strong relationships between midface or lower-face widths and anthropometrics of obesity (BMI and neck circumference) in this study confirm results from our previous study using photographic facial measurements.12 This suggests that surface facial dimensions are closely linked with obesity, at least in subjects with OSA. Similarly, tongue volume was also significantly correlated with BMI and neck circumference in our study. This is consistent with autopsy findings of the positive correlations between tongue weight/fat percentage and BMI.31 This common linkage with obesity could be, in part, the basis for the relationship between surface facial dimensions and upper airway soft tissue volumes. It is also possible that, at least in part, the relationship is related simply to body size. However, the correlation remains even when controlled for obesity (BMI), suggesting that factors in addition to adipose tissue deposition (or body size) also account for this relationship (e.g., genetic or embryonic factors).
Our use of MRI enabled us to examine the relative importance of bony and soft tissue components that comprise surface dimensions, which we performed through separate correlations of the bony and soft tissue subdivisions of the face widths (see Table 4). Correlations between surface soft tissue thickness and upper airway soft tissue volumes were noted at the level of the midface but not at the level of the lower face. This suggests that the relationship between surface soft tissues and upper airway structures may be regional on the face. Facial soft tissues are composed of mainly skeletal muscles and adipose tissue. The latter has been suggested to relate to visceral obesity32 and insulin sensitivity.33,34 We hypothesize that the distribution of facial soft tissues could be an important intermediate trait for OSA, through relationships with visceral obesity and upper airway soft tissue structures. Further studies examining the distribution of adipose/soft tissues over different regions on the face are needed to further understand these relationship.
The facial ratio provides a simple measure of the facial form. A higher ratio indicates a long-thin (leptoprosopic) facial form, whereas a lower ratio indicates a wider and shorter (euryprosopic) form. In the present study, only certain upper airway structures (soft palate and lateral pharyngeal wall) appear to positively correlate with the more leptoprosopic bony facial form. This suggests that the size of upper airway structures may be differentially related to the facial skeletal form. However, surface facial forms did not relate to upper airway structures, but the absolute dimensions of facial soft tissues were important, as discussed above. Although it has previously been suggested that facial forms are important in Caucasian subjects with OSA,36 it remains unclear whether this observation is a cause or effect phenomenon. Future studies in larger populations may be able to address the relevance of different facial forms in OSA.
A limitation of the study was the lack of a control group in our cohort. This should not influence the results of the primary aim, which was to examine correlations between measurements on MRI. However, the lack of significant linear relationship between AHI and obesity/upper airway structures may also relate to the sample selected for investigation. Relationships between MRI measurements and OSA may be better addressed using a case-control design in future studies.11 Sex and ethnic differences in craniofacial and upper airway anatomy were not examined as part of this study. However, the relative simplicity in obtaining surface facial measurements could provide a novel approach for anatomic phenotyping in future research studies of sex and ethnicity. Due to the voxel size and limited resolution of MR images, the identification of surface landmarks may be less accurate, compared with clinical methods of anthropometry. For example, identification of the landmarks exocanthion/endocanthion may be more accurate on clinical examination, compared with MR imaging. The cranial base was not included in all the MR images; therefore, orbit dimensions were unavailable in some subjects. Although the surface measurements performed were limited to linear distances between facial landmarks, they are simple to perform and can potentially be utilized for clinical applications. Future studies would need to examine the correlations between MRI measurements and those obtained clinically or on photographic images.12,13 Although such agreement would need validation, volumetric MRI measurements have previously been validated on phantom models,17 and intrarater reliability for facial photographic measurements has been shown to be excellent.12 The imaging was performed during wakefulness, and, thus, some of the upper airway findings of this study may not be identical to those during sleep (e.g., jaw or tongue position). Nevertheless, MR imaging during wakefulness is an invaluable tool for defining the size of upper airway structures. Because the spin-echo MRI scans occurred over several minutes, resulting in averaging of data over many respiratory cycles, we were also unable to assess dynamic airway changes during inspiration and expiration.
In summary, this study demonstrates that there is a relationship between surface facial dimensions and upper airway structures in subjects with OSA. Surface facial metrics capture phenotypic information that relates to measures of obesity and upper airway anatomy. The relative simplicity in obtaining surface facial measurements could provide a novel approach for anatomical phenotyping in future research.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Jin Qian, Dr. Andrew Ng, Dr. Belinda Liu, and the sleep laboratory staff for their assistance. We thank Dr. Francois-Louis Comyn for the assistance in manuscript preparation.
REFERENCES
- 1.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome: II. Soft tissue morphology. J Laryngol Otol. 1989;103:293–7. doi: 10.1017/s0022215100108746. [DOI] [PubMed] [Google Scholar]
- 2.Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:347–52. doi: 10.1164/ajrccm.161.2.9810091. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840–51. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson KA, Ono T, Lowe AA, Ryan CF, Fleetham JA. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108:375–81. doi: 10.1378/chest.108.2.375. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:260–5. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Riley R, Powell N. Obstructive sleep apnea and abnormal cephalometric measurements. Implications for treatment. Chest. 1984;86:793–4. doi: 10.1378/chest.86.5.793. [DOI] [PubMed] [Google Scholar]
- 8.Lowe AA, Fleetham JA, Adachi S, Ryan CF. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 1995;107:589–95. doi: 10.1016/s0889-5406(95)70101-x. [DOI] [PubMed] [Google Scholar]
- 9.Miles PG, Vig PS, Weyant RJ, Forrest TD, Rockette HE., Jr Craniofacial structure and obstructive sleep apnea syndrome--a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop. 1996;109:163–72. doi: 10.1016/s0889-5406(96)70177-4. [DOI] [PubMed] [Google Scholar]
- 10.Riha RL, Brander P, Vennelle M, Douglas NJ. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep. 2005;28:315–20. [PubMed] [Google Scholar]
- 11.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 12.Lee RWW, Chan ASL, Grunstein RR, Cistulli PA. Craniofacial phenotyping in obstructive sleep apnea – A novel quantitative photographic approach. Sleep. 2009;32:37–45. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RWW, Petocz P, Prvan T, Chan ASL, Grunstein RR, Cistulli PA. Prediction of obstructive sleep apnea with craniofacial photographic analysis. Sleep. 2009;32:46–52. [PMC free article] [PubMed] [Google Scholar]
- 14.Chan A, Schwab R, Zeng B, et al. Mandibular advancement splints and upper airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2008;177:A939. [Google Scholar]
- 15.Chan A, Sutherland K, Schwab R, et al. Mandibular advancement splints and upper airway anatomy in obstructive sleep apnea. Sleep Biol Rhythms. 2008;6:A55. [Google Scholar]
- 16.Kolar JC. Craniofacial anthropometry: Practical measurement of the head and face for clinical, surgical, and research use. Springfield, Ill., U.S.A: C.C. Thomas; 1997. [Google Scholar]
- 17.Welch KC, Foster GD, Ritter CT, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25:532–42. [PubMed] [Google Scholar]
- 18.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 19.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 21.Deane SA, Cistulli PA, Ng AT, Zeng BA, Petocz P, Darendeliler MA. Comparison of Mandibular Advancement Splint and Tongue Stabilizing Device in Obstructive Sleep Apnea: A Randomized Controlled Trial. Sleep. 2009;32:648–53. doi: 10.1093/sleep/32.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redline S, Tishler PV. The genetics of sleep apnea. Sleep Med Rev. 2000;4:583–602. doi: 10.1053/smrv.2000.0120. [DOI] [PubMed] [Google Scholar]
- 23.Schwab RJ. Genetic determinants of upper airway structures that predispose to obstructive sleep apnea. Respir Physiolo Neurobiol. 2005;147:289–98. doi: 10.1016/j.resp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Sadler TW, Langman J. Langman's Medical Embryology. 10th ed. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 25.Solow B, Ovesen J, Nielsen PW, Wildschiodtz G, Tallgren A. Head posture in obstructive sleep apnoea. Eur J Orthod. 1993;15:107–14. doi: 10.1093/ejo/15.2.107. [DOI] [PubMed] [Google Scholar]
- 26.Frankel R, Frankel C. Orofacial Orthopedics with the Function Regulator. Karger, Basel: 1989. [Google Scholar]
- 27.Tamari K, Shimizu K, Ichinose M, Nakata S, Takahama Y. Relationship between Tongue Volume and Lower Dental Arch Sizes. Am J Orthod Dentofacial Orthop. 1991;100:453–8. doi: 10.1016/0889-5406(91)70085-B. [DOI] [PubMed] [Google Scholar]
- 28.Yoo E, Murakami S, Takada K, Fuchihata H, Sakuda M. Tongue volume in human female adults with mandibular prognathism. J Dent Res. 1996;75:1957–62. doi: 10.1177/00220345960750120701. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z-J, Shcherbatyy V, Gu G, Perkins JA. Effects of tongue volume reduction on craniofacial growth: A longitudinal study on orofacial skeletons and dental arches. Arch Oral Biol. 2008;53:991–1001. doi: 10.1016/j.archoralbio.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuiki S, Isono S, Ishikawa T, et al. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108:1009–15. doi: 10.1097/ALN.0b013e318173f103. [DOI] [PubMed] [Google Scholar]
- 31.Nashi N, Kang S, Barkdull GC, Lucas J, Davidson TM. Lingual fat at autopsy. Laryngoscope. 2007;117:1467–73. doi: 10.1097/MLG.0b013e318068b566. [DOI] [PubMed] [Google Scholar]
- 32.Levine JA, Ray A, Jensen MD. Relation between chubby cheeks and visceral fat. N Engl J Med. 1998;339:1946–7. doi: 10.1056/NEJM199812243392619. [DOI] [PubMed] [Google Scholar]
- 33.Sierra-Johnson J, Johnson BD. Facial fat and its relationship to abdominal fat: a marker for insulin resistance? Med Hypotheses. 2004;63:783–6. doi: 10.1016/j.mehy.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Sierra-Johnson J, Johnson BD, Bailey KR, Turner ST. Relationships between insulin sensitivity and measures of body fat in asymptomatic men and women. Obes Res. 2004;12:2070–7. doi: 10.1038/oby.2004.258. [DOI] [PubMed] [Google Scholar]
- 35.Banabilh SM, Suzina AH, Dinsuhaimi S, Samsudin AR, Singh GD. Craniofacial obesity in patients with obstructive sleep apnea. Sleep and Breathing. 2009;13:19–24. doi: 10.1007/s11325-008-0211-9. [DOI] [PubMed] [Google Scholar]
- 36.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]