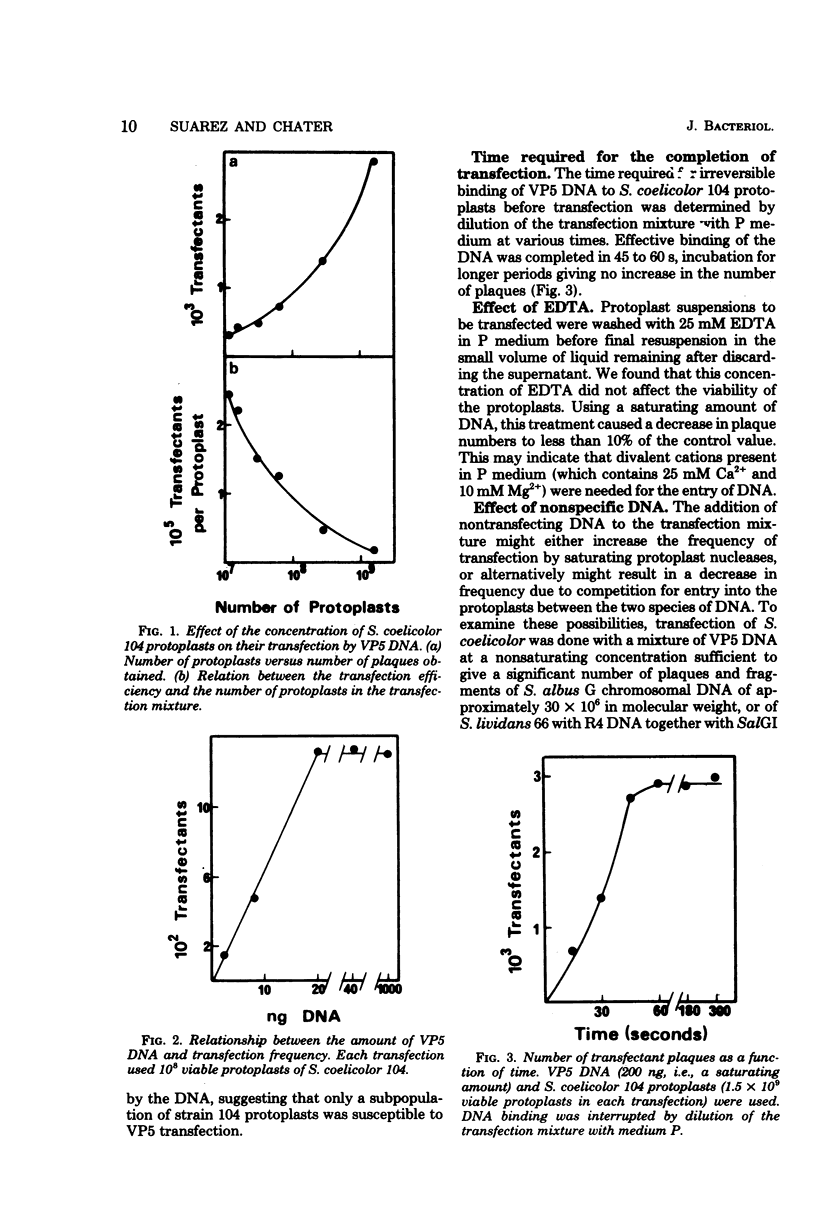
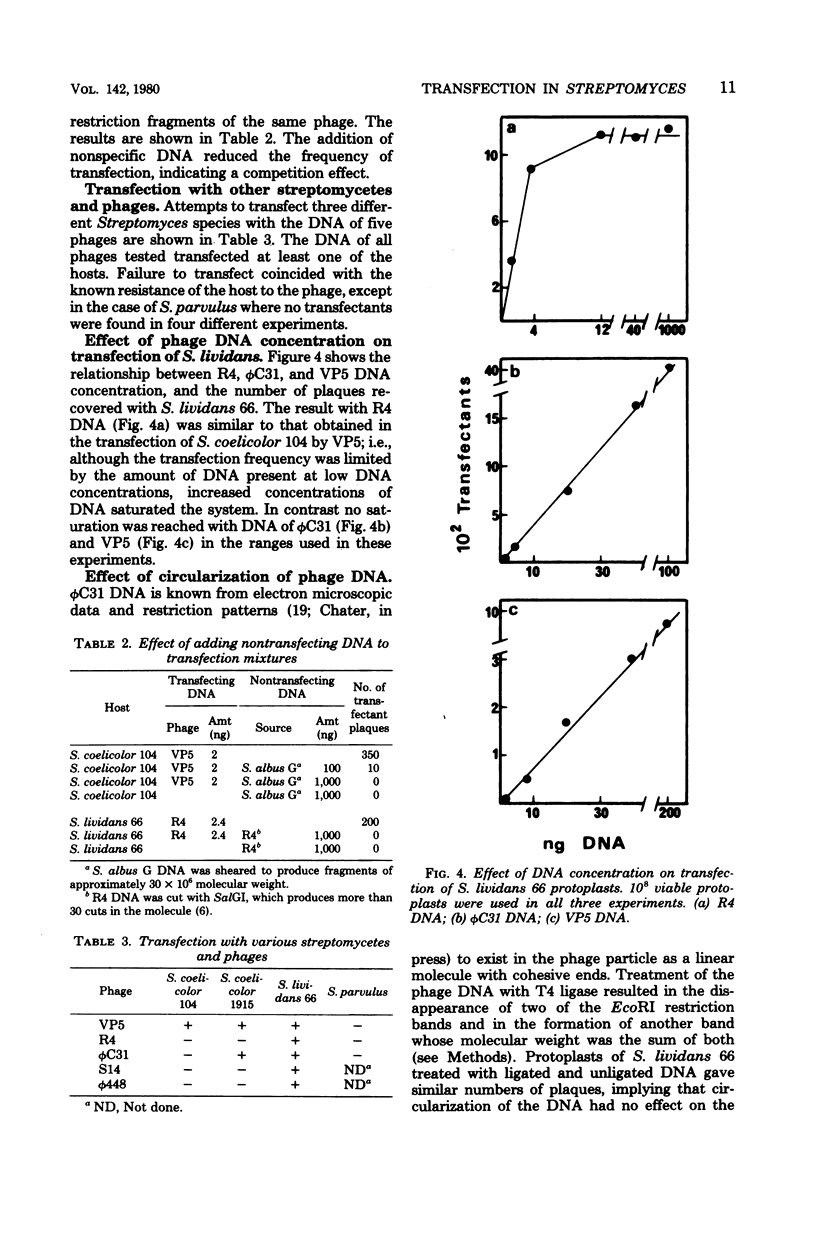
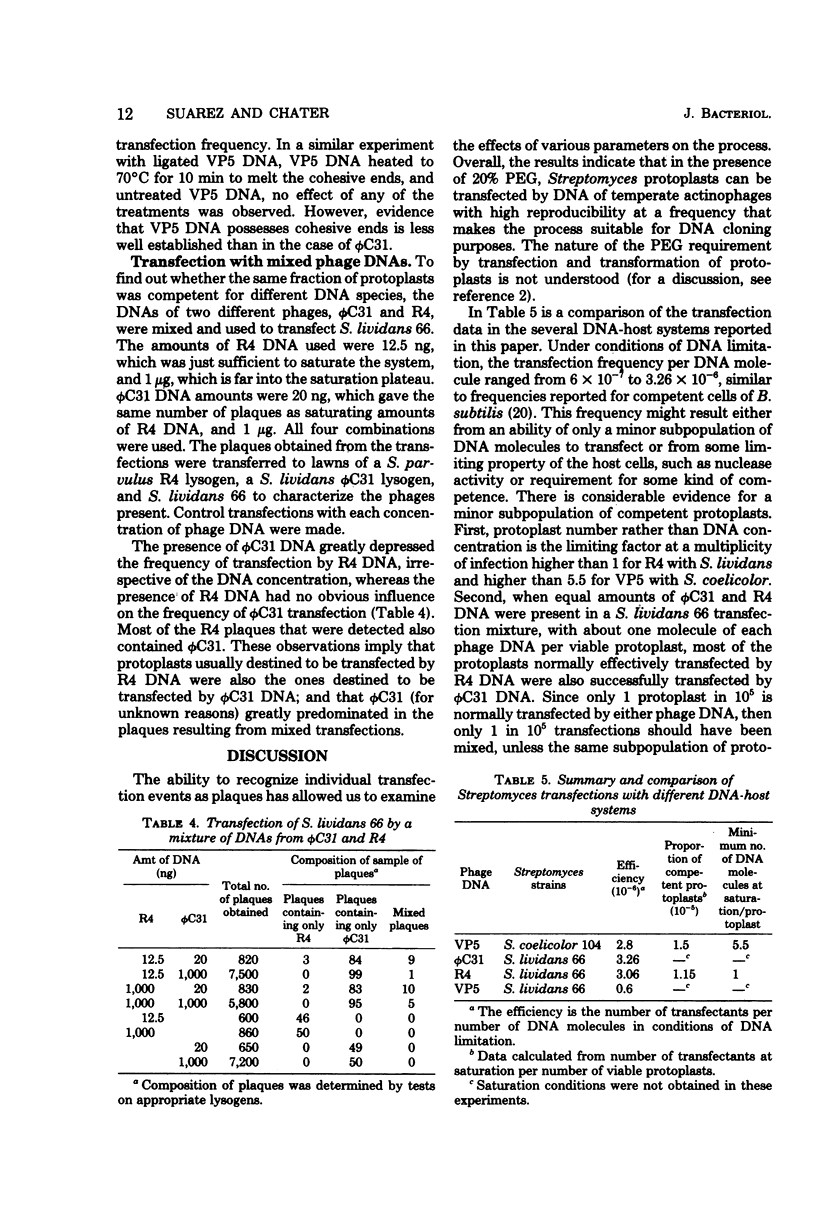
Abstract
In the presence of polyethylene glycol (concentration optimum 20%), protoplasts of appropriate Streptomyces strains could be transfected by deoxyribonucleic acid (DNA) of five temperate phages (phi C31, VP5, R4, phi 448, and S14) belonging to four different immunity groups. Quantitation of transfection was made possible by plating the transfection mixture with excess uninfected protoplasts in soft agar overlays on protoplast regeneration medium so that plaques were easily detected. Optimum frequencies of transfection in the ranges of 10(-6)/DNA molecule and 10(-5)/viable protoplast were invariably obtained. It appeared that single DNA molecules initiated transfection events, and that the conformation of the DNA (i.e., circular or linear) was not important. Inhibition of transfection by ethylenediaminetetraacetic acid suggested that divalent cations were also observed. A minor subpopulation of protoplasts appeared to be particularly sensitive to transfection (i.e., "competent") in some DNA-host combinations. In such cases the size of this subpopulation was the major limiting factor in obtaining high transfection frequencies. The same protoplast
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzinger R. Transfection of Enterobacteriaceae and its applications. Microbiol Rev. 1978 Mar;42(1):194–236. doi: 10.1128/mr.42.1.194-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Wilde L. C. Restriction of a bacteriophage of Streptomyces albus G involving endonuclease SalI. J Bacteriol. 1976 Nov;128(2):644–650. doi: 10.1128/jb.128.2.644-650.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Bacterial protoplast fusion: recombination in fused protoplasts of Streptomyces coelicolor. Mol Gen Genet. 1978 Jul 4;162(3):307–317. doi: 10.1007/BF00268856. [DOI] [PubMed] [Google Scholar]
- Joenje H., Venema G. Different nuclease activities in competent and noncompetent Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):25–33. doi: 10.1128/jb.122.1.25-33.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinkova V., Roelants P., Mergeay M. Transfection in Streptomyces virginiae [proceedings]. Biochem Soc Trans. 1977;5(4):941–943. doi: 10.1042/bst0050941. [DOI] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Hamana K., Umezawa H. Factors affecting infection of protoplasts with deoxyribonucleic acid of actinophage PK-66. J Virol. 1968 Jul;2(7):686–691. doi: 10.1128/jvi.2.7.686-691.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Early stages in DNA binding and uptake during genetic transformation of pneumococci. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1493–1498. doi: 10.1073/pnas.71.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner T. A., Spatz H. C. Transfection in B. subtilis. Curr Top Microbiol Immunol. 1973;62:61–88. doi: 10.1007/978-3-642-65772-6_3. [DOI] [PubMed] [Google Scholar]



