Abstract
The formation of the musculoskeletal system represents an intricate process of tissue assembly involving heterotypic inductive interactions between tendons, muscles and cartilage. An essential component of all musculoskeletal systems is the anchoring of the force-generating muscles to the solid support of the organism: the skeleton in vertebrates and the exoskeleton in invertebrates. Here, we discuss recent findings that illuminate musculoskeletal assembly in the vertebrate embryo, findings that emphasize the reciprocal interactions between the forming tendons, muscle and cartilage tissues. We also compare these events with those of the corresponding system in the Drosophila embryo, highlighting distinct and common pathways that promote efficient locomotion while preserving the form of the organism.
Keywords: Drosophila, Vertebrate, Bone-tendon interaction, Muscle-tendon interaction, Tendon development
Introduction
The formation of complex tissues during embryonic development requires continuous bidirectional communication between cells of different origins to allow precise tissue assembly. The musculoskeletal system, which comprises muscles, tendons (see Box 1) and bones, represents a fascinating example in which the accurate assembly of distinct cell types is crucial for the efficient movement, as well as for the stability, of the entire organism.
Box 1. An overview of Drosophila and vertebrate tendons
Drosophila tendons. Specialized cells of ectodermal origin, which upon differentiation express arrays of polarized microtubules that stretch between the basal side of the tendon at the myotendinous junction and the apical side facing the cuticle. These microtubules are necessary for tendon function and provide the necessary elasticity for the tendon cell to resist muscle contraction.
Vertebrate tendons. Connective tissues that transmit the force generated by muscle contraction to the skeleton. The tendons integrate with the muscle and skeletal tissues through specialized structures termed the myotendinous junction and the enthesis, respectively, that provide flexible but robust and resilient anchor points. To support the complex requirements for connections through the various structures of the musculoskeletal system, the tendons are structurally diverse, ranging from thin fascia-like structures to the long cord-like tendons of the limbs and tail. Tendons are fibrous tissues with the structural hallmark of tightly packed bundles of collagen fibrils that occupy most of the volume of the mature tendon and provide it with the unique combination of a tensile strength and flexibility (Benjamin and Ralphs, 1997).
Although much data have accumulated that describe the specification, patterning and differentiation of muscles and skeletal tissues during vertebrate embryonic development (Kablar and Rudnicki, 2000; Olsen et al., 2000; Pownall et al., 2002; Towers and Tickle, 2009), the origin, patterning and differentiation of tendons have only recently begun to be elucidated (Brent and Tabin, 2002; Tozer and Duprez, 2005). Questions regarding the assembly of the musculoskeletal tissues, emphasizing the cross-regulatory interactions between muscle and tendon, or cartilage and tendon, and their specific differentiation programs, are only now being addressed in molecular terms.
Invertebrates, such as Drosophila, lack an internal skeleton, and their movement is based on the precise connectivity between muscles and the exoskeleton. Intriguingly, tendon-like cells develop within the ectoderm of the Drosophila embryo and play an active role in musculoskeletal tissue assembly (Volk, 1999). These cells share functional properties with vertebrate tendons, including their elastic nature and the induction of a myotendinous junction (see Glossary, Box 2). In view of recent findings regarding vertebrate tendon development, it is interesting to compare the principles of musculoskeletal tissue assembly in vertebrate and invertebrate development and highlight the similarities and differences in cellular and molecular processes utilized by the two systems.
Box 2. Glossary Autopod.
The most distal subdivision of a tetrapod limb (hand or foot).
Avulsion fracture. A fracture occurring when a tendon, ligament, joint capsule or muscle is pulled from a bone, tearing away a fragment of the bone mass.
Branchiomeric muscles. Cranial muscles.
Deltoid tuberosity. A bone ridge located on the shaft of the humerus to which the deltoid muscle attaches.
Founder cells. A single cell produced by asymmetric division of a progenitor mesodermal cell that promotes fusion of neighboring myoblasts to produce a multinucleated muscle cell.
Hemi-adherens junctions. Junctions created between a cell and the extracellular matrix (ECM).
Inside-outside integrin signaling. When cytoplasmic proteins bind to the cytoplasmic tail of an integrin β subunit to change the conformation of the ectodomain of both integrin α and β subunits, so elevating their affinity for various ECM ligands.
Myotendinous junction. The junction that connects muscles to tendon cells. It is composed of ECM components, secreted into the space between the two cell types, that bind to integrin receptors on these cells.
Myotome. A dorsolateral compartment of the somite that gives rise to the trunk and limb muscles.
Sclerotome. Ventromedial compartment of the somite that gives rise to skeletal tissue.
Syndetome. An early compartment of tendon progenitors in somites, occupying a dorsolateral stripe of sclerotome cells at the junction between two abutting myotomes.
Tenocytes. Differentiated tendon cells.
Zeugopod. The middle subdivision of a tetrapod limb.
Here, we discuss recent results regarding the specification of tendons and the molecular mechanisms involved in their initial determination in vertebrate and invertebrate (Drosophila) development. We then describe the molecular players that regulate the targeting of muscles to tendons and the formation of the myotendinous junction in Drosophila. This is followed by a discussion of recently discovered mechanisms that mediate the encounter between tendons and cartilage and the formation of the tendon-bone insertion (enthesis, see Box 3). Finally, the process of tendon differentiation is described, with a focus on its dependence on communication with the muscle and cartilage tissues.
Box 3. The enthesis
The enthesis is a site where a tendon, ligament or muscle inserts into a bone and it exhibits a unique and intricate composition. Starting at the tendon side, the first zone exhibits tendon properties, including aligned type I collagen fibers and the proteoglycan decorin (Ralphs et al., 1998). The second zone comprises fibrocartilage that contains type II collagen, with only small amounts of type I collagen. The ECM of zone 2 contains, in addition, type III collagen, aggrecan and decorin (Fukuta et al., 1998; Kumagai et al., 1994; Thomopoulos et al., 2003). Next, zone 3 contains mineralized fibrocartilage that includes type II and type X collagens as well as aggrecan (Fukuta et al., 1998; Kumagai et al., 1994; Ralphs et al., 1998; Thomopoulos et al., 2003). Finally, zone 4 is bony and composed mostly of mineralized type I collagen.
Tendon determination: autonomous versus non-autonomous induction
The precise connectivity between muscles, tendons and bones is crucial for optimal locomotion of the organism, yet the pathways involved in this process are poorly understood. Several mechanisms could underlie the accurate connectivity between these three tissues. First, parallel independent development of each tissue, followed by induction of connectivity between a given tissue and its counterpart tissue. Second, in an alternative mechanism, an initial independent induction of each tissue might occur as in the first scenario, but only cells that are correctly connected are able to complete their differentiation into muscles, tendons or bones. In a third possible mechanism, cells that initiate the differentiation program of one tissue (for example, cartilage) could later trans-differentiate into the fate of another tissue (for example, tendons).
Below, we discuss how the above-mentioned strategies are utilized in vertebrate and invertebrate embryos to achieve specificity and accuracy in the construction of the musculoskeletal system.
Tendon induction in the Drosophila embryo
Findings from the Drosophila embryo suggest that an initial induction of tendon progenitors occurs first, and that only later in development do these tendon progenitors differentiate into mature tendons in a muscle-dependent fashion, implicating the second strategy described above. Although Drosophila do not contain cartilage or bone tissue, the muscles are connected to specialized muscle attachment cells termed tendon cells that are part of the epidermal cell layer. These cells, together with the cuticle that they secrete, form the exoskeleton (Volk, 1999; Schnorrer and Dickson, 2004).
Early tendon determination is independent of muscle cells
The intricate pattern of tendon cells within the Drosophila embryonic ectoderm emerges in parallel to muscle founder cell (see Glossary, Box 2) determination. One of the earliest genes that induce tendon progenitor cells within the ectoderm encodes the epidermal growth factor (Egf)-like transcription factor StripeB, one of the two isoforms produced by the stripe gene (Frommer et al., 1996; Volk and VijayRaghavan, 1994). StripeB is less active than the StripeA isoform in terms of their ability to promote downstream gene expression following their overexpression. stripeA expression is activated at a later developmental stage, following the interaction of the tendon with the muscle (Volohonsky et al., 2007). stripeB transcription is promoted by signaling pathways involved in the patterning of the embryonic ectoderm, including the Wingless (Wg), Hedgehog (Hh), Notch and Egf receptor (Egfr) pathways (Hatini and DiNardo, 2001). stripe expression is directly induced by the Wg and Hh signaling pathways, as both Pangolin and Cubitus interruptus (Ci) binding sites are functional in the stripe promoter region (Piepenburg et al., 2000). Consequently, StripeB positively regulates its own transcription, as its overexpression promotes the expression of β-galactosidase from an enhancer trap inserted in the stripe promoter region (Becker et al., 1997). Once activated, at stage 11-12 of embryonic development, the ectodermal cells are transformed into tendon progenitor cells, directing the correct targeting of the muscle cells that migrate towards the Stripe-positive tendon cells (Fig. 1A).
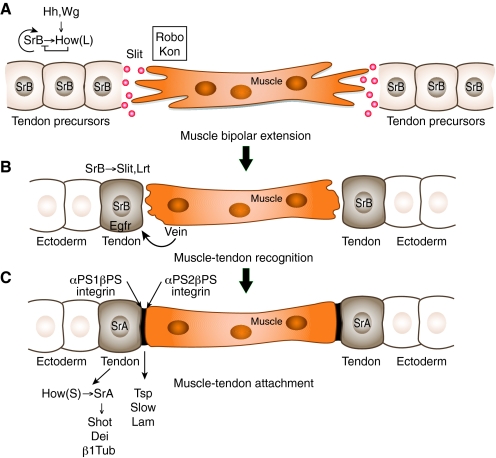
Fig. 1.
Different stages in Drosophila muscle-tendon interactions during embryogenesis. (A) In the first stage, tendon progenitors are defined in the ectoderm by the induction of StripeB (SrB) expression by the products of segment polarity genes, such as Hedgehog (Hh) and Wingless (Wg). StripeB expression is maintained at a low level as a result of post-transcriptional repression by the RNA-binding protein How(L). SrB regulates its own expression positively, as well as the expression of its inhibitor How(L). Tendon progenitors secrete Slit and provide initial cues for directing muscle bipolar migration. The muscle responds to Slit through Robo receptors. In addition, Kon-tiki (Kon) contributes to the migration of the muscles. (B) In a second step, the muscle signals to the tendon through epidermal growth factor receptor (Egfr) activation by means of the neuregulin-like secreted ligand Vein to initiate tendon differentiation and elevate SrB expression. SrB further induces the expression of Slit and Lrt; Lrt is required to arrest muscle migration. Tendon precursors that do not bind muscles lose SrB expression and become ectoderm cells. (C) In a third step, the myotendinous junction (black) is formed through muscle-specific αPS2βPS integrin association with the tendon-secreted ECM component Thrombospondin (Tsp) and its regulator Slow. Laminin (Lam) binds the αPS1βPS tendon-specific integrin. At this stage, How(S) is elevated in the cytoplasm of the tendon cell, promoting the expression of StripeA (SrA), which is essential to induce tendon terminal differentiation by induction of Short stop (Shot), Delilah (Dei), and β1 Tubulin (β1Tub).
Stripe also mediates the induction of adult tendon cells in the fly thorax (Fernandes et al., 1996). Interestingly, stripe expression in the thorax antagonizes the expression of achaete-scute proneural genes, inhibiting sensory organ precursor formation in the areas of future muscle attachment sites. Thus, Stripe expression divides the future adult fly thorax into a Stripe-positive domain, in which tendon cells develop, and a Stripe-negative domain, where sensory bristles form (Usui et al., 2004).
Several genes involved in muscle targeting to tendon cells are positively regulated by Stripe activity in the embryo, including slit, Thrombospondin (Tsp), Leucine-rich tendon-specific protein (Lrt) and slowdown (slow) (Chanana et al., 2009; Gilsohn and Volk, 2010a; Gilsohn and Volk, 2010b; Kramer et al., 2001; Subramanian et al., 2007; Wayburn and Volk, 2009). Interestingly, although Stripe is sufficient to induce their expression, some of these genes are expressed at low level even in stripe mutant embryos, raising the possibility that segment polarity genes, such as hh, wg, or both, initially activate a set of tendon-specific genes, including stripeB. StripeB then maintains and amplifies the expression of these genes, as well as its own transcription, transforming these ectodermal cells into tendon progenitor cells (Fig. 1B). However, the final differentiation of these progenitor cells depends on a specific interaction with muscles.
Post-transcriptional downregulation of StripeB levels in the tendon progenitor cells is provided by the long isoform of the RNA-binding protein Held out wings [How(L)], which is both necessary and sufficient to reduce stripe mRNA levels. How(L) itself is a target of StripeB (Nabel-Rosen et al., 1999; Nabel-Rosen et al., 2002). Thus, How(L) creates a negative-feedback loop that counteracts StripeB auto-activation, leading to the maintenance of StripeB at low levels in the progenitor tendon cells, inhibiting their subsequent differentiation.
The muscle-dependent signal required for the differentiation of tendon progenitors into fully mature tendon cells is provided by Vein, a neuregulin-like secreted ligand of the Egfr pathway. Following muscle binding, Vein accumulation at the muscle-tendon junction site activates the Egfr pathway specifically in the muscle-bound tendon progenitor, driving it to differentiate into a mature tendon cell (Fig. 1B) (Yarnitzky et al., 1997).
Thus, the initial determination of Drosophila tendon progenitor cells in the ectoderm takes place sequentially. The initial weak signal is initiated by segment polarity genes, such as hh and wg. Then, the signal is strengthened as a result of StripeB activity, which is positively autoregulated, but also maintained at low levels as a result of the post-transcriptional inhibitory activity of How(L). In this manner, the tendon progenitor cells produce the necessary signals for attracting muscle cells towards the attachment sites; however, the cells are not fully committed as tendon cells and their final differentiation is still dependent on their subsequent interaction with muscles, consistent with the second strategy mentioned above.
Induction of tendon progenitors in vertebrate embryos
Development of the musculoskeletal system in vertebrate embryos diverges from that of flies with the addition of a cartilaginous skeleton and the characteristic multicellular nature of tendons that connect the muscles to their respective skeletal insertions. Direct observations of early events in tendon formation became possible with the finding that the bHLH transcription factor scleraxis (Scx) is a distinctive marker of tendon cells from early embryonic stages and throughout development (Brent et al., 2003; Cserjesi et al., 1995; Schweitzer et al., 2001). A universal feature that emerged from lineage studies, from analyzing early Scx expression and from studies of tendon development in muscle-less limbs, is that, throughout the vertebrate body, tendons and cartilage arise from a common mesodermal compartment, which is different from the myogenic compartment (Chai et al., 2000; Kardon, 1998; Kontges and Lumsden, 1996; Tozer and Duprez, 2005). The proximity of the embryonic origin of tendon and cartilage progenitors might reflect an interrelated evolutionary history of these tissues.
Moreover, although the major molecular regulators of tendon induction and differentiation may be shared throughout the vertebrate body, the cellular dynamics and tissue interactions directing these processes are different in the three major sections of the body. We will therefore discuss the induction of trunk, limb and head tendons separately.
Axial tendon induction depends on a myotomal signal
The early somite is subdivided into a ventromedial sclerotome (see Glossary, Box 2), which gives rise to skeletal tissues, and a dorsolateral dermomyotome (see Glossary, Box 2), which gives rise to both the dermis and the myotome, the myogenic compartment. The Scx-expressing tendon progenitors are concentrated in a subdomain of the sclerotome, the syndetome (see Glossary, Box 2) (Brent et al., 2003). Interestingly, a recent study in Xenopus embryos (della Gaspera et al., 2009) identified early expression of the MADS box transcription factor Mef2c in the syndetome. Mef2c expression preceded that of Scx in these cells, and the overexpression of Mef2c resulted in a modest induction of Scx, suggesting a possible early role for Mef2c in the induction of tendon progenitors in Xenopus (della Gaspera et al., 2009).
The separation between cartilage-forming sclerotome cells and the tendon-forming syndetome is maintained by mutual repression. Expression of Pax1, the prototypic sclerotome marker, is repressed during syndetome induction in chick embryos and, conversely, overexpression of Pax1 in the sclerotome inhibits Scx expression (Brent et al., 2003). These observations suggest the existence of multipotential tendo-chondro progenitors in the early sclerotome, a notion further supported by the expansion of Scx expression in Sox5–/–;Sox6–/– double-mutant mouse embryos, in which cartilage differentiation is compromised (Brent et al., 2005).
The syndetome is located at the expected site for progenitors of a tissue that connects muscles to the skeleton, being at the junction between two neighboring myotomes and the sclerotome (Fig. 2A). The proximity to the myotome reflects a dependence of syndetome induction on a signal from the myotome. Indeed, both removal of the dermomyotome in chick embryos and failure of myotome differentiation in Myod1–/–;Myf5–/– double-mutant mouse embryos result in the absence of Scx expression, whereas no effect has been detected on sclerotome induction (Brent et al., 2005; Brent et al., 2003).
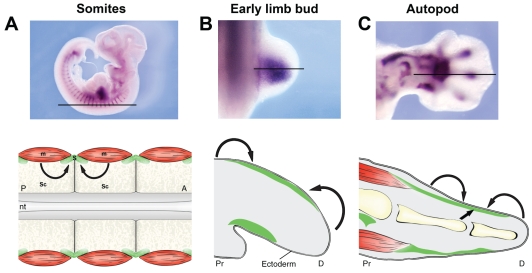
Fig. 2.
Tissue interactions required for tendon progenitor induction in vertebrate embryos. Induction of tendon progenitors, identified as Scx-expressing cells, depends on a unique set of tissue interactions in different parts of the embryo. Each panel shows tendon progenitor distribution by whole-mount in situ hybridization (ISH) with an Scx probe. The line across each upper image shows the orientation of the section schematized beneath, which highlights the relevant tissue interactions (with tendon progenitors shown in green; muscle progenitors in red; cartilage in yellow). (A) Whole-mount Scx ISH on E10.5 mouse embryo and a schematic of a frontal trunk section, showing somite pairs (squares) and the neural tube (gray). Skeletal tissue derives from the sclerotome (Sc) of somites, whereas the musculature arises from the myotome (m). The tendon progenitors are found in the syndetome (S, green), a stripe of sclerotome cells at the junction between two adjacent myotomes. Scx expression in syndetome cells is induced by FGFs secreted from the adjacent myotomes (arrows). (B) Whole-mount Scx ISH on E10.5 mouse limb bud and a schematized sagittal section through the limb bud. In the early limb bud, Scx is expressed in mesodermal cells directly under the dorsal and ventral ectoderm. Scx expression at this stage depends on ectoderm (curved arrows) and not on a signal from the myoblasts or from prechondrogenic cells. (C) Whole-mount Scx ISH on E12.5 mouse limb and a schematized sagittal section through the autopod. In the differentiating autopod, Scx is expressed in sub-ectodermal mesoderm along the differentiating skeletal elements. Scx expression along the differentiating digits can be induced by a signal from the skeletal condensations (straight arrow), and the sub-ectodermal position of the tendon progenitors suggests a role for the ectoderm (curved arrows) in tendon induction as well. A, anterior; D, distal; P, posterior; Pr, proximal; nt, neural tube.
The tendon-inducing activity of the myotome is associated with fibroblast growth factors (FGFs) emanating from the myotome: Fgf4 and Fgf8 in chick, and Fgf4 and Fgf6 in mouse embryos (Brent et al., 2005; Brent et al., 2003; Brent and Tabin, 2004; Tozer and Duprez, 2005). Several studies have demonstrated that FGF signaling is both necessary and sufficient for the induction of the syndetome. FGF signaling is a potent inducer of Scx and other tendon markers in chick embryos (Brent et al., 2005), and downregulation of FGF signaling in chick embryos results in the reduction or loss of Scx expression (Brent et al., 2003; Brent and Tabin, 2004). Fine-tuning of syndetome promotion by FGF signaling is achieved by the induction of dual specificity protein phosphatase 6 (Mkp3; also known as Dusp6), which attenuates in a negative-feedback loop the range and intensity of ERK and MAPK activity induced by FGF signaling (Brent and Tabin, 2004; Smith et al., 2005). Interestingly, while most of the studies implicating FGF signaling in tendon induction have been performed in chick embryos, and inhibition of FGF signaling in trunk cultures of mouse embryos resulted in loss of Scx expression, there is no report to date of a specific tendon disruption in mice bearing mutations that affect FGF signaling, which is likely to reflect the common redundancy in FGF ligands and receptors and the need for a targeted deletion of multiple ligands or receptors to demonstrate a specific role in tendon induction.
Recent studies have also implicated transforming growth factor β (TGFβ) signaling as a major regulator of tendon induction. Activation of TGFβ signaling leads to a robust induction of Scx and additional tendon markers, including tenomodulin, a type II transmembrane protein and an inhibitor of angiogenesis (Lorda-Diez et al., 2009; Oka et al., 2008; Pryce et al., 2009). Furthermore, disruption of TGFβ signaling (in Tgfb2/Tgfb3 double-mutant mouse embryos) results in the complete loss of all tendon tissue. Interestingly, the syndetome is not disrupted in these embryos, and loss of tendon progenitors is detected only at embryonic day (E) 12.5, a developmental stage at which the tendon progenitors align between the differentiating muscle and cartilage tissues (Fig. 3B). Moreover, expression of Tgfβ2, the major TGFβ ligand in this process, is detected only in the differentiating muscles and cartilage. It was therefore suggested that TGFβ signals from the differentiating muscle and cartilage recruit a second wave of tendon progenitors that are likely to contribute to the establishment of connections between the forming tendon, muscle and skeletal tissues (Pryce et al., 2009).
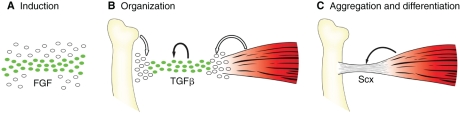
Fig. 3.
The main stages and regulators of tendon induction and differentiation in vertebrate embryos. The induction and differentiation of tendon progenitors occur in three distinct stages (A-C, in which Scx-expressing tendon progenitors are represented in green, and mesenchymal cells in white). (A) Induction. The initial induction of Scx-expressing tendon progenitors is associated with FGF signaling, but the myotome in somites is the only identified source to date. In somites and digits, the progenitors are induced at or near their functional position between the myogenic and skeletogenic cells, but in the early limb bud and branchial arches the site of progenitor induction is not related to their final destination. (B) Organization. In an E12.5 mouse embryo, tendon progenitors throughout the embryonic body organize as loose cellular aggregations between the differentiating muscle and skeletal tissues. This transition depends on TGFβ signaling, which mediates the recruitment of additional tendon progenitors by the muscle and cartilage tissues to position and integrate the tendon progenitors with their interacting musculoskeletal tissues (white arrows). In addition, TGFβ ligands expressed by the tendon progenitors are likely to contribute to the maintenance of the tenoblastic identity of the tendon progenitors (black arrow). (C) Aggregation and differentiation. By E13.5, the tendon progenitors condense and organize into structurally distinct tendons that connect to the muscle and cartilage. In some, but not all, tendons tenocyte differentiation depends on Scx function. In most tissues, tendon differentiation depends on the presence of muscles (arrow), but the extensor and flexor tendons that extend into the autopod differentiate as structurally distinct tendons even in the absence of muscles.
The interdependence of musculoskeletal tissues in the somite is therefore complex, beginning with syndetome induction by a signal from the myotome, followed by a second wave of induction that is likely to emanate from both muscle and cartilage tissue.
Progressive induction of limb tendon progenitors shows a differential dependence on limb tissues
The dynamics that characterize the development of limb tendons are inherently different from those of trunk tendons. In the early limb buds, the progenitors of the musculoskeletal tissues are not separated in discrete compartments, and the migrating myoblasts and Scx-expressing cells are physically mixed in the dorsal and ventral domains of the limb bud (for reviews, see Edom-Vovard and Duprez, 2004; Edom-Vovard et al., 2002; Schweitzer et al., 2001; Tozer and Duprez, 2005). Moreover, unlike in somites, initial Scx expression is not at a distinct position relative to the muscle or skeletal progenitors (Fig. 2B).
In contrast to the myoblasts that migrate into the limb bud from the ventrolateral lip of the dermomyotome, limb tendons arise from the lateral plate mesoderm. This observation was initially based on the presence of tendon progenitors in chick embryo limb buds subjected to embryonic manipulations that prevent somitic contributions (such as coelomic grafts) (Kardon, 1998; Kieny and Chevallier, 1979; Shellswell and Wolpert, 1977). Similarly, all tendon cells were labeled when the limb bud-specific Prx1-cre transgenic mouse, in which Cre is expressed under the control of the paired-related homeobox 1 (Prx1; also known as Prrx1 – Mouse Genome Informatics) promoter, was used to label the limb bud mesoderm and its descendants (Logan et al., 2002; Pryce et al., 2009).
The induction of Scx expression in the limb bud progresses from proximal to distal regions. Scx is initially detected in dorsal and ventral sub-ectodermal patches (Murchison et al., 2007; Schweitzer et al., 2001) (Fig. 2B). By E12.5, mouse tendon progenitors in the proximal elements of the limb undergo a major dynamic reorganization and align between the differentiating muscles and cartilage. By E13.5, the progenitors condense and differentiate into overtly distinct tendons (Fig. 3). Concurrently, beginning at E12.5, tendon progenitors are also induced in the sub-ectodermal mesoderm of the forming autopod (Fig. 2C). Interestingly, unlike the other tendon progenitors in the limb bud, the autopod progenitors are induced near the eventual position of the tendons along the forming digits (Schweitzer et al., 2001).
The origin of signals essential for tendon induction in the developing limb bud is drastically different from that of the somites. Studies of tendon induction in mouse and chick limb buds that lack muscle show that muscles are not essential for the induction, or for the initial organization, of tendon progenitors in the limb bud. The early expression of Scx and tenascin C (Tnc), an extracellular matrix (ECM) protein that was originally used as an early tendon marker, is normal in mutant mouse embryos that have muscle-less limbs [such as in Pax3 or Myf5/MyoD (Myod1 – Mouse Genome Informatics) mouse mutants] and is normal in chick embryos following coelomic grafts. Signals from the muscles are, however, essential for tendon differentiation at subsequent stages (Bonnin et al., 2005; Edom-Vovard et al., 2002; Eloy-Trinquet et al., 2009; Kardon, 1998; Schweitzer et al., 2001), implicating the second strategy described above. The only tissue so far implicated in progenitor induction in the limb bud is the ectoderm. Ectoderm ablation experiments in chick embryos have demonstrated its essential role for the sub-ectodermal induction of tendon progenitors (Schweitzer et al., 2001).
Interestingly, it has also been suggested that cartilage might play a role in tendon induction in the autopod (Hurle et al., 1990). This is because, as reported by Hurle et al., the late removal of interdigital ectoderm resulted in the differentiation of a cartilage element in the interdigital space, which was followed by the induction of a tendon along the new cartilage element.
The tendon-inducing activities of FGF and TGFβ signaling are effective in limb buds as well. As in somites, the ectopic activation of FGF signaling in the chick limb bud in ovo, and the application of TGFβ-containing beads to mouse limbs in organ culture, result in the induction of Scx and other tendon markers (Edom-Vovard et al., 2002; Pryce et al., 2009). Moreover, limb tendon progenitors are dependent on TGFβ signaling and could not be detected in the limb of Tgfb2/3 double mutants at E12.5. However, the specific molecular identity of the tendon-inducing activity remains unknown (Eloy-Trinquet et al., 2009; Pryce et al., 2009). Robust expression of Tgfβ2 in differentiating skeletal condensation in the limb bud might be associated with tendon progenitor induction. However, in Tgfb2 mouse mutants, or following the complete ablation of TGFβ signaling in mouse limb bud mesenchyme, tendon progenitors were first induced and only subsequently lost, suggesting that additional molecular activities direct the induction of tendon progenitors, and that TGFβ signaling might be involved in the interactions between the tendon progenitors and their respective skeletal and muscle partners (Pryce et al., 2009). Finally, Fgf4 expression has been detected at the extremities of limb muscles in chick embryos; however, this expression does not correspond to the distinct sites of Scx induction (Edom-Vovard et al., 2001; Edom-Vovard et al., 2002; Eloy-Trinquet et al., 2009).
Thus, several strands of evidence indicate that the induction of tendon progenitors in the limb bud is modular and differs significantly from that of the trunk.
Cranial tendons depend on muscles for differentiation but not induction
The development of cranial tendons has received the least attention to date. Lineage studies in chick and mouse embryos have shown that cranial tendons and cartilage are derived from the cranial neural crest, whereas the muscles differentiate from the mesodermal core of the branchial arches (Chai et al., 2000; Couly et al., 1992; Kontges and Lumsden, 1996; Trainor et al., 1994).
To study the dependence of cranial tendons on signals from muscles, tendon development was evaluated in T-box 1 (Tbx1) null mouse mutants, in which branchiomeric muscles (see Glossary, Box 2) fail to form or are severely reduced in size (Grenier et al., 2009; Grifone et al., 2008). As in the limb, cranial tendon progenitor induction was found to not depend on muscle, and Scx distribution was only slightly reduced by E12.5. However, by E15.5, all cranial tendons disappear in the Tbx1–/– mutant, demonstrating that cranial tendon differentiation depends on an interaction with branchiomeric muscle (as in the second strategy above).
The dependence of tendon induction on signals from neighboring tissues is thus complex and variable (Fig. 2). Whereas the induction of the syndetome depends on a signal from the myotome, the induction of limb and cranial tendon progenitors is independent of muscle. In limb buds, the initial sub-ectodermal induction of tendon progenitors is ectoderm dependent, whereas the cartilage might be important for induction of tendon progenitors in the autopod. Finally, the dependence on TGFβ signaling is likely to reflect a second wave of progenitor recruitment that underlies the connection between the forming tendon and the skeletal and muscle insertion sites.
Muscle targeting and anchoring to tendons and myotendinous junction formation in Drosophila
Whereas the initial determination of muscles and tendons appears to be autonomous in Drosophila, the subsequent targeting of muscles to tendons requires signaling to occur between these two cell types. In Drosophila, muscle migration towards tendons depends on several factors, including the initial polarity of the muscle cell, local signals available during muscle migration, target recognition and signals involved in terminating migration. Owing to the limited amount of information available on these processes in both vertebrates and invertebrates, it is too early to speculate as to how conserved these processes are in these two systems. However, the gradual construction of the myotendinous junction, which is mediated primarily by integrin-dependent adhesion in both systems, might reflect a high degree of similarity between invertebrates and vertebrates.
At stage 12-13 of embryonic development, following the fusion of the myoblasts to distinct founder cells, the myotubes acquire a characteristic polarity in which the edges of the cells are directed to either anterior-posterior or dorsal-ventral positions (Schnorrer and Dickson, 2004). By following individual GFP-labeled myotubes, it is possible to detect the migration path of muscle cells towards their targeted tendon cells (Schnorrer et al., 2007). In this manner, it is possible to distinguish between mutations that affect migration per se from those that affect muscle attachment to tendons. Although both these defects result in the rounding up of muscle cells, defects in muscle migration appear at earlier developmental stages (Schnorrer et al., 2007).
A unique aspect of muscle migration towards target tendon cells is its bipolar extension towards two tendon cells located at its two opposite ends. In Drosophila embryos, live imaging of single muscle cells during their migration suggests a simultaneous bi-directional extension of the muscle cell (Schnorrer and Dickson, 2004) (Fig. 1A). How do the muscle ends respond in opposite directions to guiding signals? It is possible that the initial extension of the two muscle ends reflects the intrinsic polarity of this cell type, independent of any external signals. Only when the two muscle ends are distant enough from each other might they respond to short-range signals provided by the tendon cells located at the segment border. Such a scenario might take place during vertebrate muscle migration, where, in a manner similar to that of Drosophila, the muscles are connected at their two ends to attachment sites.
Several signaling pathways involved in axon guidance have been described that mediate muscle guidance towards tendon cells as well. These include the Slit-Robo and the Derailed receptor tyrosine kinase pathways (Callahan et al., 1996; Kramer et al., 2001; Ypsilanti et al., 2010). Muscles express the receptor Robo, and its ligand Slit is secreted from tendon cells and from the ventral cord midline. Interestingly, whereas both the Slit-Robo and Derailed pathways repress axon guidance, they appear to mediate the attraction of muscles towards their target tendon cells (an exception to this is the ventral muscles, which are repelled from the ventral midline owing to Slit activity secreted at the midline).
Recently, an additional novel protein complex expressed by the ventral longitudinal muscles was demonstrated to mediate muscle migration towards tendon cells. This complex includes the transmembrane protein Kon-tiki (Perdido), its cytoplasmic partner the PDZ domain protein Grip, and the cell-surface protein Echinoid (Estrada et al., 2007; Schnorrer et al., 2007; Swan et al., 2006; Swan et al., 2004). Grip and kon-tiki mutants share a similar phenotype in which the muscles do not extend towards the tendon cells during their migration, suggesting that they both mediate a positive attractive cue sensed by the muscle ends. The nature of this signal has not been elucidated.
In summary, the unique bipolar extension of the muscle ends in Drosophila might be dependent on short-range signals provided by the tendon cells.
Drosophila myotendinous junction formation
In both vertebrates and invertebrates, the correct construction of the myotendinous junction is essential for force transmission and to counteract muscle contraction by the skeletal elements by means of tendon cells. The Drosophila myotendinous junction consists of hemi-adherens junctions formed between integrin heterodimers assembled on the muscle and tendon membrane surfaces, together with ECM proteins deposited in between these cells (Brown, 2000). These ECM proteins `glue' the two cell types together through integrin receptors associated with the actin cytoskeleton in the cytoplasm of each cell. The glue-like ECM material provides elastic properties to the myotendinous junction and its unique ultrastructural organization is essential for proper force transmission (Brown, 2000).
Studies in Drosophila reveal the sequence of events associated with myotendinous junction formation. Two types of integrin heterodimers mediate the formation of this junction, namely the integrin receptors αPS1βPS on the tendon cell and αPS2βPS on the muscle side (Bokel and Brown, 2002; Brown, 2000). They appear to interact with distinct types of ECM proteins: the tendon-specific αPS1βPS heterodimer interacts with laminin (Gotwals et al., 1994; Martin et al., 1999), whereas the muscle-specific αPS2βPS heterodimer interacts with Tsp (Chanana et al., 2007; Subramanian et al., 2007) and Tiggrin (Fogerty et al., 1994). Laminin and Tsp are secreted from the tendon cells, and Tiggrin is secreted from the muscle cell. Non-functional myotendinous junctions observed in integrin, laminin and Tsp Drosophila mutants lead to a complete dissociation of muscles from tendons, promoting embryonic lethality. The most severe muscle detachment phenotype is obtained in embryos mutant for either αPS2βPS or its ECM ligand Tsp. Lack of laminin, and/or of αPS1βPS, leads to a less severe muscle detachment phenotype, suggesting that the adhesion of the muscle to the tendon-associated ECM is a crucial aspect of the function of the myotendinous junction.
The earliest event in the construction of the Drosophila myotendinous junction is the tendon-specific secretion of Tsp, which precedes muscle attachment and the assembly of PS integrins on the muscle and tendon surfaces. While migrating, the muscle does not respond to Tsp, even when ectopically expressed; however, once the muscle ends approach the tendon cell, PS2 integrin gradually accumulates at the muscle ends, presumably as a result of inside-out integrin signaling, which, following association of its cytoplasmic tail with the cytoskeletal linker protein Talin (also known as Rhea – FlyBase), enhances the affinity of its ectodomain for Tsp (Bokel and Brown, 2002; Tanentzapf and Brown, 2006). Recently, a secreted tendon-specific protein, Slow, was shown to modulate the responsiveness of the muscle integrins to Tsp, presumably through its association with Tsp (Gilsohn and Volk, 2010a). Lack of slow leads to a severe locomotion phenotype in homozygous larvae and in an inability to fly in adult slow homozygous mutant escapers. A similar phenotype is also obtained when muscle integrin receptors are overexpressed in the muscle cells (Tanentzapf and Brown, 2006). Therefore, the gradual construction of the myotendinous junction, as well as the correct assembly of the ECM, are crucial for proper musculoskeletal function (Brown, 2000).
Recently, components of the integrin-mediated adhesion apparatus, including talin 1 and talin 2, as well as the laminin integrin receptors α7β1D and α7Bβ1D, were shown to actively mediate vertebrate myotendinous junction formation, and their absence was shown to lead to myopathies in humans (Conti et al., 2008; Conti et al., 2009).
Arrest of muscle migration
The arrival of the muscle cell at the target tendon cell and the formation of the myotendinous junction are temporally and spatially coupled. However, it is not clear how tendon recognition, arrest of muscle migration and initiation of myotendinous junction formation are coordinated at the molecular level. The transmembrane protein Kon-tiki has been shown to interact genetically with the tendon-specific αPS1βPS integrin receptors (Estrada et al., 2007). Such an interaction might provide the muscle cell with a signal to arrest its migration. The phenotype of kon-tiki mutants is first detected during migration of the muscles; however, an additional role in migration arrest cannot be excluded. In wild-type Drosophila embryos, Lrt accumulates in the tendon membrane at the junctional site following the arrival of the migrating muscle cell at its target, and functionally interacts with Robo receptors on the muscle cell. Lack of Lrt leads to the presence of extra-membrane extensions on migrating muscle cells, implying aberrant muscle targeting and/or defects in the arrest of muscle migration. Moreover, Lrt overexpression stalls muscle extension towards tendon cells, supporting its central role in promoting muscle targeting to tendon cells (Wayburn and Volk, 2009).
One possible mechanism for the arrest of muscle migration is the initiation of myotendinous junction formation. During muscle migration, the muscle is insensitive to ectopic expression of the ECM protein Tsp (Gilsohn and Volk, 2010a). A possible explanation is that integrin receptors are not expressed on the membrane of migrating muscle cells. The arrival of the muscle at its target tendon cell leads to the initial accumulation of muscle integrin receptors, responsiveness to Tsp, and the accumulation of integrin at the muscle ends. These initial adhesion events might represent a signal for the muscle to arrest its migratory behavior and to initiate the formation of the myotendinous junction. A finding supporting this possibility is that overexpression of integrin in the muscle during its migration leads to aberrant muscle migratory behavior (Gilsohn and Volk, 2010a).
In summary, recent findings in Drosophila have shed light on how a migrating muscle cell recognizes its target tendon, and have shown that the initial formation of the myotendinous junction depends on the function of certain highly conserved proteins, which might play similar roles during myotendinous junction formation in the vertebrate embryo.
Tendon-bone attachment in vertebrates
The attachment of tendons to the internal skeleton is unique to vertebrates. The formation of this junction is essential for musculoskeletal functionality, as it transmits muscle-generated force to the skeleton. The biology behind the development of a functional tendon-skeleton attachment unit is largely unknown. Nevertheless, several studies have provided a histological and, to some extent, molecular description of the attachment unit, known as the enthesis (Benjamin et al., 2002; Benjamin et al., 2006; Thomopoulos et al. 2003). Based on these studies, the enthesis can be approximately divided into four zones (see Box 3). It is tempting to view these zones, with their different cellular and extracellular properties, as a gradient that shifts from tendinous to cartilaginous-bony tissue.
The molecular mechanisms that regulate the formation of the cellular and ECM protein gradient that constitute the different enthesis zones are largely unknown. However, a cell-lineage study in mice has demonstrated that, similarly to the chondrocytes and osteoblasts that form the skeleton, cells at the attachment site are descendants of Sox9-expressing cells (Akiyama et al., 2005). This is an intriguing observation as it suggests that cells comprising the different zones of the enthesis share common progenitors. The immediate implication of this finding is that the molecular mechanism that underlies the cellular gradient of the enthesis operates by allowing these Sox9-positive progenitor cells to adopt different cell fates according to their position, implicating the third strategy of transdifferentiation described above.
An intriguing recent study suggests a role for TGFβ signaling in this process (Lorda-Diez et al., 2009). TGFβ signaling has been implicated in the induction of both the cartilage and tendon cell fates and in the direct regulation of Sox9 and Scx expression. The ability of TGFβ signaling to divert cells in micromass cultures from Sox9-expressing prechondrogenic cells to tendon cells was associated in this study with the induction of the transcriptional repressors Tgif1 and SnoN (Skil – Mouse Genome Informatics), which selectively reduce Sox9 expression levels (Lorda-Diez et al., 2009).
An additional interesting common feature of the skeleton and the enthesis is that cells at the tendon side of the enthesis express genes, such as those encoding collagen type II alpha 1 (Col2a1), Indian hedgehog (Ihh), parathyroid hormone-related peptide receptor (PTHrPR; Pth1r – Mouse Genome Informatics) and collagen type X alpha 1 (Col10a1), all of which are the hallmarks of the bone growth plate (Kronenberg, 2003; Thomopoulos et al., 2003). The growth plate is located near the ends of long bones and consists of chondrocytes that undergo a well-defined and highly controlled differentiation program, leading to the replacement of cartilage by ossified bone. One can speculate that the growth plate module was selected as a cellular mechanism for bone ridge formation to allow the growth of the mineralized part of the enthesis.
In many instances, the fourth `bony' zone of the enthesis is part of a bone protrusion that provides a stable anchoring point for muscles, inserted into the skeleton by means of tendons. Bone protrusions are divided into two groups: articular and non-articular. Examples of articular eminences are found in the heads of the humerus and femur. Non-articular eminences are located along the bone shaft and termed according to their shape (e.g. ridge, crest, tuberosity). Most of the mechanical load applied to the skeleton, as generated by muscle contraction and transduced by tendons, initially encounters the bone ridge structures. A reasonable assumption that would explain the complex multi-zone structure of the bone-tendon attachment site and its ending in a bone ridge is that these structures absorb and dissipate some of the stress concentrated at the interface between the hard and soft tissues, thereby diminishing the risk of avulsion fractures (see Glossary, Box 2) (Benjamin et al., 2002; Biewener et al., 1996).
Studies performed over the past century have established the contribution of the mechanical load to skeletal development, and specifically to the attachment site (Hall and Herring, 1990; Hamburger, 1939; Hamburger, 1940; Hosseini and Hogg, 1991; Pai, 1965; Rot-Nikcevic et al., 2006; Tremblay et al., 1998). Muscle contraction was demonstrated to regulate bone ridge development, as well as tendon strength and structure (Ralphs et al., 1998; Thomopoulos et al., 2003). Recently, however, it has been reported that bone ridge development is a biphasic process, consisting of initiation and growth phases (Fig. 4). The initiation phase is muscle independent, whereas the subsequent growth phase, which unfolds through chondrocyte proliferation and the formation of a miniature growth plate, depends on muscle contraction. The finding that bone ridge initiation is muscle independent requires that there be a different mechanism to regulate the initiation phase. Indeed, it has been shown that Scx-positive cells are necessary for bone ridge initiation. This suggests that at the core of this novel mechanism is a signal that emanates from the tendon side of the enthesis and governs bone ridge formation (Blitz et al., 2009).
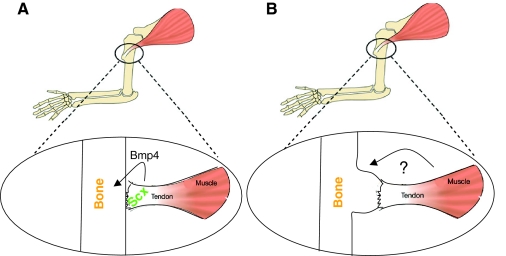
Fig. 4.
Bone ridge formation proceeds via a biphasic process. (A,B) Schematics of the mouse forelimb skeleton, illustrating the involvement of tendons and muscles in bone ridge formation. The attachment site area (circled) is magnified beneath. According to the biphasic model, tendons regulate the initiation of tuberosity (see Glossary, Box 2) (A) and muscles control its subsequent growth (B). (A) The molecular mechanism that underlies tendon regulation of bone ridge initiation involves the bHLH transcription factor Scx, which regulates Bmp4 expression in tendon cells. Next, upon binding of Bmp4 to its receptor Alk3 (Bmpr1a) in chondrocytes, a signaling cascade is activated, eventually leading to the initiation of a bone ridge. (B) The mechanism whereby muscle contraction promotes bone ridge growth remains to be uncovered.
Several signaling pathways are known to regulate chondrocyte differentiation, notably bone morphogenetic proteins (BMPs) and members of the TGFβ superfamily (Yoon et al., 2005). The finding that Scx regulates the expression of Bmp4 in tendon cells at the attachment site has implicated Bmp4 as the signal emanating from the tendon side of the attachment unit to regulate bone ridge formation. The loss of numerous bone ridges in mice in which Bmp4 expression was blocked in Scx-positive cells confirms this hypothesis (Blitz et al., 2009). Interestingly, not all bone ridges are lost in the Bmp4-depleted limbs. This observation suggests that either there are other BMPs that play a redundant role in bone ridge initiation or that there is more than one mechanism that regulates this process.
These recent studies underscore the centrality of the regulatory interaction between tendon and skeleton cells during the development of the attachment unit. This notion raises several fundamental questions regarding the establishment of the attachment site. For example, which cells respond to the signal from tendon cells to initiate bone ridge formation and where are they located? By what mechanism do tendons attach to bones? Given their morphological variation, do all bone ridges develop through endochondral bone formation, or are other mechanisms, such as periosteal growth, involved? Finally, and perhaps most interestingly, what is the mechanism that underlies the cellular gradient in the attachment site? These questions should be addressed in future research in this field.
Regulation of tendon differentiation
Tendons are structurally diverse, ranging from thin fascia-like layers of connective tissue to the long cord-like tendons of the limb and tail (Benjamin et al., 2008; Benjamin and Ralphs, 1997). To form these structures, the loosely organized tendon progenitors condense and aggregate as they differentiate into structurally distinct functional tendons (Murchison et al., 2007). Tendon differentiation also involves the generation of the myotendinous junction at one end of the tendon and the enthesis at the skeletal insertion to establish an integrated and functional musculoskeletal system. Tendon differentiation and the emergence of overtly distinct tendons occur as a rapid transition that sweeps across the embryo at E13.5 of mouse development and at approximately Hamburger-Hamilton stage 28 (HH28) in chick embryos (Bonnin et al., 2005; Murchison et al., 2007; Schweitzer et al., 2001). The embryos begin to display spontaneous movements shortly after the tendons differentiate, suggesting that, although tendons eventually transmit force through extracellular bundles of collagen fibers, the cohesiveness of the cellular interactions formed in the differentiating tendons is sufficient for the propagation of force through what is a mostly cellular structure.
Tendon differentiation depends on musculature interactions
In Drosophila, tendon differentiation is induced by an Egfr ligand secreted from the muscles. The corresponding scenario in vertebrate embryos was evaluated in muscle-less limbs generated through coelomic grafts in chick embryos or in Pax3 mouse mutants in which myoblast migration into the limb bud is disrupted. In both cases, the induction and early distribution of tendon progenitors appeared normal, but subsequent tendon differentiation was profoundly disrupted. Expression of Scx and Tnc was completely lost in the proximal parts of muscle-less limbs at the onset of tendon differentiation, and no tendons developed in the arm or leg in later stages (Bonnin et al., 2005; Eloy-Trinquet et al., 2009; Kardon, 1998; Schweitzer et al., 2001).
The absolute dependence of tendon differentiation and maintenance in the proximal parts of the limb on interactions with muscles is sharply contrasted by the normal development of tendons within the autopod in these limbs. The distal segments of the major extensor and flexor tendons that extend along the digits are not affected by the absence of muscles and differentiate to form well-organized tendons. This striking result highlights the modular nature of limb tendons (Kardon, 1998). These tendons, however, degenerate at later stages, suggesting that tendon maintenance might also be dependent on the interaction with muscle or the mechanical load applied by the muscle. In chick embryos, the modular nature of these tendons has been directly visualized by detection of digit, hand and arm sections of the tendons as separate elements before they fuse to give rise to complete tendons (Kardon, 1998). As the muscles for the major tendons that extend into the autopod reside in the zeugopod (see Glossary, Box 2), the distal segments of these tendons develop with no muscle interactions even in wild-type limbs, and it is therefore not surprising that these tendon segments develop despite the absence of muscles within the limbs.
The dependence of tendon differentiation on interaction with muscles is thus not an obligatory aspect of tendon differentiation, and the essential muscle-dependent signal might be replaced by input from a different source, which is likely to be the cartilage in the developing hand. However, a recent report of the muscle-dependent differentiation of cranial tendons further reinforces a near-universal requirement for muscle-tendon interactions during tendon differentiation (Grenier et al., 2009).
The molecular nature of the interaction between the muscle and tendon at this stage has not been elucidated. However, the expression of Fgf4 at the extremities of limb muscles and of FGF target genes (e.g. sprouty) at the extremities of limb muscles in differentiating tendons, combined with the ability of exogenous FGF to induce Scx in muscle-less limbs, suggest the possible involvement of FGF signaling in tendon differentiation (Edom-Vovard et al., 2002; Eloy-Trinquet et al., 2009). An intriguing aspect of this model is the suggestion that tendon differentiation in both vertebrate and Drosophila embryos occurs through the activation of a tyrosine kinase receptor in the tendon progenitors by a ligand secreted from the muscle. In vertebrates, however, the muscle signal is likely to affect mostly the differentiating tenocytes (see Glossary, Box 2) at the myotendinous junction. It is therefore possible that tenocyte differentiation through the length of the tendon depends on the propagation of cellular interactions from a solid anchor in the form of the myotendinous junction. A similar essential role for the cartilage in tendon differentiation has not been shown to date, suggesting that the effect on tendon differentiation could be unique to muscles or that a mutant with an adequate disruption of cartilage differentiation has not been identified so far.
Scleraxis is a crucial regulator of tenocyte differentiation and aggregation
The only direct molecular regulator of tenocyte differentiation identified to date is the transcription factor Scx (Murchison et al., 2007). Scx mouse mutants are viable, but their movement is severely restricted, with limited use of the paws and complete loss of tail movement. This phenotype is associated with a severe disruption of distinct groups of tendons and a limited growth of other tendons. Interestingly, despite the early expression of Scx in tendon progenitors, the induction and distribution of tendon progenitors are not disrupted in the absence of Scx. The underlying determinants of whether tendons are dependent on Scx function remain elusive, but it has been straightforward to categorize the tendons into groups based on the requirement for Scx. Thus, the tendons that project to the distal body parts (for example, the tail and the distal tips of the digits) are severely disrupted or lost in Scx mutant embryos, whereas the myotendinous junctions, entheses, ligaments and a few groups of tendons remain functional in Scx mutants, even if their size is reduced (Murchison et al., 2007).
Tendon disruption in Scx mouse mutants is manifest at the time of tendon differentiation at E13.5, and the common feature in all affected tendons is a failure of condensation and aggregation of the tendon progenitors. The expression of some genes depends on Scx function, including those encoding tenomodulin, collagen XIV and collagen I (Espira et al., 2009; Lejard et al., 2007; Murchison et al., 2007; Shukunami et al., 2006). Based on the mutant phenotype, it is likely that Scx regulates adhesion proteins or genes involved in the control of cellular adhesion that might regulate the capacity of the tendon cells to aggregate during tendon differentiation; to date, the identity of such genes remains elusive.
Conclusions
The dependence of tendon differentiation on the interaction with muscle represents a convergence of function in both systems and suggests that other aspects of muscle-tendon interaction that have not been elucidated at the molecular level so far, such as the mechanism of muscle targeting to tendon cells and of the gradual construction of the myotendinous junction, might reveal a significant level of conservation.
Outstanding issues and questions for this field to investigate in the future include the following. (1) The identification of transcription factors that promote the specification of tendon progenitors and of factors that cooperate with Scx to regulate tendon differentiation. Do post-transcriptional events play a major role in these processes, as they do in Drosophila? (2) Elucidation of the specific functions of FGF and TGFβ signaling in the heterotypic interactions between the muscle and skeletal tissues and the differentiating tendons. Is there a cross-talk between these signaling cascades and are additional signals employed in these processes? (3) Identification of the reciprocal effect of tendons on the skeletal and muscle tissues. (4) Establishment of the cellular and molecular events that promote the formation of the enthesis and the myotendinous junction. (5) Identification of the mechanism that underlies the targeting of tendon cells by muscles. In vertebrates, are there direct correlates to the Drosophila processes of guided migration, arrest of cellular migration and the establishment of the myotendinous junction?
Injuries and degenerative conditions in tendons and ligaments represent almost half of the musculoskeletal injuries treated in orthopedic clinics. These conditions often lead to surgery and to considerable morbidity owing to a limited healing process and to a long-term failure of tissue functionality. Efforts to improve clinical procedures and bioengineering approaches for replacement tissues are hampered by the very limited knowledge of the molecular regulators of these tissues and of the cellular dynamics operating during the process of tendon differentiation. A little-explored challenge that is crucial for the successful application of tendon development studies to the clinical setting is to the define the similarities and differences between the embryonic process of tendon development and those of the mature and injured tendon.
Finding answers to the questions above is essential not only for elucidating an intriguing developmental process that encompasses the construction of the musculoskeletal system, but also because it is likely to improve the clinical treatment of tendon and ligament injuries.
Acknowledgments
We thank S. Schwarzbaum and Development editorial staff for editorial assistance and Spencer Watson for graphical support. The authors are supported by several grant agencies, including the Israeli Science Foundation (T.V.), the NIH, NIAMS (R.S.). Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
References
- Akiyama H., Kamitani T., Yang X., Kandyil R., Bridgewater L. C., Fellous M., Mori-Akiyama Y., de Crombrugghe B. (2005). The transcription factor Sox9 is degraded by the ubiquitin-proteasome system and stabilized by a mutation in a ubiquitin-target site. Matrix Biol. 23, 499-505 [DOI] [PubMed] [Google Scholar]
- Becker S., Pasca G., Strumpf D., Min L., Volk T. (1997). Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development 124, 2615-2622 [DOI] [PubMed] [Google Scholar]
- Benjamin M., Ralphs J. R. (1997). Tendons and ligaments – an overview. Histol. Histopathol. 12, 1135-1144 [PubMed] [Google Scholar]
- Benjamin M., Kumai T., Milz S., Boszczyk B. M., Boszczyk A. A., Ralphs J. R. (2002). The skeletal attachment of tendons-tendon `entheses'. Comp. Biochem. Physiol. A Physiol. 133, 931-945 [DOI] [PubMed] [Google Scholar]
- Benjamin M., Toumi H., Ralphs J. R., Bydder G., Best T. M., Milz S. (2006). Where tendons and ligaments meet bone: attachment sites (`entheses') in relation to exercise and/or mechanical load. J. Anat. 208, 471-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Kaiser E., Milz S. (2008). Structure-function relationships in tendons: a review. J. Anat. 212, 211-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener A. A., Fazzalari N. L., Konieczynski D. D., Baudinette R. V. (1996). Adaptive changes in trabecular architecture in relation to functional strain patterns and disuse. Bone 19, 1-8 [DOI] [PubMed] [Google Scholar]
- Blitz E., Viukov S., Sharir A., Shwartz Y., Galloway J. L., Pryce B. A., Johnson R. L., Tabin C. J., Schweitzer R., Zelzer E. (2009). Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokel C., Brown N. H. (2002). Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 3, 311-321 [DOI] [PubMed] [Google Scholar]
- Bonnin M. A., Laclef C., Blaise R., Eloy-Trinquet S., Relaix F., Maire P., Duprez D. (2005). Six1 is not involved in limb tendon development, but is expressed in limb connective tissue under Shh regulation. Mech. Dev. 122, 573-585 [DOI] [PubMed] [Google Scholar]
- Brent A. E., Tabin C. J. (2002). Developmental regulation of somite derivatives: muscle, cartilage and tendon. Curr. Opin. Genet. Dev. 12, 548-557 [DOI] [PubMed] [Google Scholar]
- Brent A. E., Tabin C. J. (2004). FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131, 3885-3896 [DOI] [PubMed] [Google Scholar]
- Brent A. E., Schweitzer R., Tabin C. J. (2003). A somitic compartment of tendon progenitors. Cell 113, 235-248 [DOI] [PubMed] [Google Scholar]
- Brent A. E., Braun T., Tabin C. J. (2005). Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 132, 515-528 [DOI] [PubMed] [Google Scholar]
- Brown N. H. (2000). Cell-cell adhesion via the ECM: integrin genetics in fly and worm. Matrix Biol. 19, 191-201 [DOI] [PubMed] [Google Scholar]
- Callahan C. A., Bonkovsky J. L., Scully A. L., Thomas J. B. (1996). derailed is required for muscle attachment site selection in Drosophila. Development 122, 2761-2767 [DOI] [PubMed] [Google Scholar]
- Chai Y., Jiang X., Ito Y., Bringas P., Jr, Han J., Rowitch D. H., Soriano P., McMahon A. P., Sucov H. M. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671-1679 [DOI] [PubMed] [Google Scholar]
- Chanana B., Graf R., Koledachkina T., Pflanz R., Vorbruggen G. (2007). AlphaPS2 integrin-mediated muscle attachment in Drosophila requires the ECM protein Thrombospondin. Mech. Dev. 124, 463-475 [DOI] [PubMed] [Google Scholar]
- Chanana B., Steigemann P., Jackle H., Vorbruggen G. (2009). Reception of Slit requires only the chondroitin-sulphate-modified extracellular domain of Syndecan at the target cell surface. Proc. Natl. Acad. Sci. USA 106, 11984-11988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F. J., Felder A., Monkley S., Schwander M., Wood M. R., Lieber R., Critchley D., Muller U. (2008). Progressive myopathy and defects in the maintenance of myotendinous junctions in mice that lack talin 1 in skeletal muscle. Development 135, 2043-2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F. J., Monkley S. J., Wood M. R., Critchley D. R., Muller U. (2009). Talin 1 and 2 are required for myoblast fusion, sarcomere assembly and the maintenance of myotendinous junctions. Development 136, 3597-3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly G. F., Coltey P. M., Le Douarin N. M. (1992). The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 114, 1-15 [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Brown D., Ligon K. L., Lyons G. E., Copeland N. G., Gilbert D. J., Jenkins N. A., Olson E. N. (1995). Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 121, 1099-1110 [DOI] [PubMed] [Google Scholar]
- della Gaspera B., Armand A. S., Sequeira I., Lecolle S., Gallien C. L., Charbonnier F., Chanoine C. (2009). The Xenopus MEF2 gene family: evidence of a role for XMEF2C in larval tendon development. Dev. Biol. 328, 392-402 [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F., Duprez D. (2004). Signals regulating tendon formation during chick embryonic development. Dev. Dyn. 229, 449-457 [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F., Bonnin M., Duprez D. (2001). Fgf8 transcripts are located in tendons during embryonic chick limb development. Mech. Dev. 108, 203-206 [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F., Schuler B., Bonnin M. A., Teillet M. A., Duprez D. (2002). Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247, 351-366 [DOI] [PubMed] [Google Scholar]
- Eloy-Trinquet S., Wang H., Edom-Vovard F., Duprez D. (2009). Fgf signaling components are associated with muscles and tendons during limb development. Dev. Dyn. 238, 1195-1206 [DOI] [PubMed] [Google Scholar]
- Espira L., Lamoureux L., Jones S. C., Gerard R. D., Dixon I. M., Czubryt M. P. (2009). The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J. Mol. Cell. Cardiol. 47, 188-195 [DOI] [PubMed] [Google Scholar]
- Estrada B., Gisselbrecht S. S., Michelson A. M. (2007). The transmembrane protein Perdido interacts with Grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development 134, 4469-4478 [DOI] [PubMed] [Google Scholar]
- Fernandes J. J., Celniker S. E., VijayRaghavan K. (1996). Development of the indirect flight muscle attachment sites in Drosophila: role of the PS integrins and the stripe gene. Dev. Biol. 176, 166-184 [DOI] [PubMed] [Google Scholar]
- Fogerty F. J., Fessler L. I., Bunch T. A., Yaron Y., Parker C. G., Nelson R. E., Brower D. L., Gullberg D., Fessler J. H. (1994). Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development 120, 1747-1758 [DOI] [PubMed] [Google Scholar]
- Frommer G., Vorbruggen G., Pasca G., Jackle H., Volk T. (1996). Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 15, 1642-1649 [PMC free article] [PubMed] [Google Scholar]
- Fukuta S., Oyama M., Kavalkovich K., Fu F. H., Niyibizi C. (1998). Identification of type II, IX and X collagens at the insertion site of the bovine achilles tendon. Matrix Biol. 17, 65-73 [DOI] [PubMed] [Google Scholar]
- Gilsohn E., Volk T. (2010a). Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development 137, 785-794 [DOI] [PubMed] [Google Scholar]
- Gilsohn E., Volk T. (2010b). A screen for tendon-specific genes uncovers new and old components involved in muscle-tendon interaction. Fly (Austin) 4, 149-153 [DOI] [PubMed] [Google Scholar]
- Gotwals P. J., Fessler L. I., Wehrli M., Hynes R. O. (1994). Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc. Natl. Acad. Sci. USA 91, 11447-11451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier J., Teillet M. A., Grifone R., Kelly R. G., Duprez D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE 4, e4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifone R., Jarry T., Dandonneau M., Grenier J., Duprez D., Kelly R. G. (2008). Properties of branchiomeric and somite-derived muscle development in Tbx1 mutant embryos. Dev. Dyn. 237, 3071-3078 [DOI] [PubMed] [Google Scholar]
- Hall B. K., Herring S. W. (1990). Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol. 206, 45-56 [DOI] [PubMed] [Google Scholar]
- Hamburger V. (1939). The development and innervation of transplanted limb primordia of chick embryos. J. Exp. Zool. 80, 347-389 [Google Scholar]
- Hamburger V. (1940). The primary development of the skeleton in nerveless and poorly innervated limb transplants of chick embryos. Physiol. Zool. 13, 367-384 [Google Scholar]
- Hatini V., DiNardo S. (2001). Divide and conquer: pattern formation in Drosophila embryonic epidermis. Trends Genet. 17, 574-579 [DOI] [PubMed] [Google Scholar]
- Hosseini A., Hogg D. A. (1991). The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J. Anat. 177, 169-178 [PMC free article] [PubMed] [Google Scholar]
- Hurle J. M., Ros M. A., Ganan Y., Macias D., Critchlow M., Hinchliffe J. R. (1990). Experimental analysis of the role of ECM in the patterning of the distal tendons of the developing limb bud. Cell Differ. Dev. 30, 97-108 [DOI] [PubMed] [Google Scholar]
- Kablar B., Rudnicki M. A. (2000). Skeletal muscle development in the mouse embryo. Histol. Histopathol. 15, 649-656 [DOI] [PubMed] [Google Scholar]
- Kardon G. (1998). Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019-4032 [DOI] [PubMed] [Google Scholar]
- Kieny M., Chevallier A. (1979). Autonomy of tendon development in the embryonic chick wing. J. Embryol. Exp. Morphol. 49, 153-165 [PubMed] [Google Scholar]
- Kontges G., Lumsden A. (1996). Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229-3242 [DOI] [PubMed] [Google Scholar]
- Kramer S. G., Kidd T., Simpson J. H., Goodman C. S. (2001). Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science 292, 737-740 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332-336 [DOI] [PubMed] [Google Scholar]
- Kumagai J., Sarkar K., Uhthoff H. K., Okawara Y., Ooshima A. (1994). Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. J. Anat. 185, 279-284 [PMC free article] [PubMed] [Google Scholar]
- Lejard V., Brideau G., Blais F., Salingcarnboriboon R., Wagner G., Roehrl M. H., Noda M., Duprez D., Houillier P., Rossert J. (2007). Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J. Biol. Chem. 282, 17665-17675 [DOI] [PubMed] [Google Scholar]
- Logan M., Martin J. F., Nagy A., Lobe C., Olson E. N., Tabin C. J. (2002). Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77-80 [DOI] [PubMed] [Google Scholar]
- Lorda-Diez C. I., Montero J. A., Martinez-Cue C., Garcia-Porrero J. A., Hurle J. M. (2009). Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J. Biol. Chem. 284, 29988-29996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Zusman S., Li X., Williams E. L., Khare N., DaRocha S., Chiquet-Ehrismann R., Baumgartner S. (1999). wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J. Cell Biol. 145, 191-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison N. D., Price B. A., Conner D. A., Keene D. R., Olson E. N., Tabin C. J., Schweitzer R. (2007). Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697-2708 [DOI] [PubMed] [Google Scholar]
- Nabel-Rosen H., Dorevitch N., Reuveny A., Volk T. (1999). The balance between two isoforms of the Drosophila RNA-binding protein how controls tendon cell differentiation. Mol. Cell 4, 573-584 [DOI] [PubMed] [Google Scholar]
- Nabel-Rosen H., Volohonsky G., Reuveny A., Zaidel-Bar R., Volk T. (2002). Two isoforms of the Drosophila RNA binding protein, how, act in opposing directions to regulate tendon cell differentiation. Dev. Cell 2, 183-193 [DOI] [PubMed] [Google Scholar]
- Oka K., Oka S., Hosokawa R., Bringas P., Jr, Brockhoff H. C., 2nd, Nonaka K., Chai Y. (2008). TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev. Biol. 321, 303-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Reginato A. M., Wang W. (2000). Bone development. Annu. Rev. Cell Dev. Biol. 16, 191-220 [DOI] [PubMed] [Google Scholar]
- Pai A. C. (1965). Developmental genetics of a lethal mutation, muscular dysgenesis (Mdg), in the mouse. Ii. Developmental analysis. Dev. Biol. 11, 93-109 [DOI] [PubMed] [Google Scholar]
- Piepenburg O., Vorbruggen G., Jackle H. (2000). Drosophila segment borders result from unilateral repression of hedgehog activity by wingless signaling. Mol. Cell 6, 203-209 [PubMed] [Google Scholar]
- Pownall M. E., Gustafsson M. K., Emerson C. P., Jr (2002). Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 18, 747-783 [DOI] [PubMed] [Google Scholar]
- Pryce B. A., Watson S. S., Murchison N. D., Staverosky J. A., Dunker N., Schweitzer R. (2009). Recruitment and maintenance of tendon progenitors by TGF{beta} signaling are essential for tendon formation. Development 136, 1351-1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralphs J. R., Benjamin M., Waggett A. D., Russell D. C., Messner K., Gao J. (1998). Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J. Anat. 193, 215-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot-Nikcevic I., Reddy T., Downing K. J., Belliveau A. C., Hallgrimsson B., Hall B. K., Kablar B. (2006). Myf5–/–:MyoD–/– amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev. Genes Evol. 216, 1-9 [DOI] [PubMed] [Google Scholar]
- Schnorrer F., Dickson B. J. (2004). Muscle building; mechanisms of myotube guidance and attachment site selection. Dev. Cell 7, 9-20 [DOI] [PubMed] [Google Scholar]
- Schnorrer F., Kalchhauser I., Dickson B. J. (2007). The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev. Cell 12, 751-766 [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Chyung J. H., Murtaugh L. C., Brent A. E., Rosen V., Olson E. N., Lassar A., Tabin C. J. (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855-3866 [DOI] [PubMed] [Google Scholar]
- Shellswell G. B., Wolpert L. (1977). The pattern of muscle and tendon development in the chick wing. Vertebrate Limb and Somite Morphogenesis (ed. D. A. Ede, J. R. Hincliffe and M. Balls), pp. 71-86 Cambridge, UK: Cambridge University Press; [Google Scholar]
- Shukunami C., Takimoto A., Oro M., Hiraki Y. (2006). Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 298, 234-247 [DOI] [PubMed] [Google Scholar]
- Smith T. G., Sweetman D., Patterson M., Keyse S. M., Munsterberg A. (2005). Feedback interactions between MKP3 and ERK MAP kinase control scleraxis expression and the specification of rib progenitors in the developing chick somite. Development 132, 1305-1314 [DOI] [PubMed] [Google Scholar]
- Subramanian A., Wayburn B., Bunch T., Volk T. (2007). Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development 134, 1269-1278 [DOI] [PubMed] [Google Scholar]
- Swan L. E., Wichmann C., Prange U., Schmid A., Schmidt M., Schwarz T., Ponimaskin E., Madeo F., Vorbruggen G., Sigrist S. J. (2004). A glutamate receptor-interacting protein homolog organizes muscle guidance in Drosophila. Genes Dev. 18, 223-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan L. E., Schmidt M., Schwarz T., Ponimaskin E., Prange U., Boeckers T., Thomas U., Sigrist S. J. (2006). Complex interaction of Drosophila GRIP PDZ domains and Echinoid during muscle morphogenesis. EMBO J. 25, 3640-3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Brown N. H. (2006). An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat. Cell Biol. 8, 601-606 [DOI] [PubMed] [Google Scholar]
- Thomopoulos S., Williams G. R., Gimbel J. A., Favata M., Soslowsky L. J. (2003). Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res. 21, 413-419 [DOI] [PubMed] [Google Scholar]
- Towers M., Tickle C. (2009). Growing models of vertebrate limb development. Development 136, 179-190 [DOI] [PubMed] [Google Scholar]
- Tozer S., Duprez D. (2005). Tendon and ligament: development, repair and disease. Birth Defects Res. C Embryo Today 75, 226-236 [DOI] [PubMed] [Google Scholar]
- Trainor P. A., Tan S. S., Tam P. P. (1994). Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos. Development 120, 2397-2408 [DOI] [PubMed] [Google Scholar]
- Tremblay P., Dietrich S., Mericskay M., Schubert F. R., Li Z., Paulin D. (1998). A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev. Biol. 203, 49-61 [DOI] [PubMed] [Google Scholar]
- Usui K., Pistillo D., Simpson P. (2004). Mutual exclusion of sensory bristles and tendons on the notum of dipteran flies. Curr. Biol. 14, 1047-1055 [DOI] [PubMed] [Google Scholar]
- Volk T. (1999). Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 15, 448-453 [DOI] [PubMed] [Google Scholar]
- Volk T., VijayRaghavan K. (1994). A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development 120, 59-70 [DOI] [PubMed] [Google Scholar]
- Volohonsky G., Edenfeld G., Klambt C., Volk T. (2007). Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development 134, 347-356 [DOI] [PubMed] [Google Scholar]
- Wayburn B., Volk T. (2009). LRT, a tendon-specific leucine-rich repeat protein, promotes muscle-tendon targeting through its interaction with Robo. Development 136, 3607-3615 [DOI] [PubMed] [Google Scholar]
- Yarnitzky T., Min L., Volk T. (1997). The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev. 11, 2691-2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y. S., Lee J. H., Hwang S. C., Choi K. S., Yoon G. (2005). TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 24, 1895-1903 [DOI] [PubMed] [Google Scholar]
- Ypsilanti A. R., Zagar Y., Chedotal A. (2010). Moving away from the midline: new developments for Slit and Robo. Development 137, 1939-1952 [DOI] [PubMed] [Google Scholar]