Abstract
Background
Age differences may help to explain discrepancies in medical care received by cancer patients near death.
Objectives
Understanding age differences in advanced cancer patients' end-of-life experiences.
Design
NCI and NIMH funded multi-site prospective cohort study.
Participants
396 deceased cancer patients, mean age (58.6 ± 12.5), in the Coping with Cancer study.
Measurements
Baseline interviews (Treatment Preference) and 1 week postmortem chart reviews (Treatment Received).
Results
14.1% of patients were 20–44 years old, 54.0% were 45–64 years old, and 31.8% were ≥65 years old. Compared to younger patients, middle-aged patients wanted less life-prolonging care (OR 0.32; CI 0.16-0.64). In the last week of life, older patients were less likely to undergo ventilation (OR 0.27; CI 0.07-1.00) than younger patients. Middle-aged patients who preferred life-prolonging care were less likely to receive it than younger patients (OR 0.21; CI 0.08-0.54), but were more likely to avoid unwanted life-prolonging care (OR 2.38; CI 1.20-4.75) than younger patients. Older patients were less likely to receive desired life-prolonging care than younger patients (OR 0.23; CI 0.08-0.68), however, they were not more likely to avoid unwanted life-prolonging care than younger patients (OR 1.74; CI 0.87-3.47).
Conclusions
Likelihood of a patient's treatment preference being consistent with care differ by age and treatment preferences. Older patients preferring life-prolonging therapies are less likely to receive them than younger patients; middle-aged patients who want to avoid life-prolonging care are more likely to do so than younger patients. Both findings have implications for patients' quality-of-death, indicating a need for further research.
Introduction
Terminally ill patient treatment preferences and treatments received differ by age.1–8 Age differences help explain discrepancies in treatments provided to cancer patients.9–17 Study to Understand Prognoses and Preferences for Outcomes and Treatments (SUPPORT), a well known end-of-life care study on hospitalized patients with a 6 month prognosis,18 showed that age was associated with decreased desire for—and receipt of—aggressive treatments.19,20 Although numerous subsequent studies treated age as a covariate among the terminally ill, less is known about specific age differences in patient treatment preferences and treatment received, particularly among advanced cancer patients. Even less is known about how age affects the likelihood a patient will receive treatment consistent with their preferences. As the US population ages a heightened understanding of how age influences health care for terminally ill patients is needed.
Coping with Cancer (CwC), a study of advanced cancer patients followed through death, recruited a diverse, primarily outpatient sample. Older studies drew data from inpatient settings. Over the last decade advanced cancer patients, particularly older aged patients, received care more frequently in outpatient settings, increasing the need to reexamine advanced cancer patient end-of-life care.
This analysis sought to identify advanced cancer patient age differences in treatment preferences, treatment received and goal attainment (the likelihood that a patient's treatment preference will be consistent with their treatment received). The a priori hypothesis predicted that older age would be associated with lower rates of preference for life-prolonging treatment, decreased receipt of life-prolonging care, and higher rates of goal attainment.
Methods
Study sample
CwC, an NCI- and NIMH-funded prospective, longitudinal, multi-site, cohort study, recruited advanced cancer patients from August 2002 to October 2008. Eligibility criteria included: diagnosis of advanced cancer, expectation of less than 6 months to live; age at least 20 years; and adequate stamina to complete the interview. Using interviews and chart reviews, patient data were collected at baseline and then at postmortem, roughly 4 months after the baseline interview (median survival time = 146 days). Details on this study, including recruitment and eligibility, are published elsewhere.21 Of 1035 eligible patients, 723 patients (69.9%) consented and enrolled in the study. Reasons for nonparticipation (N = 302) included “not interested” (N = 120), “other” (N = 69), or “caregiver refuses” (N = 39). Apart from increased rates of higher education among participants (p = 0.003), there were no differences in the socio-demographic characteristics between participants and non-participants. As of May 2008, 396 of the 723 participating subjects were deceased with postmortem data. The deceased cohort (N = 396) did not differ significantly (p < 0.05 was considered significant), by psychological distress or rates of psychiatric disorders, from other participants. This cohort was more debilitated (e.g., had worse performance status and higher symptom burden) and more likely to have characteristics associated with lower socioeconomic status (e.g., younger, less educated, uninsured, and self identified as an ethnic minority). These findings are consistent with prior studies showing increased cancer mortality rates among patients of lower socioeconomic status.22,23 Of 396 deceased patients, 327 had complete baseline data on the question “If you could choose, would you prefer a course of treatment that focused on extending life as much as possible, even if it meant more pain and discomfort.”
Measures
Interviews were conducted by trained research staff in English or Spanish. Patient race/ethnicity was self-reported as non-Hispanic White, non-Hispanic Black, and Hispanic (hereafter referred to as White, Black, and Hispanic). Other patient-reported socio-demographic characteristics included gender, marital status, education, religion, insurance status and Medicare/Medicaid enrollment status. The Karnofsky scale (scale 0–100, where 0 = “dead” and 100 = “asymptomatic”) and the Charlson Index of Co-morbidity (scale 0–37, where 0 = no co- morbid conditions and 37 = the maximal possible co-morbid conditions) assessed functional status.
Independent variables
Age was examined as a continuous variable, to determine the presence of a difference, and categorical variable, to better define that difference. Categorically, age was divided into three groups, younger (20 to 44 years), middle (45 to 64 years), and older aged (≥65 years) Categorical variables were analyzed by separately comparing older, middle and younger aged advanced cancer patients. These categories, used in relatively recent secondary data analyses conducted on age differences in end-of-life care in the SUPPORT study, allow for comparisons between the results of the two data sets.17,19,20
Dependent variables
Patient treatment preferences
Direct questions assessed individual patient treatment preferences at end-of-life, e.g., “Would you take chemotherapy and risk side effects such as nausea, eating problems, hair loss, weakness, fatigue, bowel problems, or have to spend more time in the hospital if it would keep you alive: 2 years, 1 year, 6 months, 3 months, 1 month, 1 week?” Patients desiring chemotherapy that would only extend life one week were categorized as preferring life- prolonging care. 1 week was the selected cut off since desiring chemotherapy that kept the patient alive 1 week was the most aggressive option available for the patient to choose and because it best approximated the median split for patient response to this question for the overall CwC sample. For other life-prolonging interventions, any preference for the intervention was categorized as desiring life prolonging care. Patients were also asked, e.g. “If you could choose, would you prefer 1) a course of treatment that focused on extending life as much as possible, even if it meant more pain and discomfort, or 2) on a plan of care that focused on relieving pain and discomfort as much as possible, even if that meant not living as long?” Patients preferring option 1 were considered to prefer life-prolonging care. This question determined patient “extend life” treatment preference in goal attainment analysis.
End-of-life care received
Treatment type and location of death were obtained from postmortem chart reviews.
Patient goal attainment
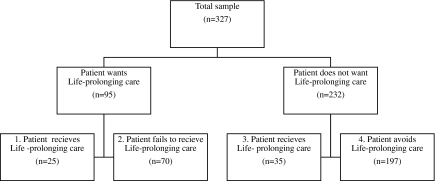
Successful goal attainment was defined as patients who wanted and received or did not want and avoided life-prolonging care. Previous literature suggests age differences in patient goal attainment (comparisons between treatment preference and treatment received).20,24 The likelihood of patients' baseline treatment preferences (i.e. life-prolonging vs. non life-prolonging), matching patients' care received (objective outcomes recorded in patients' charts) were compared across age groups. Patients were placed into 1 of 4 categories (See Figure): group 1 wanted life-prolonging care and received it; group 2 wanted life-prolonging care but did not receive it; group 3 did not want life-prolonging care but received it; group 4 did not want life-prolonging care and did not receive it. Successful goal attainers, like group 1, got what they wanted or, like group 4, avoided what they did not want. Unsuccessful goal attainers (groups 2 and 3), while distinct patient populations, had care inconsistent with their treatment preferences.
FIG. 1.
Goal attainment grouping.
Statistical analysis
T-test, Cochran-Mantel-Haenszel, and χ2 test statistics were used, as appropriate, to test for significant differences in socio-demographic characteristics, recruitment sites and cancer type between the three age groups at baseline. Logistic regression models were used to compare age as a continuous variable and age groups by patients' treatment preferences, location of death and care received at end-of-life. All models were adjusted individually for confounders using backward selection. Each dependent variable was compared between age groups and also age as a continuous variable in a separate statistical model evaluating all variables listed in Table 1 as possible confounders. Based on the results, the least powerful non-statistically significant (p > .05) variables were removed from each estimated model and the analysis rerun. This process continued until remaining variables from Table 1 were all statistically significant (p < .05) confounders. Cox proportional hazards models were applied to examine differences in probability of survival between the age groups. The assumption of proportional hazards was tested and adequately met in this sample. All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Table 1.
Characteristics of Sample Patients (n = 396)
| |
Descriptive statistics |
Comparative analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sociodemographic variables | Total sample n = 396 | Older Aged (≥65) n = 126 | Middle-aged (45–64) n = 214 | Younger aged (20–44) n = 56 | t or χ2 | DF | p-value | ||||
| Age, M (SD) | 58.6 | (12.5) | 72.4 | (5.7) | 55.8 | (5.7) | 38.1 | (6.1) | |||
| Education, M (SD) | 12.6 | (4.0) | 12.3 | (5.1) | 12.9 | (3.3) | 12.5 | (3.6) | 0.9 | 2 | 0.41* |
| Karnofsky, M (SD) | 64.9 | (16.1) | 61.9 | (16.0)a | 66.9 | (15.6)a | 64.0 | (17.5) | 3.8 | 2 | 0.02 |
| CC M (SD) | 8.3 | (2.7) | 10.0 | (2.7)ac | 8.0 | (2.1)ab | 5.9 | (1.9)bc | 65.7 | 2 | <0.001† |
| Male, n (%) | 221 | (55.8) | 75 | (59.5) | 118 | (55.1) | 28 | (50.1) | 1.5 | 2 | 0.47 |
| Health Insurance, n (%) | 239 | (61.9) | 95 | (77.9)ac | 116 | (55.8)a | 28 | (50.0)c | 19.9 | 2 | <0.001 |
| Medicare, n (%) | 170 | (43.9) | 102 | (84.3)ac | 48 | (22.8)a | 20 | (36.4)c | 119.8 | 2 | <0.001 |
| Married, n (%) | 244 | (62.4) | 75 | (61.0) | 134 | (63.2) | 35 | (62.5) | 0.2 | 2 | 0.92 |
| Race, n (%) | 25.0 | 8 | 0.002 | ||||||||
| White | 260 | (65.7) | 92 | (73.0)c | 144 | (67.3)b | 24 | (42.9)cb | 16.2 | 2 | <0.001 |
| Hispanic | 58 | (14.7) | 18 | (14.3)c | 23 | (10.8)b | 17 | (30.4)cb | 13.7 | 2 | 0.001 |
| Black | 7 | (17.9) | 14 | (11.1) | 44 | (20.6) | 13 | (23.2) | 6.1 | 2 | 0.05 |
| Asian | 5 | (1.3) | 2 | (1.6) | 2 | (0.9) | 1 | (1.8) | 0.4 | 2 | 0.81 |
| Other | 2 | (0.5) | 0 | (0.0) | 1 | (0.7) | 1 | (4.0) | 4.2 | 2 | 0.13 |
| Religion, n (%) | 20.8 | 14 | 0.11 | ||||||||
| Catholic | 145 | (36.6) | 50 | (39.7) | 74 | (34.6) | 21 | (37.5) | 0.9 | 2 | 0.63 |
| Protestant | 70 | (17.7) | 25 | (19.8) | 40 | (18.7) | 5 | (8.9) | 3.5 | 2 | 0.17 |
| Jewish | 20 | (5.1) | 12 | (9.5)a | 7 | (3.3)a | 1 | (1.8) | 7.9 | 2 | 0.02 |
| Muslim | 5 | (1.3) | 2 | (1.6) | 3 | (1.4) | 0 | (0.0) | 0.9 | 2 | 0.65 |
| Pentecostal | 9 | (2.3) | 2 | (1.6) | 5 | (2.3) | 2 | (3.6) | 0.7 | 2 | 0.71 |
| Baptist | 57 | (14.4) | 13 | (10.3) | 34 | (15.9) | 10 | (17.9) | 2.6 | 2 | 0.27 |
| Other | 71 | (17.9) | 19 | (15.1) | 37 | (17.3) | 13 | (26.8) | 3.7 | 2 | 0.15 |
| None | 19 | (4.8) | 3 | (2.4) | 14 | (6.5) | 2 | (3.6) | 3.2 | 2 | 0.20 |
| Site, n (%) | 52.9 | 12 | <0.001 | ||||||||
| Yale | 81 | (20.5) | 24 | (19.1) | 46 | (21.5) | 11 | (19.6) | 0.3 | 2 | 0.87 |
| VACT | 25 | (6.3) | 12 | (9.5) | 12 | (5.6) | 1 | (1.8) | 4.3 | 2 | 0.12 |
| MSK | 30 | (7.6) | 11 | (8.7) | 15 | (7.0) | 4 | (7.1) | 0.4 | 2 | 0.83 |
| Simmons | 32 | (8.1) | 10 | (7.9) | 15 | (7.0) | 7 | (12.5) | 1.8 | 2 | 0.40 |
| Parkland | 152 | (38.4) | 27 | (21.4)ac | 94 | (43.9)a | 31 | (55.4)c | 24.7 | 2 | <0.001 |
| DFCI/MGH | 9 | (2.3) | 1 | (0.8) | 7 | (3.3) | 1 | (1.8) | 2.2 | 2 | 0.33 |
| NHOH | 67 | (16.9) | 41 | (32.5)ac | 25 | (11.7)a | 1 | (1.8)c | 36.0 | 2 | <0.001 |
| Diagnosis, n (%) | 12.5 | 10 | 0.25 | ||||||||
| Lung cancer | 85 | (21.5) | 28 | (22.2) | 50 | (23.4) | 7 | (12.5) | 3.5 | 2 | 0.18 |
| Colorectal cancer | 57 | (14.8) | 18 | (14.3) | 32 | (15.0) | 7 | (12.5) | 0.3 | 2 | 0.87 |
| Breast cancer | 42 | (10.6) | 8 | (6.4) | 26 | (12.2) | 8 | (14.3) | 3.4 | 2 | 0.19 |
| Pancreatic cancer | 37 | (9.6) | 16 | (12.7) | 19 | (8.9) | 2 | (3.6) | 4.4 | 2 | 0.11 |
| Other GI cancer | 54 | (13.6) | 16 | (12.7) | 28 | (13.1) | 10 | (17.9) | 0.7 | 2 | 0.70 |
| Other cancers | 121 | (30.6) | 40 | (31.8) | 59 | (27.6) | 22 | (39.3) | 3.0 | 2 | 0.22 |
CC -Charlson Comorbidity; VACT -Veteran's Association of Connecticut; MSK -Memorial Sloan Kettering; DFCI -Dana Farber Cancer Institute; MGH -Massachusetts General Hospital; NHOH -New Hampshire Oncology-Hematology; GI -Gastrointestinal.
All outcomes for continuous variables were calculated using the t-test.
All dichotomous comparisons were calculated using the Fisher Chi square test. If expected cell count was less than 5, the Cochran-Mantel-Haenszel General Association Statistics were used.
indicates a significant (p < .0167) difference between older aged and middle-aged patients.
indicates a significant (p < .0167) difference between middle-aged and younger aged patients.
indicates a significant (p < .0167) difference between older aged and younger aged patients.
Results
Sample characteristics are shown in Table 1. The mean age for older aged patients was 72.4 years (SD = 5.7 years), for middle-aged patients 55.8 years (SD = 5.7 years) and for younger aged patients 38.1 years (SD = 6.1 years). Most variables remained similar across age groups, though differences existed. Comparing patient treatment preference for life-prolonging care (Table 2) to treatment received (Table 3) yielded 4 goal attainment groups that fell into 1 of 2 categories: 1) successful goal attainment (N = 222); 2) unsuccessful goal attainment (N = 105). Successful goal attainers were patients who desired life- prolonging treatment and then got it (N = 25) or were patients who did not want and then avoided life-prolonging care (N = 197) (See Figure). All age-comparisons in tables 2, 3, and 4 were adjusted for possible confounders.
Table 2.
Age Group Comparisons of Baseline Patient Treatment Preferences (n = 396)
| |
Unadjusted descriptive statistics |
Adjusted group comparisons¶ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline variables | Total sample n = 396 | Older aged (≥65) n = 126 | Middle-aged (45–64) n = 214 | Younger aged (20–44) n = 56 | Older aged vs middle-aged | Middle-aged vs younger aged | Older aged vs younger aged | |||||||
| Pt Treatment Preference‡ | n | (%) | n | (%) | n | (%) | n | (%) | OR | (CI) | OR | (CI) | OR | (CI) |
| Ventilator | 86 | (24.2) | 23 | (20.7) | 42 | (21.8) | 21 | (40.4) | 0.93 | (0.42-1.65) | 0.41 | (0.21-0.79) | 0.39 | (0.19-0.79) |
| Chemo 1 wk before death | 194 | (55.0) | 57 | (52.3) | 103 | (53.7) | 34 | (65.4) | 1.29 | (0.75-2.22) | 0.75 | (0.37-1.53) | 0.78 | (0.37-1.66) |
| Extend life§ | 95 | (29.1) | 29 | (27.9) | 43 | (24.6) | 23 | (47.9) | 1.34 | (0.74-2.41) | 0.32 | (0.16-0.64) | 0.49 | (0.23-1.02) |
| Against ICU death | 131 | (37.1) | 46 | (42.2) | 70 | (36.3) | 15 | (29.4) | 0.85 | (0.48-1.51) | 1.18 | (0.57-2.41) | 0.95 | (0.41-2.21) |
| Pt Completed DNR Order | 149 | (33.2) | 51 | (46.0) | 79 | (40.9) | 19 | (36.5) | 1.51 | (0.67-1.98) | 1.08 | (0.56-2.08) | 0.83 | (0.34-2.02) |
| Tell Life Expectancy | 262 | (72.8) | 81 | (72.3) | 148 | (75.5) | 33 | (63.5) | 0.82 | (0.49-1.40) | 1.99 | (1.00-3.95) | 1.38 | (0.67-2.85) |
Pt-Patient; psych-psychological; wk-week; chemo-chemotherapy; DNR-Do Not Resuscitate; ICU- Intensive Care Unit; TI –Terminal Illness.
All values in bold and italicized are at least p < .05.
Patients were asked a series of questions regarding their preferences for treatment at the end of life. Desiring the use of a ventilator to extend life for any period of time was recorded as preferring aggressive care. Desiring non palliative chemotherapy to extend life for one week or less was considered preference for aggressive care. Against ICU death was a dichotomous measuring of patients' perception of dying in an ICU setting as a bad death. Tell Life Expectancy assessed patients' desire to know exactly when they would die if that information was available.
Complete data on patient preference to extend life (N = 327) fell short of the total sample size (N = 396). Extend life (If you could choose, would you prefer a course of treatment that focused on extending life as much as possible, even if it meant more pain and discomfort)
Adjustments were made by running all Table 1 variables as possible confounders using backwards selection.
Table 3.
Age Group Comparisons of End-of-Life Care (n = 396)
| |
Unadjusted descriptive statistics |
Adjusted group comparisons† |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postmortem variables | Total sample n = 396 | Older aged (≥65) n = 126 | Middle-aged (45–64) n = 214 | Younger aged (20–44) n = 56 | Older aged vs middle-aged | Middle-aged vs younger aged | Older aged vs younger aged | |||||||
| Life-Prolonging Treatment | n | % | n | % | n | % | n | % | OR | (CI) | OR | (CI) | OR | (CI) |
| ICU | 37 | (9.4) | 9 | (7.2) | 20 | (9.4) | 8 | (14.3) | 0.81 | (0.35-1.88) | 0.57 | (0.23-1.42) | 0.29 | (0.09-0.92) |
| Ventilator | 28 | (7.1) | 5 | (4.0) | 17 | (8.0) | 6 | (10.7) | 0.51 | (0.18-1.43) | 0.72 | (0.27-1.93) | 0.27 | (0.07-1.00) |
| Chemotherapy | 25 | (6.3) | 8 | (6.4) | 14 | (6.5) | 3 | (5.4) | 0.51 | (0.18-1.46) | 1.46 | (0.40-5.40) | 1.20 | (0.31-4.69) |
| Feeding tube | 31 | (7.9) | 10 | (8.0) | 13 | (6.1) | 8 | (14.6) | 1.33 | (0.57-3.13) | 0.38 | (0.15-0.98) | 0.51 | (0.19-1.37) |
| Resuscitation | 31 | (4.1) | 1 | (0.8) | 10 | (4.7) | 5 | (8.9) | 0.16 | (0.02-1.29) | 0.50 | (0.17-1.54) | 0.08 | (0.01-0.72) |
| Location of Death | ||||||||||||||
| ICU | 28 | (7.1) | 5 | (4.0) | 15 | (7.0) | 8 | (14.3) | 0.59 | (0.20-1.71) | 0.41 | (0.16-1.07) | 0.20 | (0.06-0.70) |
| Hospital | 86 | (21.7) | 22 | (17.5) | 48 | (22.4) | 16 | (28.6) | 0.72 | (0.40-1.27) | 0.70 | (0.35-1.37) | 0.53 | (0.25-1.11) |
| Home | 219 | (55.3) | 71 | (56.4) | 119 | (55.6) | 29 | (51.8) | 1.00 | (0.61-1.65) | 1.37 | (0.73-2.57) | 1.24 | (0.60-2.57) |
| Nursing Home | 16 | (4.0) | 7 | (5.6) | 9 | (4.2) | 0 | (0.0) | 1.59 | (0.55-4.62) | ----- | ------------- | ----- | ------------- |
| Hospice | 47 | (11.9) | 21 | (16.7) | 23 | (10.8) | 3 | (5.4) | 1.45 | (0.72-2.92) | 2.03 | (0.53-7.76) | 4.09 | (0.91-18.33) |
| Survival* | mdn | (Q1-Q3) | mdn | (Q1-Q3) | mdn | (Q1-Q3) | mdn | (Q1-Q3) | HR | (CI) | (CI) | HR | HR | (CI) |
| Survival time (in days) | 146 | (62-312) | 125 | (68-297) | 157 | (61-313) | 143 | (60-347) | 0.88 | (0.68-1.15) | 1.07 | (0.78-1.49) | 0.74 | (0.50-1.08) |
ICU -Intensive Care Unit; EOL-End of Life; Pt-Patient; psych-psychological; mdn-Median Q1 -Lower Quartile (25%); Q3 -Upper Quartile (75%); HR-Hazard Ratio.
All values in bold are at least p < .05.
Survival time was measured from the baseline assessment of advanced cancer patients with a 6 month prognosis.
Adjustments were made by running all Table 1 variables as possible confounders using backwards selection.
Table 4.
Age Group Comparisons of End-of-Life Goal Attainment (n = 327)*
| |
Unadjusted descriptive statistics |
Adjusted group comparisons† |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline variables | Total sample n = 327 | Older aged (≥65) n = 104 | Middle-aged (45–64) n = 175 | Younger aged (20–44) n = 48 | Older aged vs middle-aged | Middle-aged vs younger aged | Older aged vs younger aged | |||||||
| Goal Attainment | n | (%) | n | (%) | n | (%) | n | (%) | OR | CI | OR | CI | OR | CI |
| Successful Goal Attainment | 222 | (65.9) | 70 | (67.3) | 119 | (68.0) | 33 | (68.8) | 0.76 | (0.42-1.34) | 1.03 | (0.49-2.13) | 0.84 | (0.39-1.84) |
| Wanted and Received LPT | 25 | (7.4) | 6 | (5.8) | 9 | (5.1) | 10 | (20.8) | 1.00 | (0.34-2.98) | 0.21 | (0.08-0.54) | 0.23 | (0.08-0.68) |
| Did Not Want and Avoided LPT | 197 | (58.5) | 64 | (61.5) | 110 | (62.9) | 23 | (47.9) | 0.90 | (0.53-1.53) | 2.38 | (1.20-4.75) | 1.74 | (0.87-3.47) |
| Unsuccessful Goal Attainment | 105 | (31.2) | 34 | (32.7) | 56 | (32.0) | 15 | (31.3) | 1.33 | (0.74-2.36) | 0.98 | (0.47-2.03) | 1.25 | (0.58-2.70) |
| Wanted But Did Not Receive LPT | 70 | (20.8) | 23 | (22.1) | 34 | (19.4) | 13 | (27.1) | 1.27 | (0.14-0.70) | 0.62 | (0.29-1.31) | 0.86 | (0.37-2.02) |
| Did Not Want But Received LPT | 35 | (10.4) | 11 | (10.6) | 22 | (12.6) | 2 | (4.2) | 0.82 | (0.38-1.77) | 3.31 | (0.75-14.60) | 3.29 | (0.64-16.83) |
GA- Goal Attainment; LPT- Life-Prolonging Treatment.
All values in bold are at least p < .05.
Goal Attainment has 4 categories: 1) patient wants and receives life-prolonging treatment 2) patient wants but does not receive life-pronging treatment 3) patient does not want but fails to avoid life-prolonging treatment 4) Patient does not want and avoids life-prolonging treatment. Patient in categories 1 & 4 were grouped as successful Goal Attainment while groups 2 &3 were grouped as unsuccessful Goal Attainment.
Goal Attainment sample was limited by sample size of the “Extend Life” measure of patient treatment preference (N = 327).
Adjustments were made by running all Table 1 variables as possible confounders using backwards selection.
Age differences in sample characteristics, dependent variables, and goal attainment
Older aged vs middle-aged patients
Older aged patients were more likely to receive lower Karnofsky scores but higher Charlson Comorbidity scale scores. Older aged patients were more likely to have health insurance and self-identify as Jewish than middle-aged patients (Table 1). Baseline treatment preference data (Table 2) showed that older and middle-aged patients did not differ significantly by treatment preference. In analysis of postmortem data (Table 3) care received by older aged and middle-aged patients did not differ significantly. Goal attainment analysis (Table 4) showed no significant differences between older patients' likelihood of being successful goal attainers compared to that of middle-aged patients with similar treatment preferences.
Middle-aged vs younger aged patients
Middle-aged and younger aged patients did not significantly differ on Karnofsky scores, but middle-aged patients were more likely to score higher on the Charlson Comorbidity scale (indicating relatively more comorbid conditions). Middle-aged patients were more likely to be White but less likely to be Hispanic than younger aged patients (Table 1). Analysis of treatment preference (Table 2) showed that middle-aged patients were less likely to prefer life-prolonging care or use of a ventilator to extend life than younger aged patients. In analysis of postmortem data (Table 3) it was found that middle-aged patients were less likely to have received life-prolonging care involving a feeding tube than younger aged patients. Goal attainment analysis (Table 4) showed that middle-aged patients initially wanting life-prolonging care were less likely than younger aged patients, with similar treatment preferences, to receive it. Conversely, middle- aged patients not wanting life-prolonging care were more likely than younger aged patients, with similar preferences, to successfully avoid life-prolonging care.
Older aged vs younger aged patients
Older aged patients did not significantly differ from younger aged patients in their Karnofsky scores but were more likely to score higher on the Charlson Comorbidity scale. Older aged patients were more likely to have health insurance than younger aged patients. Older aged patients were more likely to be White but less likely to be Hispanic than younger aged patients. Analysis of baseline treatment preference data (Table 2) showed that older patients were less likely to desire life-prolonging treatment involving a ventilator than younger aged patients. Analysis of postmortem data (Table 3) shows that older patients were less likely to receive ventilator therapy or resuscitation to sustain life. Goal attainment analysis (Table 4) showed that older patients wanting life-prolonging care were less likely than younger aged patients, with similar treatment preferences, to receive it (Table 4).
Age as a continuous variable
Using age as a continuous variable, preference to extend life (O.R. 0.97; p < .01), to do so with a ventilator (O.R. 0.97; p = 0.001), or with chemotherapy (O.R. 0.98; p = .01) was decreased with age. For treatment received likelihood of an ICU death (O.R. 0.96; p < .05) was decreased marginally with age while likelihood of a nursing home death (O.R. 1.06; p < 0.05) or hospice death (O.R. 1.03; p < .05) was increased with age. Likelihood of a patient wanting and then receiving life-prolonging care (OR 0.94; p < .001) was decreased with age while likelihood of not wanting and avoiding life-prolonging care (OR 1.02; p = .017) was increased with age.
Discussion
This study revealed that older and middle-aged advanced cancer patients desired and received less life-prolonging care than younger aged cancer patients. Younger aged patients wanting life-prolonging care were more likely to receive it compared to middle-aged or older adults. But, younger aged patients not desiring life-prolonging care were less likely to receive care consistent with their treatment preference compared to middle-aged or older adults.
The original hypothesis that advanced cancer patient age would be associated with: a) less desire for life-prolonging treatment; b) a lower likelihood of receiving life-prolonging treatment; and c) increased goal attainment was not completely supported by the results. As expected, middle and older aged patients were less likely to desire and receive life-prolonging treatment than younger aged patients. Previous research indicated that older and middle-aged patients would differ in desire for, or receipt of, life-prolonging care.9–20 These differences were not present using age as a categorical variable but were present across the full sample using age as a continuous variable. The age groups are particularly helpful in comparing CwC to prior work done on the SUPPORT study.17,19,20 The CwC, is a more recent study, indicating possible cohort effects of demographic and or practice pattern shifts over time regarding interventions in Table 2. Additionally, the CwC, recruited heavily from outpatient facilities. Advanced cancer patients, particularly those over 65 years old, often receive outpatient cancer care instead of inpatient hospital care. This study's results might be more applicable to current populations of advanced cancer patients; although replication of these findings among the inpatient advanced cancer patient subgroup is needed to determine the extent these findings can be generalized.
Goal attainment compares individual patient preference to patient outcome; successful goal attainers get what they want (group 1) or avoid what they do not want (group 4) (See Figure). Successful goal attainment was differentiated into subgroups to determine if age affected the patient's likelihood of getting wanted care or avoiding unwanted care. Among patients wanting to avoid life-prolonging care, the likelihood of care consistent with patients' treatment preferences increased with age. But, among patients preferring life-prolonging care, the likelihood of care consistent with patients' treatment preference decreased with age. Stark age differences between successful goal attainment sub-groups suggest that physicians' age biases may influence the likelihood of patient care being consistent with treatment preferences.17
Previous studies show physicians of elderly advanced cancer patients can be inconsistent in their understanding of patients' treatment preference.26–29 Older aged patients wanting life-prolonging care and young patients not wanting it, challenges the societal norms that a young persons' death is unacceptable relative to the acceptability of an older person's death. In this study, treatment differed by patient preference and age despite adjusting for confounders like Karnofsky and Charlson comorbidity. Age bias, while not a surprising conclusion, needs further examination. Although not honoring known patient preferences would appear in conflict with notions that honoring patient treatment preference is essential to quality end-of-life care, honoring futile treatment preferences could potentially cause harm by increasing suffering among patients and families. Research is needed to determine how age bias affects quality-of-death outcomes in the four goal attainment subgroups. Results did not fully support the hypothesis that both subgroups of goal attainment would increase with age, but they revealed groups of patients at risk of receiving care inconsistent with treatment preference. Previous arguments asserted that care consistent with patient's wishes, aggressive or palliative, is an ethical imperative and important for ensuring a good death.30–35 A good death, free from avoidable distress and suffering and consistent with patients' wishes,34 is important for patients, their families and the health care system.36 Researching subgroups defined by age and treatment preference might help to predict quality-of-death.
This study has some noteworthy limitations. Although power to detect a difference by age group for the low rate dichotomous outcomes (e.g., relative rarity of resuscitation) was limited, significant associations were still observed, demonstrating the robustness of the results. Although the cohort studied in this paper had a lower education level than surviving CwC participants and education was included as a potential confounder in data analysis models, the lower level of education among CwC nonparticipants highlights the need for replication in less educated samples. A single assessment determined patient preference, which could change over time. Forthcoming research within the CwC patient sample indicates that treatment preference did not significantly change with survival time from baseline assessments nor with Karnofsky scores (Wright et al., in press).
Overall results for goal attainment remained stable among patients who acknowledge that they are dying; demonstrating the stability of patient treatment preference over time in the CwC dataset.37 Recent studies have shown small, but significant decreases in willingness to undergo life-prolonging therapy among critically ill geriatric patients.35 Studies have also shown that patient treatment preferences and course of treatment vary over time.38 These studies were not specific for advanced cancer patients and even acknowledged significant differences between cancer and non-cancer patients (i.e. decreased desire for burdensome care, lower proportions of inconsistent treatment trajectories, and greater likelihood of receiving preferred aggressive care) in their patient samples.34,38 Cancer is distinct from many diseases; it has a “death sentence” connotation that could affect advanced cancer patients' treatment preferences. More cancer specific research on treatment preference and goal attainment is needed to define advanced cancer patients with respect to age differences.
Cancer deaths are on a trajectory to become the leading cause of death among older adults, increasing the importance of identifying advanced cancer patients at greatest risk for a bad death. To that end, the benefits of honoring patient treatment preferences warrant further investigation. Successful goal attainers and unsuccessful goal attainers are two categories comprised of four distinct patient populations. Among non successful goal attainers it is important to note that patient autonomy in medicine is defined by the right to decline treatment, not by a right to receive desired treatment. Further research is needed to differentiate these four subgroups by their associations with patient outcomes like quality-of-life and quality-of-death. Despite limitations, this study's identification of at risk advanced cancer patient populations offers new insight and opportunities for improving end-of-life care. Educating clinicians about the existence, influence and prevalence of age bias on end-of-life care offers a first step towards limiting its potentially negative effects on patient care. Awareness of the increased risks for misinterpreting certain patients' treatment preference (e.g. older patients wanting life-prolonging care and younger patients not wanting life-prolonging care) could help identify such patients and help physicians be prepared to guide conversations on end-of-life treatment options with them.
Acknowledgments
This research was supported in part by the following grants to Dr. Prigerson: MH63892 from the National Institute of Mental Health and CA106370 from the National Cancer Institute; the Center for Psycho-Oncology and Palliative Care Research, Dana-Farber Cancer Institute. Research was also supported by NIH Grant AG026781-04 and the American Federation for Aging Research.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Frankl D. Oye RK. Bellamy PE. Attitudes of hospitalized patients toward life support: A survey of 200 medical inpatients. Am J Med. 1989;86:645–648. [PubMed] [Google Scholar]
- 2.Yarzebski J. Goldberg RJ. Gore JM. Alpert JS. Temporal trends and factors associated with pulmonary artery catheterization in patients with acute myocardial infarction. Chest. 1994;105:1003–1008. doi: 10.1378/chest.105.4.1003. [DOI] [PubMed] [Google Scholar]
- 3.Gurwitz JH. Osganian V. Goldberg RJ. Chen ZY. Gore JM. Alpert JS. Diagnostic testing in acute myocardial infarction: Does patient age influence utilization patterns? The Worcester Heart Attack Study. Am J Epidemiol. 1991;134:948–957. doi: 10.1093/oxfordjournals.aje.a116179. [DOI] [PubMed] [Google Scholar]
- 4.Bearden DM. Allman RM. Sundarum SV. Burst NM. Bartolucci AA. Age-related variability in the use of cardiovascular imaging procedures. J Am Geriatr Soc. 1993;41:1075–1082. doi: 10.1111/j.1532-5415.1993.tb06455.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal GE. Fortinsky RH. Differences in the treatment of patients with acute myocardial infarction according to age. J Am Geriatr Soc. 1994;42:826–832. doi: 10.1111/j.1532-5415.1994.tb06553.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleming C. D'Agostino RB. Selker HP. Is coronary-care-unit admission restricted for elderly patients? A multicenter study. Am J Public Health. 1991;81:1121–1126. doi: 10.2105/ajph.81.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evers M. Meier D. Morrison S. Assessing differences in care needs and service utilization in geriatric palliative care patients. J Pain Symptom Manage. 2002;23:424–432. doi: 10.1016/s0885-3924(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 8.Hirakawa Y. Masuda Y. Kuzuya M. Iguchi A. Uemura K. Age-related differences in care receipt and symptom experience of elderly cancer patients dying at home: Lessons from the DEATH project. Geriatr Gerontol Int. 2007;7:34–40. [Google Scholar]
- 9.Yancik R. Ganz P. Varricchio G. Conley B. Perspectives on comorbidity and cancer in older patients: Approaches to expand the knowledge base. J Clin Oncol. 2001;19:1147–1151. doi: 10.1200/JCO.2001.19.4.1147. [DOI] [PubMed] [Google Scholar]
- 10.Ganz PA. Interaction between the physician and the older patient: The oncologist's perspective. Cancer. 1997;80:1323–1325. doi: 10.1002/(sici)1097-0142(19971001)80:7<1323::aid-cncr19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Silliman RA. Troyan SL. Guadagnoli E. Kaplan SH. Greenfield S. The impact of age, marital status and physician-patient interactions on the care of older women with breast carcinoma. Cancer. 1997;80:1326–1334. [PubMed] [Google Scholar]
- 12.Blanchard CG. Labrecque MS. Ruckdeschel JC. Blanchard EB. Information and decision-making preferences of hospitalized adult cancer patients. Soc Sci Med. 1988;27:1139–1145. doi: 10.1016/0277-9536(88)90343-7. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann JC. Wenger NS. Davis RB Teno J. Connors AF., Jr. Desbiens N. Lyn J. Phillips RS. Patient preferences for communication with physicians about end-of-life decisions. Ann Intern Med. 1997;127:1–12. doi: 10.7326/0003-4819-127-1-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Harrison J. Maguire P. Influence of age on psychological adjustment to cancer. Psychooncology. 1995;4:33–38. [Google Scholar]
- 15.Bosshard G. Nilstun T. Bilsen J. Norup M. Miccinesi G. van Delden JJ. Faist KK. van der Heide A. European End-of-Life Consortium: Forgoing treatment at the end of life in 6 European countries. Arch Intern Med. 2005;165:401–407. doi: 10.1001/archinte.165.4.401. [DOI] [PubMed] [Google Scholar]
- 16.Bradley C. Clement J. Lin C. Absence of cancer diagnosis and treatment in elderly medicaid-insured nursing home residents. J Natl Cancer Inst. 2008;100:21–23. doi: 10.1093/jnci/djm271. [DOI] [PubMed] [Google Scholar]
- 17.Hamel MB. Lynn J. Teno JM. Covinsky KE. Galanos A. Desbiens NA. Phillip RS. Age-related differences in care preferences, treatment decisions, and clinical outcomes of seriously ill hospitalized adults: Lessons from SUPPORT. J Am Geriatr Soc. 2000;48:S176–S182. doi: 10.1111/j.1532-5415.2000.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 18.The SUPPORT Principle Investigators: A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 19.Rose JH. O'Toole EE. Dawson NV. Thomas C. Connors AF., Jr. Wenger NS. Phillip RS. Hamel MB. Cohen HJ. Lynn J. Age differences in care practices and outcomes for hospitalized patients with cancer. J Am Geriatr Soc. 2000;48:S25–S32. doi: 10.1111/j.1532-5415.2000.tb03137.x. [DOI] [PubMed] [Google Scholar]
- 20.Rose JH. O'Toole EE. Dawson NV. Lawrence R. Gurley D. Thomas C. Hamel MB. Cohen HJ. Perspectives, preferences, care practices and outcomes among older and middle-aged patients with late-stage cancer. J Clin Oncol. 2004;22:4907–4917. doi: 10.1200/JCO.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Kadan-Lottick N. Vanderwerker L. Block SD. Zhang B. Prigerson H. Psychiatric disorders and mental health service use in patients with advanced cancer. Cancer. 2005;104:2872–2881. doi: 10.1002/cncr.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu KC. Miller BA. Springfield SA. Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J Natl Med Assoc. 2007;99:1092–1100. , 1102–1104. [PMC free article] [PubMed] [Google Scholar]
- 23.Albano JD. Ward E. Jemal A. Anderson R. Cokkinides VE. Murray T. Anderson R. Cokkinides VE. Murray T. Henley J. Liff J. Thun MJ. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 24.Somogyi-Zalud E. Zhong Z. Hamel MB. Lynn J. The use of life sustaining treatments in hospitalized persons age 80 and older. J Am Geriatr Soc. 2002;50:930–934. doi: 10.1046/j.1532-5415.2002.50222.x. [DOI] [PubMed] [Google Scholar]
- 25.Gripp S. Moeller S. Bolke E. Schmitt G. Matuschek C. Asgari S. Asgharzadeh F. Roth S. Budach W. Franz M. Willers R. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self rated anxiety and depression. J Clin Oncol. 2007;26:3313–3320. doi: 10.1200/JCO.2006.10.5411. [DOI] [PubMed] [Google Scholar]
- 26.Elkin E. Kim SH. Casper ES. Kissane DW. Schrag D. Desire for information and involvement in treatment decisions: Elderly cancer patient preferences and their physician's preferences. J Clin Oncol. 2007;25:5275–5280. doi: 10.1200/JCO.2007.11.1922. [DOI] [PubMed] [Google Scholar]
- 27.Stevens A-M. Gwilliam B. A'hern R. Broadley K. Hardy J. Experience in the use of the palliative care outcome scale. Support Care Cancer. 2005;13:1027–1034. doi: 10.1007/s00520-005-0815-6. [DOI] [PubMed] [Google Scholar]
- 28.Cohen SR. Boston P. Mount BM. Porterfield P. Changes in quality of life following admission to palliative care units. Palliat Med. 2001;15:363–371. doi: 10.1191/026921601680419401. [DOI] [PubMed] [Google Scholar]
- 29.Shalowitz DI. Garrett Mayer E. Wendler D. The accuracy of surrogate decision makers: a systematic review. Arch Intern Med. 2006;166:493–497. doi: 10.1001/archinte.166.5.493. [DOI] [PubMed] [Google Scholar]
- 30.Council on Scientific Affairs AMA: Good care of the dying patient. JAMA. 1996;275:474–478. [PubMed] [Google Scholar]
- 31.Washington DC: National Academy Press; 2001. Improving Palliative Care for Cancer, Institute of Medicine. [Google Scholar]
- 32.Lynn J. Measuring quality of care at end of life: A statement of principles. J Am Geriatr Soc. 1997;45:526–527. doi: 10.1111/j.1532-5415.1997.tb05184.x. [DOI] [PubMed] [Google Scholar]
- 33.Sachs GA. Ahronheim JC. Rhymes JA. Volicer L. Lynn J. Good care of dying patients: The alternative to physician assisted suicide and euthanasia. J Am Geriatr Soc. 1995;43:553–562. doi: 10.1111/j.1532-5415.1995.tb06106.x. [DOI] [PubMed] [Google Scholar]
- 34.Cosgriff JA. Pisani M. Bradely EH. O'Leary JR. Fried TR. The association between treatment preferences and trajectories of care at end-of-life. J Gen Intern Med. 2007;22:1566–1571. doi: 10.1007/s11606-007-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried T. Van Hess P. Byers AL. Towle VR. O'Leary JR. Dubin JA. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med. 2007;22:495–501. doi: 10.1007/s11606-007-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levinsky NG. Yu W. Ash A. Moskowitz M. Gazelle G. Saynina O. Emanuel EJ. Influence of age on medicare expenditures and medical care in the last year of life. JAMA. 2001;286:1349–1355. doi: 10.1001/jama.286.11.1349. [DOI] [PubMed] [Google Scholar]
- 37.Mack J. Weeks J. Wright A. Block S. Prigerson H. End-of-life discussions, goal attainment, and distress at the end of life: prediction and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried T. O'Leary J. Van Hess P. Fraenkel L. Inconsistency over time in the preferences of older persons with advanced illness for life sustaining treatment. J Am Geriatr Soc. 2007;55:1007–1014. doi: 10.1111/j.1532-5415.2007.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]