Abstract
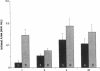
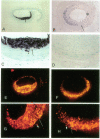
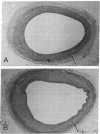
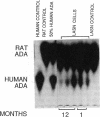
Cultured vascular smooth muscle cells (SMCs) containing retrovirally introduced genes are a potential vehicle for gene replacement therapy. Because the cultured SMCs are selected for their ability to proliferate in vitro, it is possible that the SMCs might be permanently altered and lose their capacity to respond to growth-suppressing conditions after being seeded back into blood vessels. To investigate this possibility we measured SMC proliferation and intimal thickening in balloon-injured Fischer 344 rat carotid arteries seeded with SMCs stained with the fluorescent marker 1,1'-dioctadecyl-3,3,3',3'-tetramethylindo-carbocyanine perchlorate (DiI) and infected with replication-defective retrovirus expressing human adenosine deaminase or human placental alkaline phosphatase. The majority of the seeded SMCs remained in the intima while a few of the cells appeared to migrate into the first layer of the media. Intimal SMC proliferation returned to background levels (< 0.1% thymidine labeling index) by 28 d. At late times (1 and 12 mo) the morphological appearance of the intima was the same for balloon-injured arteries with or without seeded SMC, except that the seeded arteries continued to express human adenosine deaminase or alkaline phosphatase. These results support the conclusion that cultured SMC infected with a replication-defective virus containing human adenosine deaminase or alkaline phosphatase are not phenotypically altered and do not become transformed. After seeding onto the surface of an injured artery, they stop replicating but continue to express the introduced human genes even over the long term.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnall K. M. The migration and distribution of somite cells after labelling with the carbocyanine dye, Dil: the relationship of this distribution to segmentation in the vertebrate body. Anat Embryol (Berl) 1992;185(4):317–324. doi: 10.1007/BF00188544. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. E., Kindy M. S., Sonenshein G. E. Expression of the c-myb proto-oncogene in bovine vascular smooth muscle cells. J Biol Chem. 1992 Mar 5;267(7):4625–4630. [PubMed] [Google Scholar]
- Campbell J. H., Kocher O., Skalli O., Gabbiani G., Campbell G. R. Cytodifferentiation and expression of alpha-smooth muscle actin mRNA and protein during primary culture of aortic smooth muscle cells. Correlation with cell density and proliferative state. Arteriosclerosis. 1989 Sep-Oct;9(5):633–643. doi: 10.1161/01.atv.9.5.633. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R. What controls smooth muscle phenotype? Atherosclerosis. 1981 Nov-Dec;40(3-4):347–357. doi: 10.1016/0021-9150(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Collazzo R. E., Karnovsky M. J. A morphologic and permeability study of luminal smooth muscle cells after arterial injury in the rat. Lab Invest. 1978 Aug;39(2):141–150. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fields-Berry S. C., Halliday A. L., Cepko C. L. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle J., Au Y. P., Clowes A. W., Reidy M. A. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990 Nov-Dec;10(6):1082–1087. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeau A. P., Campan M., Desgranges C. Induction of cell cycle-dependent genes during cell cycle progression of arterial smooth muscle cells in culture. J Cell Physiol. 1991 Mar;146(3):356–361. doi: 10.1002/jcp.1041460304. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D., Osborne W. R. Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood. 1989 Aug 1;74(2):876–881. [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986 Jul;103(1):171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindy M. S., Brown K. E., Sonenshein G. E. Regulation of expression of the growth-state-related genes 2F1 and 2A9 during entry of quiescent smooth muscle cells into the cell cycle. J Cell Biochem. 1991 Aug;46(4):345–350. doi: 10.1002/jcb.240460409. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Expression of actin mRNAs in rat aortic smooth muscle cells during development, experimental intimal thickening, and culture. Differentiation. 1986;32(3):245–251. doi: 10.1111/j.1432-0436.1986.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Kohler T. R., Jawien A. Flow affects development of intimal hyperplasia after arterial injury in rats. Arterioscler Thromb. 1992 Aug;12(8):963–971. doi: 10.1161/01.atv.12.8.963. [DOI] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. M., Clowes M. M., Osborne W. R., Clowes A. W., Miller A. D. Long-term expression of human adenosine deaminase in vascular smooth muscle cells of rats: a model for gene therapy. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1138–1142. doi: 10.1073/pnas.89.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Schwartz S. M., Clowes M. M., Clowes A. W. Heparin regulates smooth muscle S phase entry in the injured rat carotid artery. Circ Res. 1987 Aug;61(2):296–300. doi: 10.1161/01.res.61.2.296. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Palmer T. D., Rosman G. J., Osborne W. R., Miller A. D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz H. D., Rothman T. P., Gershon M. D. Colonization of the post-umbilical bowel by cells derived from the sacral neural crest: direct tracing of cell migration using an intercalating probe and a replication-deficient retrovirus. Development. 1991 Mar;111(3):647–655. doi: 10.1242/dev.111.3.647. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Kindy M. S., Brown K. E., Rosenberg R. D., Sonenshein G. E. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem. 1989 Apr 25;264(12):6990–6995. [PubMed] [Google Scholar]
- Reuterdahl C., Sundberg C., Rubin K., Funa K., Gerdin B. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derived growth factor B chain during wound repair in humans. J Clin Invest. 1993 May;91(5):2065–2075. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner A. S., Murphy R. A., Owens G. K. Expression of smooth muscle and nonmuscle myosin heavy chains in cultured vascular smooth muscle cells. J Biol Chem. 1986 Nov 5;261(31):14740–14745. [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Serbedzija G. N., Bronner-Fraser M., Fraser S. E. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989 Aug;106(4):809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Serbedzija G. N., Fraser S. E., Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990 Apr;108(4):605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Zwolak R. M., Adams M. C., Clowes A. W. Kinetics of vein graft hyperplasia: association with tangential stress. J Vasc Surg. 1987 Jan;5(1):126–136. [PubMed] [Google Scholar]