Abstract
Background
Abrupt discontinuation of heavy marijuana (MJ) use is associated with self-reports of sleep difficulty. Disturbed sleep is clinically important because MJ users experiencing sleep problems may relapse to MJ use to improve their sleep quality. Few studies have used polysomnography (PSG) to characterize changes in sleep architecture during abrupt abstinence from heavy MJ use.
Methods
We recorded PSG measures on Nights 1, 2, 7, 8, and 13 after abrupt MJ discontinuation in 18 heavy MJ users residing in an inpatient unit.
Results
Across abstinence, Total Sleep Time (TST), Sleep Efficiency (SEff), and amount of REM sleep declined, while Wake after Sleep Onset (WASO) and Periodic Limb Movements (PLM) increased. Furthermore, quantity (joints/week) and duration (years) of MJ use were positively associated with more PLMs.
Conclusion
The treatment of sleep disturbance is a potential target for the management of cannabis use disorders since poor sleep could contribute to treatment failure in heavy MJ users.
Keywords: sleep, polysomnography, periodic leg movements, marijuana, abstinence, substance abuse, withdrawal
INTRODUCTION
Marijuana (MJ) use has been increasing nationally, particularly among middle-aged “baby boomers” and adolescents (NSDUH, 2006 (1). Currently 11 million Americans used MJ at least once in the past year, and over 2 million use MJ daily. The prevalence of cannabis use disorders is also increasing (2). Except for alcohol use, MJ use accounted for the largest percentage of drug abuse treatment admissions (15.9%) in 2004. Unfortunately, clinical trials show high relapse rates for those seeking treatment for cannabis use disorders, with magnitudes similar to that reported with other drugs of abuse (3). High relapse rates are associated with MJ user reports that withdrawal symptoms contribute to their failed quit attempts (4).
Sleep disturbance is a frequent complaint of about 76% of daily MJ users who abruptly discontinue their MJ use (5–7). Disturbed sleep is clinically important in the context of sustained abstinence from MJ use because about 77% of MJ users experiencing sleep problems report using tranquilizers, alcohol, or relapsing to MJ use to improve their sleep quality during a quit attempt (8). While important for initial identification of problems, the clinical utility of subjective reports of sleep disturbance is limited because such reports are often not supported by objective polysomnogram (PSG) measures of sleep (9).
Few studies have collected objective PSG measures in MJ users during short periods of untreated abstinence (10–12). Results from these studies showed changes from baseline including increased REM sleep (10;12–14) and decreased slow wave sleep (SWS) (15) one day after the sudden cessation of oral tetrahydrocannabinol (THC). While these initial reports support subjective reports of sleep disturbance in MJ users, two of the studies were limited by small sample sizes, drug administration was oral rather than smoked, and the length of abstinence was short. In a report more similar to our methods, three MJ-dependent men were studied during three days of abstinence from smoking MJ (11). Initial REM latency (iREM) and sleep efficiency (SEff) decreased during abstinence relative to baseline. Concordant with the previous studies, REM sleep (REM/%TST) increased. In addition, SWS (SWS/%TST) also increased. Subjective ratings of MJ craving and irritability increased only slightly (11). Even though this report consisted of only three MJ users across only three days of abstinence, results showed robust alterations in objective PSG measures after discontinuation of smoked MJ.
In a previous publication examining the first two nights after abrupt MJ cessation, MJ users showed less TST and less SWS on both nights and less SEff, longer initial sleep latency (ISL) and shorter REM latency on Night 2 than a control group(16). There were more PSG differences between the groups on Night 2 relative to Night 1, demonstrating that sleep in the MJ group was relatively worse on Night 2 compared to Night 1 and thus the MJ group failed to show the expected improvement in sleep after an adaptation night (i.e., first night effect).
The current study extends our earlier report to 14 days of abstinence. When compared to previous studies, (1) our sample size is larger, (2) our duration of abstinence is longer, (3) MJ users resided in an inpatient setting for the entire study, and (4) we did not administer oral THC or any other psychoactive substance during the 14-day abstinence. Based on the time course of reported withdrawal symptoms lasting at least 14 days (6;7), we hypothesized that the differences in PSG measures we demonstrated during the first two nights after abrupt discontinuation of MJ use would persist across the 14 days of abstinence.
METHODS
Participants
Recruitment
We recruited all participants using newspaper advertisements, internet announcements, and word-of-mouth. We advertised for MJ users who were not currently trying to quit or reduce their MJ use.
Screening and Intake
We screened interested individuals over the telephone, after verbal consent, using a written script (approved by the National Institutes on Drug Abuse – Intramural Research Program (NIDA-IRP), and if they met initial eligibility criteria we scheduled them for the first intake interview. At the first intake interview interested volunteers met with a member of the study research staff who gave a written and verbal detailed description of the research study to assess their willingness to participant. Prior to the comprehensive screening assessment, we obtained informed consent. During the first intake interview, participants were administered the Shipley Institute of Living Scale to estimate intelligence (IQ) (17). We obtained retrospective drug use history using the Addiction Severity Index (ASI)(18), and the Drug Use Survey (DUS)(19). We demonstrated the accuracy of our method of estimating drug use by showing that self-reports of joints per week of MJ smoked are strongly correlated with urine THC-COOH levels (r = 0.75; p< 0.05). We also collected urine for drug testing to confirm the use of MJ only and to quantify THC-COOH. Psychiatric status was ascertained with the Diagnostic Interview Schedule (DIS-IV)(20) corresponding to the Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM-IV). A trained masters or doctoral level interviewer used the DIS-IV to identify the presence of any DSM-IV Axis-I (e.g., major affective disorder, attention deficit disorder, posttraumatic stress disorder) or Axis-II (conduct disorder, antisocial personality disorder) diagnoses. One of our study physicians obtained detailed medical, sleep, and family histories, and complete physical exams including blood chemistry profiles, ECG, Hepatitis, HIV, and VDRL. If the volunteer met inclusion criteria, we contacted them and gave them a date to return for a second intake interview to sign the sleep study consent and begin the study.
Inclusion Criteria for MJ participants
To be eligible for inclusion, the volunteer must have reported the following: smoking MJ was their drug of choice and no regular use of any other illicit drugs, use of MJ for ≥2 years and smoking MJ ≥5 times per week over the past 3 months, and alcohol consumption ≤15 drinks per week. They were also required to have a urine toxicology screen positive for cannabis metabolites and negative for amphetamine, barbiturate, cocaine, methadone, opiate, PCP, and benzodiazepine at the time of screening and admission to the study. Since we were interested in determining the time course of objective PSG measures in MJ users who self-reported sleep disturbances during previous discontinuation of MJ use, for inclusion, MJ users also had to report ≥2 withdrawal symptoms on a MJ withdrawal questionnaire with ≥1 being a sleep disturbance symptom during previous discontinuation of MJ use. We included individuals with nicotine dependence; however, no participant met criteria for this diagnosis. Additional inclusion criteria included: English as his/her native language; estimated IQs ≥85; 18 to 30 years old to reduce possible age-associated effects on sleep parameters; no DIS-IV, comorbid Axis I psychiatric disorders or conduct disorder or antisocial personality disorder; no symptoms of narcolepsy, as assessed by an interview with a sleep medicine physician using a sleep history questionnaire; no medical conditions that may affect sleep architecture, no prior history of sleep disorder, or any medical condition including the following: seizure disorder, dementia, CNS infection, demyelinating disease, space-occupying lesion, movement disorder, CNS vasculitis, autoimmune disease (HIV), head injury with loss of consciousness >5 min, or congenital CNS abnormality; no history of diabetes, hypertension, or current use of medications that may affect sleep function (anxiolytics, antidepressants, stimulants, antihistamines, antipsychotics); normal hematocrit levels (HCT) (women >35%; men >39%); body mass index (BMI) < 35 for women and BMI < 32 for men because obese individuals are more prone to obstructive sleep apnea. Finally, participants were retained after the second night of the study only if their apnea-hypopnea index (AHI) was <10/h on the PSG on both Night 1 and Night 2. The AHI cutoff of 10 episodes per hour was selected based on the current literature evaluating the presence of sleep disordered breathing (SDB) in relation to established comorbid risk factors such as stroke, heart disease, congestive heart failure, and arrhythmias (21). The Results section and Table 1 provide more details on participant enrollment and characteristics.
Table 1.
Demographic, Sleep, and Drug Use Characteristics of the Marijuana (MJ) Users.
| Demographics | MJ Users (N = 18) |
|---|---|
| Age, year | 21.1 (3.1) [18–30] |
| Education, grade attained | 11.5 (0.9) [9–13] |
| Mother Education, grade attained | 12.5 (3.6) [10–16] |
| Shipley IQ | 94.4 (8.5) [85–109] |
| Male Gender, N (%) | 13 (72) |
| Usual length of time in bed, hour | 7.6 (0.57) |
| Usual length of actual sleep, hour | 6.5 (0.51) |
| SHQ Sleep Satisfaction Score+ | 29 (1.3) |
| SHQ Symptoms Related to Disturbed Sleep++ |
76 (8.2) |
| Horne-Ostberg Index (% of group) |
Morningness 22 Neither 56 Eveningness 22 |
| MJ use, joints/week | 101 (52) [40–210] |
| MJ use duration, year | 5 (3) [2–12] |
| MJ use, days/week | 7 |
| Day 1 THC-COOH (ug/dl) median | 277 |
| Day 12 THC-COOH (ug/dl) median | 25 |
| Alcohol average, drinks/week | 3 (4) [0–14] |
| Alcohol duration, year | 3 (3) [0–9] |
| Daily cigarettes smoker, N (%) | 5 (27) |
Note: Numbers are means (SD) [Ranges]; Sleep History Questionnaire (SHQ); +, ++ asked to evaluate sleep during the past few months; + the higher the score, the better the sleep satisfaction (Total possible: 42); ++ the higher the score, the more frequent the number of adverse symptoms experienced (Total possible: 420); THC-COOH analyzed on 10 MJ users and corrected for creatinine clearance.
The Institutional Review Boards of NIDA-IRP, the Johns Hopkins Medical Institutions, Joint Committee on Clinical Investigation, and the Johns Hopkins Bayview Medical Institutional Review Board approved this study. All participants provided informed consent and we paid them $907 if they completed the 14-day study. Monetary compensation conformed to the approved payment policies of the NIDA-IRP.
Data Collection
During the second intake interview, and prior to the admission date, the study coordinator met with participants and gave them sleep diaries to complete the 5 mornings prior to admission. During the same visit the participants completed the Horne-Ostberg morningness/eveningness scale (22) to define individual chronotypes (sleep-wake circadian rhythm pattern) prior to discontinuing their MJ use. The MJ users also completed a detailed Sleep History Questionnaire (SHQ) developed at the Johns Hopkins Sleep Center. Section 1 of the SHQ is composed of 6 question that ask the participants to rate their sleep quality and degree of satisfaction with their sleep/alertness from “very good” to “very poor” (Sleep Satisfaction Score). The next 84 questions (Section 2, Symptoms Related to Disturbed Sleep) characterize the sleep disturbance experienced by the individual during the past few months. We instructed the MJ participants not to deviate from their normal sleep-wake and MJ smoking routines until the time of their admission.
On the day of admission into the General Clinical Research Center (GCRC), a board certified sleep specialist (SL, CG, DN) reviewed the SHQ and screened all of our MJ users for any past history or baseline sleep problems including behaviors that may be clinically suggestive of increased leg movements. She/he asked the MJ users about a history of movements, leg kicks, being a restless sleeper with sheets in disarray in the morning, and if a bed partner had commented on observing any of these behaviors.
After admission, the MJ participants resided in the Clinical Inpatient Research Unit (CIRU) at NIDA-IRP where we enforced abstinence during 14 days. Participants were supervised by the unit staff, and urine toxicology screening was performed three-times per week. We obtained PSG recordings on Nights 1, 2, 7, 8, and 13 so that we could determine if our PSG measures change over time as a function of abstinence. We believed that these time points would be sufficient to study peak withdrawal symptoms since these are reported to begin as early as 24 hours after cessation of MJ use, peak between 4–10 days, and start to resolve after day 14 after cessation of MJ use (6;7). For each of the PSG study nights, a staff member escorted the MJ user to the GCRC sleep core facility in the early evening and brought them back to NIDA-IRP mid-morning after final waking. Time for lights out was determined by taking the median bedtime for the 5 nights prior to admission from the pre-admission sleep diaries. This method avoided putting the participants to bed at a time uncharacteristic of their normal sleep habits. Time in bed was not strictly fixed at 8 hours. Throughout the 14-day stay, no food or beverage containing caffeine was available. In addition, participants could smoke nicotine cigarettes only before 19:00 and only when escorted by a staff member to an outside designated smoking area.
Standard Polysomnography (PSG)
We obtained clinical PSG recordings, following standard methodology, on Nights 1,2,7,8, and 13 of the study. A registered polysomnography technician scored the PSG recordings for every 30-second epoch using Rechtschaffen and Kales standard criteria (23), and PLMs in sleep were scored using the American Academy of Sleep Medicine (AASM) task force criteria (24;25;25). A sleep specialist certified by the American Board of Sleep Medicine blinded to group membership reviewed all scoring on an epoch-by-epoch basis. Sleep measures included total sleep time (TST), sleep efficiency (SEff [total sleep time/time in bed ×100]), initial sleep latency (ISL [minutes from lights out to first 30 sec of any sleep stage]), wake after sleep onset (WASO [number of minutes awake during the night after initial sleep onset]), percent total time spent in REM (REM/%TST), stage 1, stage 2, stage 3 and 4 [slow wave sleep (SWS)], the total number of periodic limb movements with arousals (PLMA) and without arousals (PLM) in non-REM sleep, and PLM and PLMA indices (average number of PLM per hour of sleep without and with arousals, respectively), and disordered breathing rate per hour (AHI – Apnea/Hypopnea rate per hour). We used the AASM criteria for scoring PLMs of at least 4 consecutive leg kicks spaced 5 – 90 seconds apart with each kick having a .5 sec – 10 sec duration and amplitude of 8mu volts. Clinical sleep research protocols often exclude either the first night recording or average PSG findings from Nights 1 and 2 to adjust for the “first night effect” which is an adaptation night in the sleep lab. However, since we failed to identify a first night effect in our MJ group in our initial study and, instead, found that sleep became more disrupted as the length of MJ abstinence increased, we considered each night separately (16).
Subjective Ratings of MJ Withdrawal Craving, Mood, and Sleep Quality
Withdrawal symptoms and MJ craving were measured daily with a modified version of Budney’s MJ withdrawal symptom questionnaire (6) and a MJ craving questionnaire (26). For each questionnaire, a sum of all the ratings formed a total score. We assessed sleep quality using daily sleep diaries. Participants were asked to rate the general level of their sleep last night. The participants’ ratings ranged from very poor (scored as 1) to excellent (scored as 5). We measured psychological symptoms daily (e.g., mood and irritability) using the Profile of Mood States (POMS).
Quantitative Levels of MJ (THC-COOH)
The GCRC staff collected urine samples on the day of admission and every third day to verify abstinence. Quantitative levels of THC-COOH were determined by gas chromatography/mass spectroscopy (GC/MS) for THC and 11-OH-THC and by FPIA-fluorescence polarization immunoassay (27). We corrected the values for urinary creatinine.
Statistical Analysis
We describe the baseline characteristics of the study sample in means and standard deviations (SD) for the continuous measures and as proportions for the categorical variables. The time trend of PSG variables over the 14-day monitoring period was explored using nonparametric regression with locally weighted smoothing (lowess). To facilitate graphic display of this exploratory analysis, we added a small random variation to the time variable (i.e., night of PSG monitoring). We modeled the changes in PSG sleep parameters between Night 1 and each subsequent night in a regression model using a generalized estimating equations (GEE) approach to account for the potential within-person correlation among the outcome measures repeatedly assessed over the study period. All available PSG monitoring results were included in the analyses. All 18 MJ users in the final sample contributed PSG data. Baseline characteristics were included in the GEE models to assess their associations with the sleep outcomes within this target population. An interaction term between a baseline characteristic (i.e., age, sex, joints smoked per week) and the time variable (night of PSG) was included in the model to test the hypothesis that the baseline characteristic modified the sleep pattern over the study period if such a tendency was observed in the exploratory analyses. Since we considered all analyses in this study as exploratory, we report 2-tailed significance tests with p-values less than 0.10.
RESULTS
Demographics
We enrolled 20 MJ users into the study. We excluded two MJ users from the final sample because their PSG studies indicated sleep apnea (AHI >10) on either Night 1 or Night 2. Thus, the final sample was comprised of 18 MJ users and Table 1 presents the demographic, sleep characteristic, and drug use patterns of the group. Twenty-seven percent (5/18) of the sample met the DSM-IV diagnostic criteria for cannabis dependence and 11% (2/18) met criteria for cannabis abuse only. Sixty-one percent (11/18) of the MJ users did not meet the DSM IV diagnostic criteria for cannabis dependence or abuse. It is more difficult for a non-treatment seeking MJ user to meet DSM IV diagnostic criteria for dependence or abuse because they do not report substance-related legal, social or interpersonal problems related to their drug use.
Drug Use Characteristics
We estimated MJ use (joints smoked per week and duration of use) using (1) the ASI, (2) the DUSQ, (3) the MJ user’s report of the amount of money spent each week on MJ (US $2.00/joint reported by the Drug Enforcement Agency reports for the Baltimore area), and (4) self-reports of the number of joints (or joint equivalents) smoked per week. We have used this same methodology in our previous work (28;29). MJ users reported smoking MJ daily (7 of 7 days), smoking a mean of 101 ± 52 joints per week (median value = 84 joints/wk), and having used MJ for a mean of 5 ± 3 years. Mean age when MJ was first smoked was 14 years (± 2 years). The MJ users reported minimal alcohol use and smoked very few tobacco cigarettes. Only 5 MJ users reported smoking cigarettes daily and they reported a very mild to moderate level of addiction to nicotine on the Fagerstrom Test for Nicotine Dependence (FTND) (30) median = 4 (range = 1–7 total possible = 10).
MJ users abruptly discontinued MJ use when admitted to our research unit. When questioned about their last MJ use upon admission, all participants reported using MJ within 48 hours of admission and 15/18 smoked ≤ 24 hours of admission (8 last smoked MJ the morning of admission, 7 last smoked within 24 hours, and 3 last smoked < 48 hours prior to admission).
Time-related Change in Sleep during 14 Days of Abstinence
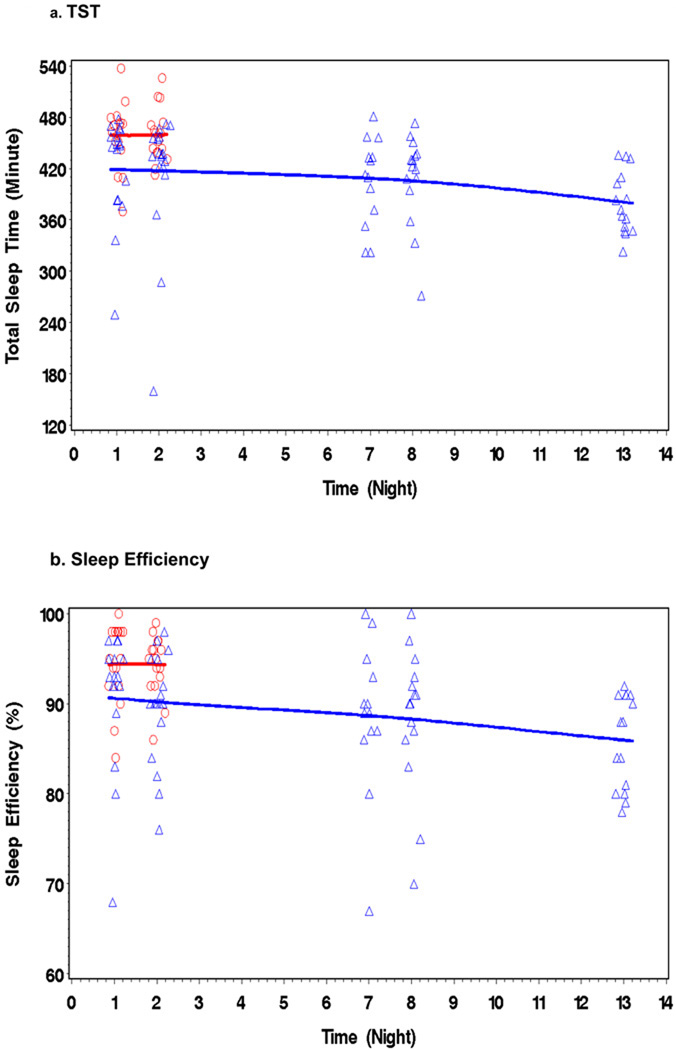
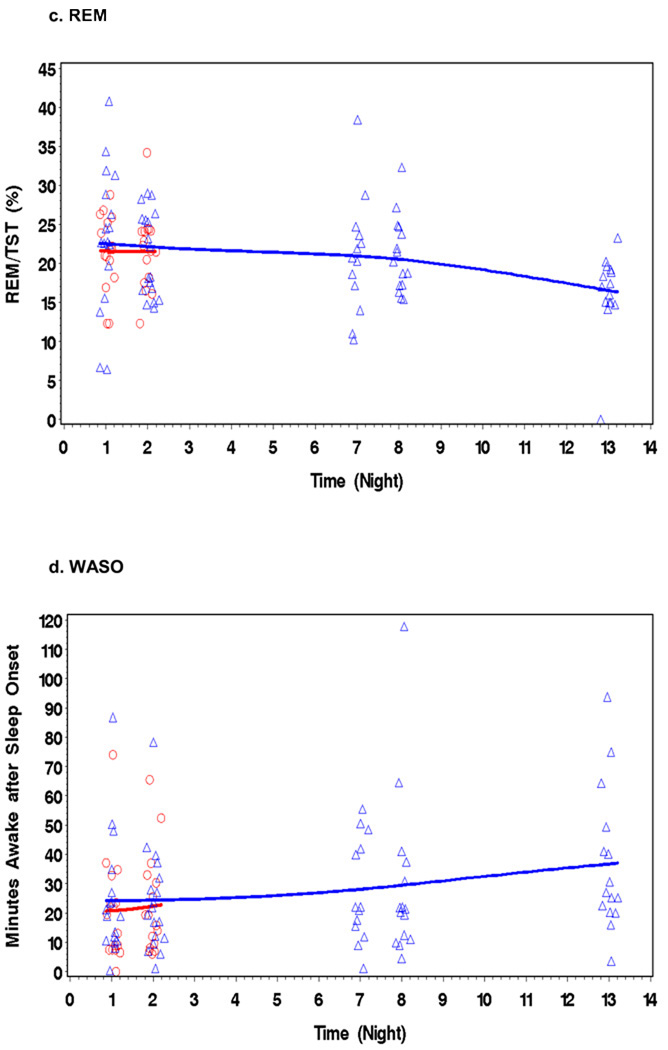
During 14 days of abstinence, MJ users showed a general trend in specific indices of sleep including reduced TST, a decline in SEff%, a reduction in REM/%TST, an increase in WASO (Figures 1a – 1d), and more PLMs. There were no significant improvements in any PSG measure of sleep. For reference only, the figure also shows the PSG results from an age-matched, drug-free comparison group (N = 16) with two nights of PSG (for more details see Ref. [16]). Data collection and PSG recording was identical for the control group. Table 2 shows the mean change values between the first PSG (Night 1) and PSG Nights 2,7,8, and 13 for each PSG measure. Some of the mean changes were significant by Nights 7 and 8, while others did not appear until Night 13. Mean values of the MJ users’ sleep variables (TST, SEff, REM, WASO) for the 5 nights of PSG studies never reached the mean values of our comparison group (16) or other published normative values for non-clinical young adults (reference values are presented at the bottom of Table 2) (31).
Figure 1.
Figures 1a – 1d/. During 14 days of abstinence, MJ users (triangles) showed deteriorations in specific indices of sleep, including reduced TST, a fall in SEff, reduced REM/%TST, and increased WASO. For reference only, the figure also shows the PSG results from an age matched-drug-free comparison group (circles) (N = 16) with two nights of PSG, (see Bolla et al., 2008 for more detail)(16).
Table 2.
Mean change in Polysomnogram values between Night 1 and Nights 2, 7, 8, and 13
| Variable | Night 1 PSG Values | Change between Night 1 and Night 2 |
Change between Night 1 and Night 7 |
Change between Night 1 and Night 8 |
Change between Night 1 and Night 13 |
|---|---|---|---|---|---|
| Total sleep time (TST), min | 422 (13.61) | −7.97 (18.21) | −10.87 (15.88) | −11.77(12.55) | −42.20(14.17)*** |
| Sleep efficiency (SEff)(TST/TIB), % | 91 (1.65) | −1.49 (1.34) | −2.06 (2.84) | −2.70 (1.38)** | −5.26 (1.93)*** |
| Initial sleep latency (ISL), min | 15 (6.47) | 5.40 (5.47) | 10.58 (12.11) | 8.77 (5.17)* | 11.37 (9.42) |
| Initial REM latency (min) | 74 (11.50) | 5.62 (12.69) | −1.9 (10.26) | −4.39 (17.47) | −2.42 (13.85) |
| Slow wave sleep (SWS)/Stage 3/4, min | 27 (5.66) | −1.82 (4.86) | 2.72 (6.48) | 12.21 (5.26)** | 3.46 (6.30) |
| SWS/%TST | 7 (1.46) | −0.19 (1.17) | 0.84 (1.57) | 2.97 (1.34)** | 1.48 (1.68) |
| Stage REM, min | 96 (7.2) | −7.14 (11.58) | −9.88 (11.44) | −8.51 (6.89) | −34.07 (8.99) *** |
| Stage REM/%TST | 23 (1.55) | −2.15 (2.17) | −2.43 (2.62) | −2.08 (1.88) | −7.00 (2.09)*** |
| Wake after sleep onset (WASO), min | 25 (4.90) | −0.60 (5.07) | 3.29 (7.67) | 5.39 (5.04) | 12.23 (5.73) ** |
| PLM index, #/hr | 1.62 (0.43) | −0.10 (0.47) | −0.86 (0.50) * | 3.45 (1.67) * | 3.75 (2.12) ** |
Note: Numbers are mean change scores (standard errors) TST = total sleep time: TIB (min): time in bed (min): PLM = periodic leg movements; Normative values are from Boselli et al., 1998 for 20–39 year olds (mean (SE): total sleep time 455(10.44); sleep efficiency 91(2.2); initial sleep latency 16(4.4), initial REM latency 76(6.3) min; wake after sleep onset 12(2.5), SWS (min) 97(7.59). Mean change between Night 1 and Nights 2,7,8,and 13 were modeled in a regression model using a generalized estimating equations (GEE) approach. r * p < 0.10; ** p < 0.05; *** p < 0.01.
Although the time since last MJ use prior to the first PSG was restricted and less than in most studies of this kind, differences in the duration of abstinence may have had a confounding effect on the observed time course of changes in sleep architecture. To address this concern, we performed further GEE analyses adjusting for “time between last MJ smoked” in three categories (i.e., Category 1: smoked MJ the morning of admission [n = 8]; Category 2: smoked MJ the within 24 hours of admission [n = 7]; Category 3: smoked 24 to 48 hours before admission [n = 3]). This additional analysis did not change any of the PSG results presented in Table 2 (i.e., PSG differences between Night 1 and subsequent nights remained significant and of the same magnitude). Due to the small sample size of Category 3 (n = 3) we were not able to model the interaction between “time since last MJ smoked” and PSG night.
Sex, age, quantity and duration of MJ use, subjective ratings of craving and withdrawal symptoms were included in the GEE models to determine if these variables predicted change in the sleep indices during the 14 nights of abstinence. We present these results below.
Sex-Related Differences in Sleep in MJ Users
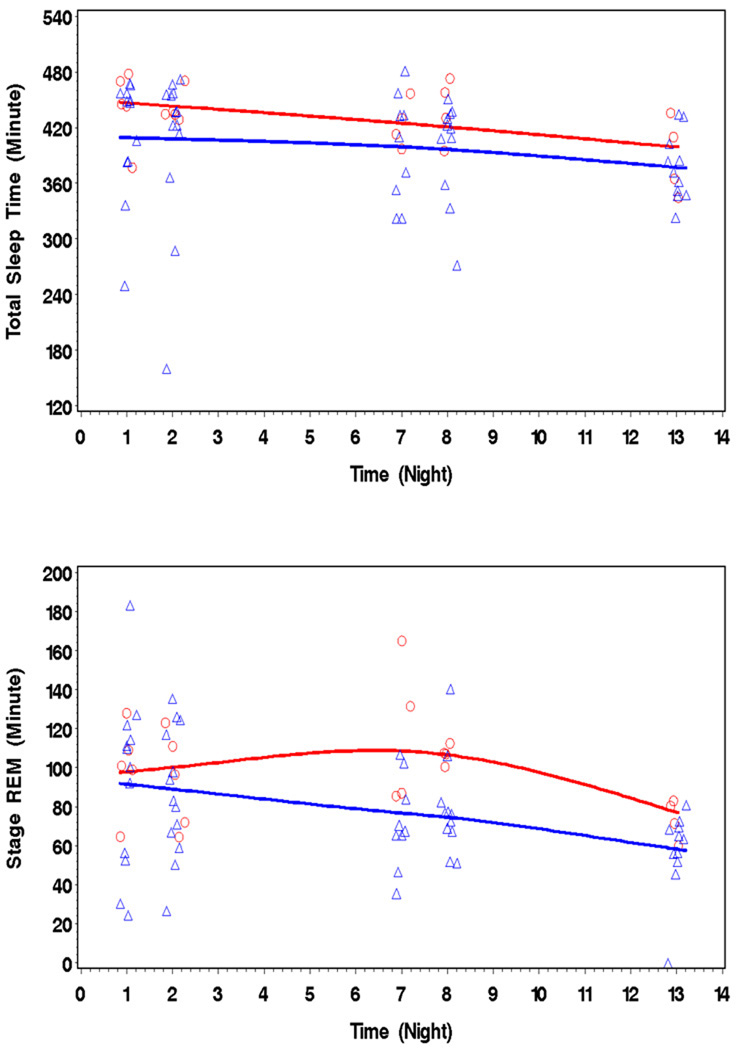
There are reports of sex-related differences in PSG sleep parameters (32) (33). Therefore, a critical factor in examining the current findings is a concern that the MJ group included a higher ratio of men to women (13 to 5) that may influence these findings. When we examined trends over the 14 nights of abstinence, MJ using women showed a significant mean difference compared to men of 28.9 min more TST and 16 min more REM sleep. Women also had 17.7 more PLMAs than men did across nights of abstinence (95%CI-39.7, 4.3), although this finding failed to reach statistical significance. Men and women experienced similar reduction in TST and REM sleep over the 14 days of the study (no sex by time interaction was found) (Figure 2). These results were age adjusted. These sex-related differences in TST and REM were not due to differences in quantity, duration, or age of first use. Women smoke 93 ± 70 joints/week and men smoked 98 ± 48 joints/week [t(16) = −.161; p = 0.87]. Duration of use was the same for women (5.6 ± 2.3 years) and men (5.1 ± 2.9 years) [t(16) = .36; p = .72) as was the age of first use (women 11.2 ±6.5 years old; men 14.7 ± 2.1 years old) [t(16) = −1.67; p = .12]. Although we only found statistically significant sex-related differences in TST and REM, we cannot rule out sex-related differences in other sleep variables because of limited sample size.
Figure 2.
MJ using women (top line - circles) showed a significant mean difference of 28.9 min more TST, 6 min more REM sleep than men (bottom line - triangles) do across nights of abstinence.
Sex-Related Differences in TST and REM during Abstinence
Age- Related Sleep Effects
Despite the narrow age range (18–30 yrs) in our study sample, we observed significant declines in SEff (on average, −0.58% per year of age increase; 95% CI −1.07, −0.9) with increased age among MJ users across the study period. The data show a significant increase of 3.38 minutes (95% CI 1.50, 5.26) in WASO per year of age increase.
Subjective Ratings of MJ Craving, Mood, and Sleep Quality
The MJ users completed daily MJ craving and withdrawal symptom questionnaires. MJ craving was low (a mean score of 40, 95% CI 33, 46, on an 84-point scale at Day 1), and craving declined significantly over time (average decline of 0.65 (SE 0.24) point per day; Z = − 2.71; p = 0.007) during the abstinence period [morning after Night 1/Day 1 (40 ± 18); morning after Night 13 (26 ± 14)], and did not correlate with any of the PSG measures. Withdrawal symptoms also decreased over time (Night 1/Day 1 (7.7 ± 4.0) to Day 13 (3.9 ± 4.0) (average decline of 0.30 (SE 0.09) point per day; Z = −3.26; p = 0.001). In addition, the MJ users did not endorse items reflecting increases in depressed mood or irritability on the POMS over the 14 days of abstinence. The sleep satisfaction score was 3.8 (+0.2) before abstinence (total possible = 5; the higher the score the more sleep satisfaction). The average sleep satisfaction score was 0.55 point lower (95% CI, −0.89, −0.21) during the abstinence period. There was on average a 0.036 point per day decline in sleep satisfaction score but it did not reach statistical significance (p = 0.59) and did not correlate with any of the PSG measures.
Relationship between Amount and Duration of MJ use and Periodic Limb Movements
After adjusting for day of PSG, age, and each other, the amount of MJ used (joints/week) was associated with higher total number of PLMs during the PSG study period (1.2 PLMs per 10 joints/week increase, p = 0.042). Similarly, longer duration of MJ use was associated with increased PLMs over the 13 days of the study (PLMs increased 0.8 per day in abstinence per year increase in MJ duration, p= 0.042). MJ duration was also a statistically significant effect modifier on the association between day in abstinence and PLMs per hour of non-REM sleep (PLM index) (on average, the PLM index increased 0.12 per day in abstinence for each additional year increase in MJ duration p = 0.046). The association between the amount of MJ used and the PLM index did not reach statistical significance. However, amount of MJ was found to be a statistically significant factor associated with total number of PLMAs during abstinence (on average 2.5 PLMAs higher per 10 joints/week increase, p = 0.0016). The association between amount of MJ and PLMA seemed to lessen as the duration of MJ use increased (p for interaction 0.056). Sex did not appear to be a statistically significant factor associated with PLM variables during abstinence.
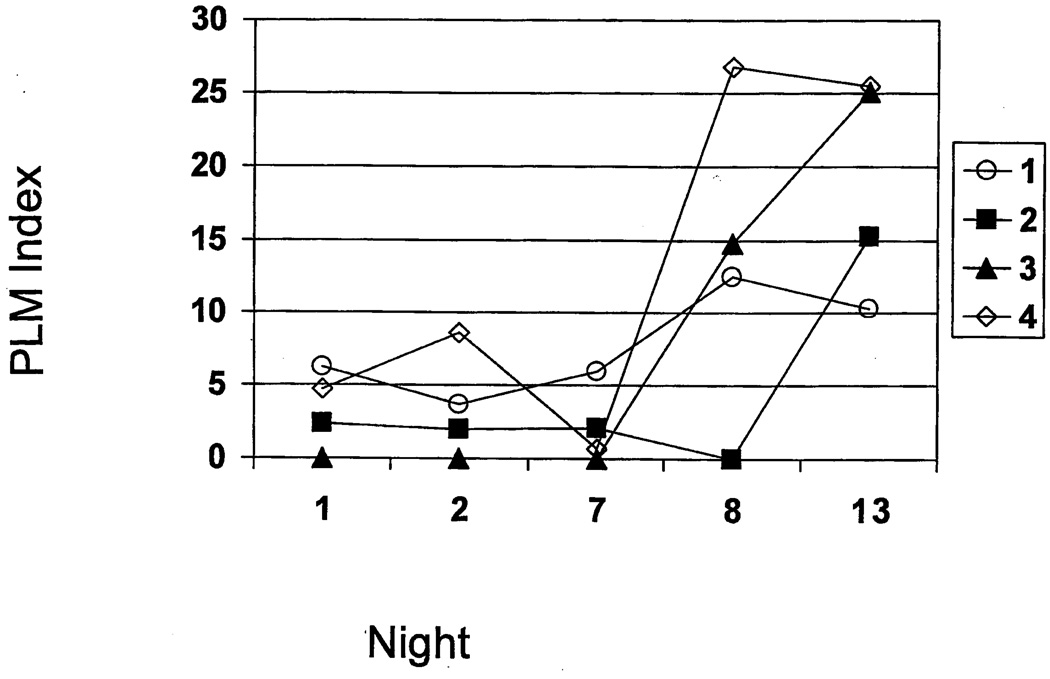
PLMs are rare in young adults with an average of less than 2/hr for 20 – 30-year-olds in one study (7/18 [39%]) (34). Our MJ users had a PLM index ranging between 1.3 – 6.8 on PSG Night 2. Four of the MJ users (22% of the group) developed a significant number of PLMs (PLM index >10) during the 13 nights of abstinence (Figure 3). These four MJ users showed lower PLM/hr on PSG Night 2 (6.2, 2.4, 0, and 4.7) that increased dramatically on Night 13 (10.3, 15.2, 25.1 and 25.5 respectively). Examining the amount of MJ smoked by these four users showed that all smoked ≥ 84 joints per week.
Figure 3.
Four of the MJ users (22% of the group) developed a significant number of PLMs (index >10) during the 14 days of abstinence.
Discussion
These data provide compelling evidence that abrupt cessation of heavy MJ use is associated with sleep disturbances and that these disturbances progress over the first two weeks of abstinence. This study also yields a number of important findings that can direct future research. First, abstinent MJ users show declines in TST, SEff%, and REM/%TST and increases in WASO and PLM across abstinence. Thus, the sleep PSG variables indicate more disrupted sleep across abstinence. Nevertheless, it is unclear whether this change relates to abstinence from MJ or reflects a return to the individual’s pre-MJ smoking sleep pattern. Second, the data show no noteworthy improvement in any PSG measure of sleep. Third, we report potentially important sex and age-related associations, and MJ dose-related effects on mean change in PSG indices during MJ abstinence. Finally, these data are important because there is limited PSG data available in this young adult age demographic.
Except for SWS, our results demonstrate a negative trend during the 14 days of abstinence for all our sleep indices. Our finding that REM/%TST decreases during abstinence is not supported by others who find increased REM/%TST during a shorter abstinence interval than ours (1 to 3 days) after high oral THC administration (11;12;15). Based on normal Horne-Ostberg Index (morningness/eveningness) questionnaire screening and normal REM latencies, we do not believe that decreased REM/%TST is due to confounders such as a phase delay. Since MJ is a REM suppressant when given acutely, it is possible that others found an initial rebound increase of REM early in abstinence because they administered high levels of oral THC. We also found significant declines in TST, SEff, and an increase in WASO not reported in the literature previously. Although no PSG studies of 14-day abstinent MJ users are available for comparison, cocaine users abruptly discontinuing use for 23 days also show reductions in TST and more WASO (35). Thus, PSG changes seem to occur across relatively long periods (at least 2 weeks) after either cocaine or MJ discontinuation. Although SWS/%TST increased marginally in a positive direction, both values were clinically abnormal [Night 1 SWS/%TST = 7.0 (5.6) versus Night 13 SWS/%TST = 8.1 (5.5)]. The mean nightly sleep duration reported by this MJ cohort prior to entering the study was 6.5 hours; therefore, one must consider that the small increase of SWS/%TST may have resulted from SWS rebound resulting from chronic sleep deprivation, which occurs preferentially over REM sleep.
Women had more TST, REM sleep and PLMAs than men during MJ abstinence. The origins of these differences between our women and men are unclear. We determined that these differences were not due to sex differences in MJ use history. Others have reported greater SEff in women than men (32) and have attributed this to more sleep-related respiratory disturbance in men. We excluded individuals with an AHI of >10 so we do not believe this was a major determinate of our sex-related PSG differences. Although specific aspects of sleep have been found to be influenced by menstrual cycle (36), because we had only five women in our sample and they were in different stages of their menstrual cycle, no meaningful patterns could be detected. Of interest, other investigators reported that abstinent cocaine using women, in similar stages of their menstrual cycle, show more TST than men, which is consistent with our observation in abstinent MJ users (35). Future studies should explore this relationship systematically.
Despite our restricted age range of 18 to 30 years old, SEff declined per year of age and WASO increased per year of age. While age-related PSG differences have been well studied in the elderly, there has been a paucity of sleep research on non-clinical young adults, (31;32), and no research has examined the interaction between MJ use and age on PSG. Expanding this research to older MJ users would help clarify the modifying role of age on sleep disturbance in abstinent MJ users.
Our MJ users report less craving and fewer withdrawal symptoms during MJ abstinence relative to baseline. We posit that craving and withdrawal symptoms were negligible because of the lack of “drug-taking” cues in our inpatient unit. Notwithstanding deteriorations in specific sleep indices, our MJ users do not report significantly less sleep satisfaction over time. This paradoxical finding is also reported in abstinent cocaine users and may be associated with dysregulation of the homeostatic sleep drive, the drive that translates increasing time awake into drive for sleep (37). This same mechanism may also explain our current findings in MJ users. Moreover, this dysregulation could potentially be a general factor-reflecting disturbance of this basic homeostatic drive that may promote recurrent drug use and relapse (38).
We also found that higher amounts of MJ used were associated with greater increases in PLMs during the 14 days of abstinence. Four of our 18 MJ users (22%) developed a PLM index >10 by Night 13 of abstinence (Figure 3). All of these MJ users smoked >85 joints per week (the group median). Moreover, longer duration of MJ use was associated with the development of more PLMs over time. There are no well-documented reports of normal PLMs in young adults, especially controlling for sleep-disordered breathing, as was done in this study. In normal adolescence the PLM index is usually <10 (39). There have been antidotal reports that MJ may actually reduce PLMs in certain individuals. Perhaps our MJ users who developed PLMs already had PLMs and their MJ use had reduced their symptoms to the point where they were not observed during the first few days of the study. Although we attempted to gather a detailed history of chronic sleep disturbances including PLM symptoms, it is possible that our MJ users denied PLM symptoms because their leg movements were masked by years of heavy MJ use. This could explain why PLMs increased over time as the levels of MJ decreased (see Table 1).
We were able to detect significant negative change in PSG indices with time in our MJ group. However, for those PSG variables that failed to show any change during abstinence, it may be that the effects were of smaller magnitude and our sample size was not large enough to detect these effects. The results of this study will not generalize to all users of MJ since our specific sample of MJ users was primarily young, reported sleep disturbance when discontinuing smoking MJ in the past, and some smoked large amounts of MJ. We studied only MJ users who reported sleep disturbance when attempting to discontinue MJ use in the past because, as a first step, we were most interested in our ability to determine if objective PSG abnormalities were present and because we believed this group would be the most similar to clinically relevant treatment seekers.
We acknowledge that it would have been better to start collecting our PSG data during a MJ use condition. This change in study design may have reduced some of the variability in our PSG data, especially during the initial PSG study nights. Nevertheless, our entire MJ group reported using MJ within 48 hours of admission and our results were unaffected after we controlled statistically for time since last use.
We believe our findings suggest potentially important associations that deserve further investigation. PSG evidence of sleep disturbance increased as levels of THC-COOH decreased, suggesting an association between declining levels of MJ and increasing sleep disturbance. However, it is impossible to determine if we may have unmasked an underlying sleep disorder, rather than a withdrawal syndrome. Although we screened very carefully for any history of symptoms suggesting a pre-existing sleep disorder and excluded participants for any abnormal initial PSG findings, it is possible that our MJ users were unable to recollect the severity of their past sleep problems because of their extensive history of MJ use. Furthermore, we also need to consider that anticipation about leaving the sleep unit could have affected some of the PSG findings on Night 13. We acknowledge these short-comings; nevertheless, this study was conducted as an initial step and is one of the few that carefully measures objective PSG sleep parameters during an extended period of abstinence.
Investigating sleep disturbance in MJ users provides evidence-based knowledge for developing prevention programs and non-pharmacological and pharmacological treatment interventions targeting the underlying mechanisms of sleep dysfunction associated with the discontinuation of daily, heavy MJ use or with other abused substances. Specifically, these findings also demonstrate that the development of specific sleep impairments may be age and sex specific. Thus, treating clinicians may need to modify their treatment programs for MJ cessation–related sleep disturbance for different ages and sexes. With treatment of the sleep dysfunction, any potentially adverse functioning consequences (i.e., poor cognitive function, daytime sleepiness) should also improve, resulting in better overall treatment outcomes in heavy MJ users.
Acknowledgment
We thank the nurses and clinical staff at NIDA-IRP and the Johns Hopkins Bayview GCRC who contributed to this project. We especially thank Ryan Vandrey, Ph.D. for editorial input and Debra Hill, B.S., and Scott Carey for computer and database support.
Funding/Support: Supported by NIH grants DA 17122(KB), the JHBMC-GCRC-CTSA (MO1 RR02719, UL1 RR 025005) and the DHH NIDA Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Karen I. Bolla, Ph.D. takes responsibility for the integrity of the data and the accuracy of the data analyses.
Conflict of Interest Disclosures: Dr. Bolla has no conflict of interest. Dr. Lesage has no conflict of interest. Dr. Gamaldo has contracted with GlaxoSmith Kline to conduct Study RXP110908. Dr. Neubauer has received Honoraria from Sanofi-Aventis and Takeda Pharmaceuticals. Dr. Wang has no conflict of interest. Mr. Funderburk has no conflict of interest. Dr. Allen has participated in the GlaxoSmith Kline grant (Study of Augmentation) at johns Hopkins; consulted and spoken for GlaxoSmith Kline, Boehringer Ingelheim, Pfizer, UCB Pharma, and Responics; and granted free use of some PAM-RL devices. Ms. David has no conflict of interest. Dr. Cadet has no conflict of interest.
Financial Disclosure: None reported
Reference List
- 1.Substance Abuse and Mental Health Services Administration. Rockville, MD: NSDUH Series H-32 ed.; Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies) 2007
- 2.Compton WM, Pringle B. Services research on adolescent drug treatment. Commentary on "The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials". J Subst Abuse Treat. 2004 Oct;27(3):195–196. doi: 10.1016/j.jsat.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. Journal of Substance Abuse Treatment. 2003 Sep;25(2):85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 4.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse 4. J Subst Abuse Treat. 2008 Dec;35(4):362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004 Nov;161(11):1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 6.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003 Aug;112(3):393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 7.Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000 Nov;8(4):483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 8.Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006 Jan;15(1):8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- 9.Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007 Oct 15;3(6):622–630. [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg I, Jones R, Walker JM, Cavness C, March J. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther. 1975 Apr;17(4):458–466. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- 11.Liguori A, Brown TW, McCall W. Sleep architecture changes during marijuana withdrawal. Sleep. 2004;27:54–55. [Google Scholar]
- 12.Jones RT, Benowitz NL, Herning RI. Clinical Relevance of Cannabis Tolerance and Dependence. Journal of Clinical Pharmacology. 1981;21(8–9):S143–S152. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg I, Jones R, Walker JM, Cavness C, March J. Effects of high dose delta-9-tetrahydrocannabinol on sleep patterns in man. Clinical Pharmacology and Therapeutics. 1975;17(4):458–466. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- 14.Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981 Aug;21(8–9 Suppl):143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 15.Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther. 1976 Jun;19(6):782–794. doi: 10.1002/cpt1976196782. [DOI] [PubMed] [Google Scholar]
- 16.Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, et al. Sleep disturbance in heavy marijuana users. Sleep. 2008 Jun 1;31(6):901–908. doi: 10.1093/sleep/31.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles, CA: Western Psychological Services; 1991. [Google Scholar]
- 18.McLellan AT, Luborsky L, Woody GE, et al. An improved evaluation instrument for substance abuse patients: the Addiction Severity Index. Journal of Nervous and Mental Disorders. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Smith SS. Addictive drug survey manual. Baltimore: NIDA Addiction Research Center; 1991. [Google Scholar]
- 20.Robins LN, Helzer JE, Croughan J, et al. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 21.Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005 Apr 1;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 22.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales Ae. A manual of standardized terminology techiques and scoring system for sleep stage of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 24.Bonnet M, Carkey D, Carskadon MA, et al. EEG arousal: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 25.Atlas Task Force of ASDA. Recording and scoring leg movements. Sleep. 1993;16:748–759. [PubMed] [Google Scholar]
- 26.Heishman SJ, Singleton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. Methods Mol Med. 2006;123:209–216. doi: 10.1385/1-59259-999-0:209. [DOI] [PubMed] [Google Scholar]
- 27.Huestis M, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- 28.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005 Jun;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Bolla K, Brown K, Eldreth D, Tate K, Cadet J-L. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Boselli M, Parrino L, Smerieri A, Terzano MG. Effect of age on EEG arousals in normal sleep. Sleep. 1998 Jun 15;21(4):351–357. [PubMed] [Google Scholar]
- 32.Roehrs T, Kapke A, Roth T, Breslau N. Sex differences in the polysomnographic sleep of young adults: a community-based study. Sleep Med. 2006 Jan;7(1):49–53. doi: 10.1016/j.sleep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Paul KN, Turek FW, Kryger MH. Influence of sex on sleep regulatory mechanisms. J Womens Health (Larchmt) 2008 Sep;17(7):1201–1208. doi: 10.1089/jwh.2008.0841. [DOI] [PubMed] [Google Scholar]
- 34.Pennestri MH, Whittom S, Adam B, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: prevalence and interval distribution. Sleep. 2006 Sep 1;29(9):1183–1187. doi: 10.1093/sleep/29.9.1183. [DOI] [PubMed] [Google Scholar]
- 35.Morgan PT, Paliwal P, Malison RT, Sinha R. Sex differences in sleep and sleep-dependent learning in abstinent cocaine users. Pharmacol Biochem Behav. 2009 Jul;93(1):54–58. doi: 10.1016/j.pbb.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007 Sep;8(6):613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006 May 20;82(3):238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Roehrs T, Johanson CE, Meixner R, Turner L, Roth T. Reinforcing and subjective effects of methylphenidate: dose and time in bed. Exp Clin Psychopharmacol. 2004 Aug;12(3):180–189. doi: 10.1037/1064-1297.12.3.180. [DOI] [PubMed] [Google Scholar]
- 39.Tayag-Kier CE, Keenan GF, Scalzi LV, Schultz B, Elliott J, Zhao RH, et al. Sleep and periodic limb movement in sleep in juvenile fibromyalgia. Pediatrics. 2000 Nov;106(5):E70. doi: 10.1542/peds.106.5.e70. [DOI] [PubMed] [Google Scholar]