Abstract
Contingent incentives can reduce substance abuse. Escalating payment schedules, which begin with a small incentive magnitude and progressively increase with meeting the contingency, increase smoking abstinence. Likewise, descending payment schedules can increase cocaine abstinence. The current experiment enrolled smokers without plans to quit in the next 6 months and compared escalating and descending payments schedules over 15 visits. In the larger incentive condition (LI, n = 39), the largest possible incentive was $100, and in the smaller incentive condition (SI, n = 18), the largest possible incentive was $32. In both conditions, more participants in the descending groups initiated abstinence. A higher proportion of participants in both the escalating and descending groups initiated abstinence in the LI than in the SI. Although participants in the descending groups had more abstinent visits during the first five contingent visits than those in the escalating groups, these differences were not maintained.
Keywords: contingency management, cigarette smoking, reinforcement schedules, smoking cessation
Contingency management has been used to decrease substance abuse and increase abstinence in a variety of populations (Higgins & Silverman, 1999; Higgins, Silverman, & Heil, 2008). In particular, contingency management can reduce cigarette smoking, as measured by breath carbon monoxide (CO) levels in adolescents (Corby, Roll, Ledgerwood, & Schuster, 2000); college students (Correia & Benson, 2006); community adults (Stitzer & Bigelow, 1982, 1983); complacent smokers (Lamb, Morral, Galbicka, Kirby, & Iguchi, 2005; Lamb et al., 2007); hard-to-treat smokers (Lamb, Morral, Kirby, Iguchi, & Galbicka, 2004); pregnant women (Higgins et al., 2004); and clinically diagnosed populations, such as individuals with schizophrenia (Roll, Higgins, Steingard, & McGinley, 1998; Tidey, O'Neill, & Higgins, 2002; see Sigmon, Lamb, & Dallery, 2008, for review). These efforts to reduce cigarette smoking are especially significant in light of reports that smoking continues to be the leading cause of morbidity and mortality in the United States (“Annual Smoking-Attributable Mortality,” 2005).
One of the most robust predictors of long-term abstinence in some smoking-cessation programs is abstinence during the first 2 weeks of the program (Garvey, Bliss, Hitchcock, Heinold, & Rosner, 1992; Gourlay, Forbes, Marriner, Pethica, & McNeil, 1994; Kenford et al., 1994; Lamb, Kirby, Morral, Galbicka, & Iguchi, 2004; Yudkin, Jones, Lancaster, & Fowler, 1996). These findings suggest that it might be useful to identify methods to increase the rate of abstinence during this critical period. The most frequent incentive schedule used to reinforce abstinence is a schedule of escalating incentive values under which longer periods of meeting the criterion result in larger incentives (e.g., Higgins et al., 1993; Roll, Higgins, & Badger, 1996; Silverman et al., 1996). Roll et al. compared the duration of sustained abstinence produced by an escalating schedule to a fixed schedule, in which incentive values remained constant throughout the intervention. The authors found that the escalating schedule, with the inclusion of a reset contingency, promoted longer durations of abstinence in smokers who initiated abstinence relative to the fixed schedule. A reset contingency specifies that when a participant fails to meet the criterion, the value of the next incentive is reset to the lowest value in the schedule. Furthermore, to encourage the resumption of abstinence after that failure, meeting the criterion a certain number of consecutive times reinstates the amount of the incentive to its previous highest amount before the reset occurred. A further experiment showed that an escalating schedule of incentive reinforcement with a reset contingency increased sustained abstinence in cigarette smokers initiating abstinence relative to groups either receiving an escalating incentive-value schedule without the reset contingency or a fixed incentive-value schedule (Roll & Higgins, 2000).
A study by Kirby, Marlowe, Festinger, Lamb, and Platt (1998) showed that a descending schedule promoted higher rates of abstinence from cocaine than an escalating schedule. The descending schedule delivered high-value incentives contingent on abstinence and then gradually decreased the value of the contingent incentives over the remainder of treatment. Escalating and descending incentive schedules have also been used in the human laboratory (Donny, Bigelow, & Walsh, 2003, 2004). In one study, participants made choices between a specific dose of cocaine and decreasing amounts of money every 60 min. As the amount of money decreased, the proportion of participants choosing the cocaine injections increased (Donny et al., 2004). By contrast, when participants made choices between a specific dose of cocaine and increasing amounts of money, cocaine choice was independent of monetary value (Donny et al., 2003). Thus, similar to the results of Kirby et al., laboratory choices for cocaine were relatively more sensitive to a descending incentive schedule than an escalating incentive schedule. However, unlike previous contingency management studies (e.g., Kirby et al.; Roll et al., 1996), participants were explicitly given a dose of cocaine (primed) before the choice procedure began. Also, the incentive increase or decrease was within the session and independent of participant choice. These procedural differences may limit the generalizability of the findings to current contingency management procedures.
There is also evidence that higher incentive magnitudes can increase abstinence (Corriea & Benson, 2006; Lamb, Morral, et al., 2004; Paxton, 1981; Stitzer & Bigelow, 1983, 1984). For example, Stitzer and Bigelow randomly assigned participants to one of four groups that offered contingent incentives between $0 and $10 per day for breath CO samples ≤50% of baseline levels. Increasing incentive magnitude led to an orderly reduction in mean breath CO and number of daytime cigarettes smoked, along with increases in the mean time since last cigarette smoked.
The current study compared the effectiveness of escalating and descending incentive schedules at initiating and maintaining smoking abstinence, as measured by breath CO over the course of 4 weeks, in smokers with no immediate plans to quit smoking. We also sought to compare different incentive magnitudes with both escalating and descending incentive schedules. In the first condition, a participant's first contingent incentive either started at $1 and increased with each successive breath CO sample meeting an abstinence criterion of <3 ppm for 15 visits or started at $100 and decreased with each successive breath CO sample meeting an abstinence criterion of <3 ppm for 15 visits. In the second condition, a separate group of participants was used. However, the contingencies were exactly the same as the first condition, except that the incentives increased or decreased only to a maximum of $32 with each successive breath CO sample meeting an abstinence criterion of <3 ppm for 15 visits. Unlike previous studies, no reset contingency was used for either the escalating or descending incentive schedule.
METHOD
Participants
Participants were 60 adults recruited via review-board-approved newspaper, radio, and Internet ads and flyers posted on bulletin boards at local college campuses. To participate, individuals had to provide a breath CO reading of ≥15 ppm at intake, report smoking at least 15 cigarettes per day for at least 2 years, not be using any other tobacco products, not have concrete plans to quit smoking in the next 6 months, be at least 18 years of age, and be able to come to the University of Texas Health Science Center (San Antonio) main campus each workday between 7:00 a.m. and 10:00 a.m. to give a breath CO sample and answer five brief questions about their smoking behavior during the previous 24 hr. Of the 60 participants who provided informed consent, two dropped out before the first incentive visit, and 50 completed the 3-week experimental period with no more than two absences. One participant in the larger incentive condition (LI, detailed below) was retroactively excluded from data analysis based on negligible salivary cotinine levels at intake. Data for the two participants who dropped out before the first visit were also not included in the analysis. Demographic information is shown in Table 1. Participants in the two groups were statistically similar on all intake measures, as judged by t and probability tests.
Table 1.
Table 1 Demographic Information
Procedure
The first 42 participants were placed in the LI, and the last 18 participants were assigned to the smaller incentive condition (SI). In each condition, participants were randomized to either the escalating or descending group by first stratifying each individual by intake breath CO level, which was assessed using a Vitolograph CO monitor. Stratification was accomplished by randomly placing the first participant in the high or low breath CO group. Subsequent participants were placed in the high or low breath CO groups depending on whether their entry breath CO level was above or below the median entry breath CO level collected to date. Participants delivering a sample on the median were assigned to the high or low breath CO groups in an alternating manner. Participants in each breath CO group were then randomized to either the escalating or descending incentive group based on a blocking procedure whereby each block of four participants had to contain two participants randomized to the escalating and two participants randomized to the descending group. This continued until 60 participants had been randomly assigned to two groups of 30 participants each.
The experimenter told each participant which condition and group he or she was assigned to immediately after he or she delivered a breath CO sample during the intake session. At intake, the experimenter gave all participants a detailed description of the experimental procedures before giving informed consent. This included a discussion on the sources of CO, the half-life of breath CO, and the reliability of providing a breath CO sample every 24 hr. Specifically, the experimenter told participants that the half-life of breath CO was 2 to 8 hr (Benowitz et al., 2002), and that most individuals could achieve a breath CO <3 ppm by not smoking for 24 consecutive hours. After giving informed consent, the experimenter asked participants to submit a saliva sample, after which they completed several forms, including a brief self-developed demographics form and a smoking history and attitudes questionnaire. The experimenter gave the participant no advice or strategies to aid attempts to cut down or quit smoking. This was done to increase the participant's attention toward the scheduled incentives instead of other determinants of smoking.
After intake all participants were expected to deliver one breath CO sample each workday (Monday through Friday, excluding holidays) between 7:00 a.m. and 10:00 a.m. at the University of Texas Health Science Center (San Antonio) main campus for 20 consecutive visits. The first 15 visits were considered the contingent incentive phase during which additional monetary incentives could be earned by delivering a breath sample of <3 ppm CO. The first contingent incentive phase visit occurred at least 24 hr but not more than 4 days (including weekends) after intake, so that each participant had adequate time to achieve a breath CO sample of <3 ppm on his or her first visit. The criterion of <3 ppm was chosen based on previous research that indicated that breath CO values in the range of 3 to 6 ppm provide the most sensitive level of measurement (i.e., minimizing both false positives and false negatives) for abstinence from smoking when breath CO tests were administered once per day (Javors, Hatch, & Lamb, 2005). The last five visits were considered the follow-up phase, and monetary incentives contingent on breath CO levels were unavailable. Participants received $1 in cash immediately after submitting a breath CO sample during each visit, regardless of their breath CO level. A missed visit resulted in a missed earning opportunity and could potentially affect the completion bonus. The completion bonus was $100 and could be earned by completing the study with no more than two missed visits during the contingent incentive phase and no missed visits during the follow-up phase. Absences arranged at least 24 hr in advance during the contingent incentive phase did not affect the completion bonus or earning opportunities. Absences with less notice were counted as missed visits and resulted in a missed earning opportunity and a potential loss of the completion bonus. Any absence during the follow-up phase was not excused and disqualified the participant for the completion bonus.
Larger Incentive Condition
Participants randomized into the escalating group in the LI could earn additional monetary incentives (contingent on providing a breath CO sample <3 ppm) during the contingent incentive phase. Incentives started at $1 and could potentially increase up to $100 according to the following visit-by-visit incentive schedule: $1, $2, $3, $4, $5, $6, $9, $12, $16, $22, $29, $40, $54, $74, $100. Submitting a breath CO sample ≥3 ppm resulted in no monetary incentive. A failed breath CO sample (≥3 ppm) did not reset the incentive amount. Thus, if a participant had earned the $9 incentive but failed to earn the $12 incentive during the next visit (with a breath CO ≥3 ppm), then the next visit during the contingent incentive phase in which the participant had a breath CO <3 ppm would result in the $12 incentive. Each earned incentive was paid in cash immediately following that day's breath CO sample.
Participants randomized into the descending group in the LI had the opposite visit-by-visit incentive schedule ($100, $74, $54, $40, $29, $22, $16, $12, $9, $6, $5, $4, $3, $2, $1) during the contingent incentive phase. Incentives started at $100 and could potentially decrease to $1. Submitting a breath CO sample ≥3 ppm resulted in no monetary incentive. The incentive amount was not reset after a failed breath CO sample. Each earned incentive was paid in cash immediately following that day's breath CO sample. The total incentive amount available was $377 for both contingent incentive groups.
Smaller Incentive Condition
The procedure for the SI was the same as in the LI, except that the incentive magnitude was smaller. Participants randomized to the escalating group could earn incentives that started at $1 and could potentially increase up to $32 according to the following visit-by-visit incentive schedule: $1, $2, $2, $3, $3, $4, $6, $6, $8, $10, $13, $16, $20, $25, $32. Participants randomized into the descending group had the opposite visit-by-visit incentive schedule ($32, $25, $20, $16, $13, $10, $8, $6, $6, $4, $3, $3, $2, $2, $1) during the contingent incentive phase. Incentives started at $32 and could potentially decrease to $1. The total incentive amount available was $151 for both contingent incentive groups in the SI.
In the LI, participants in the descending group earned a mean of $242.53 over the course of the 15-visit incentive period. Participants in the escalating group earned, on average, $49.80 over that same period. In the SI, participants in the descending group earned a mean of $48.22. Participants in the escalating group earned a mean of $16.78.
Data Analysis
Kruskal-Wallis one-way analyses of variance were used to examine the influence of each schedule of incentive reinforcement on the mean total number of visits with a breath CO <3 ppm, mean number of visits during the first five visits of the contingent incentive phase with a breath CO <3 ppm, longest duration of consecutive visits with a breath CO <3 ppm, mean number of visits during the follow-up phase with a breath CO <3 ppm, and continuous demographic variables. Chi-square tests were used to test the difference between groups on the proportion of participants able to deliver a breath CO <3 ppm on the 1st day of the contingent incentive phase and to initiate abstinence at any point during the incentive phase. Except for the demographic variables, all tests were one tailed, with the hypothesis that participants in the descending incentive contingency group would have larger means, medians, or proportions than participants in the escalating incentive contingency group. Because of the small number of participants in the SI, Fisher's exact likelihood tests were calculated if a cell was smaller than 5.
RESULTS
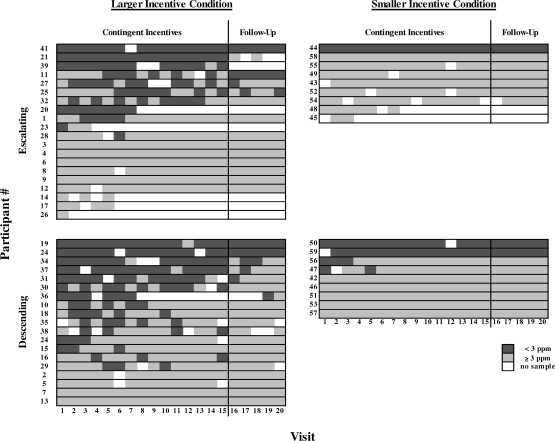
Figure 1 shows the effect of the incentive contingency on each participant's breath CO level across the 20 visits. In general, there is a larger concentration of darker gray at the beginning of the contingent incentive phase for the descending groups relative to the escalating groups, showing that more participants in the descending groups met the breath CO criterion more frequently during the beginning of the incentive phase. On average, participants in the descending groups had a greater number of visits meeting the breath CO criterion during Visits 1 through 5 than did participants in the escalating groups (Kruskal-Wallis; χ2 = 4.06, df = 1, p = .04). However, many participants in the descending groups that met the breath CO criterion early did not maintain this low breath CO level, as shown by the similar amounts of darker gray between the descending and escalating groups by the end of the incentive phase. The mean number of visits meeting the breath CO criterion across the entire incentive phase of the experiment (Visits 1 through 15) was not statistically different between the escalating and descending groups (Kruskal-Wallis; χ2 = 2.26, df = 1, p = .13).
Figure 1.
Event records of participants in the descending and escalating groups. The left panel represents participants in the larger incentive condition (LI), and the right panel represents participants in the smaller incentive condition (SI). Individual participant numbers are given on the ordinate, and visit number is shown on the abscissa. The darker gray areas represent visits with a breath CO <3 ppm, lighter gray areas represent visits with a breath CO ≥3 ppm, and white areas represent missed visits. The black vertical line after Visit 15 separates the contingent incentive phase from the follow-up phase.
During the five-visit follow-up phase, a smaller number of participants met the breath CO criterion relative to the contingent incentive phase in both conditions (Figure 1). In the LI, more participants in the descending group (n = 6) had at least one follow-up visit with a breath CO <3 ppm than participants in the escalating group (n = 4). In the SI, two participants in the descending group and one participant in the escalating group met the breath CO criterion at least once. Combining both conditions, the mean number of visits meeting the breath CO criterion during follow-up was not statistically different between the escalating and descending groups (Kruskal-Wallis; χ2 = 0.88, df = 1, p = .35).
Overall, participants in the escalating groups missed more scheduled visits than those in the descending groups. In the LI, five participants in the escalating group dropped out of the study during the contingent incentive phase, whereas only one participant in the descending group dropped out during the contingent incentive phase (Fisher's exact one tailed; p = .10). In the SI, two participants in the escalating group dropped out during the incentive phase, whereas no participants dropped out of the descending group. The proportion of participants dropping out of the escalating groups was higher than in the descending groups (Fisher's two tailed; p = .05).
The cumulative proportion of participants in each incentive schedule group that delivered a breath CO sample of <3 ppm on at least one visit over the course of the study showed a similar pattern to the total number of abstinent breath CO criterion visits. In the LI, almost half of the participants (n = 9) in the descending group delivered a breath CO sample <3 ppm on Visit 1, whereas only one fourth (n = 5) of the participants in the escalating group met this criterion (Fisher's exact one-tailed probability; χ2 = 2.12, df = 1, p = .13). However, the increase in the cumulative proportion of participants who met the breath CO criterion over the remainder of the contingent incentive phase was approximately the same in both the escalating and descending groups. The cumulative proportion meeting the breath CO criterion reached an asymptote between Visits 5 and 6 for the descending and escalating groups, respectively. Thus, for both groups, no further abstinence was initiated after the 1st week of contingent incentives. There was a similar trend in the SI. Three of the nine participants in the descending groups produced a breath CO sample <3 ppm on the first visit, whereas only one participant in the escalating group met the breath CO criterion. Participants in the SI stopped initiating abstinence earlier than those in the LI, with no participants producing a breath CO sample <3 ppm after Visit 2 in the descending incentive group.
Overall, the cumulative proportion of participants who delivered at least one breath CO sample <3 ppm throughout the incentive phase was greater in the descending groups than in the escalating groups (χ2 = 4.03, df = 1, p = .04). Of the 28 participants in the descending groups, 19 (68%) delivered a breath CO sample <3 ppm at some point during the incentive phase. By contrast, 12 (41%) of the 29 participants in the escalating groups delivered at least one breath CO sample <3 ppm. Likewise, by comparing the cumulative proportion of participants with at least one breath CO sample <3 ppm between conditions, we found that more participants in LI delivered one or more breath CO samples <3 ppm than in the SI (χ2 = 7.51, df = 1, p = .006). Of the 39 participants in the LI, 26 (67%) had at least one breath CO sample <3 ppm, whereas only five (28%) of 18 participants did so in the SI.
DISCUSSION
The present experiment suggests that a descending incentive schedule may promote higher rates of abstinence than an escalating incentive schedule in smokers with no concrete plans to quit smoking. This is similar to the results of Kirby et al. (1998) with cocaine-dependent adults who were seeking to quit. However, because the descending schedules began with a larger incentive than the escalating schedules, it is difficult to determine whether the greater magnitude or the descending schedule controlled abstinence initiation. Indeed, a greater proportion of participants in the LI met the breath CO criterion relative to those in the SI. These results are consistent with previous literature that has shown increasing rates of smoking abstinence with increasing magnitudes of incentives (Corriea & Benson, 2006; Lamb, Morral, et al., 2004; Stitzer & Bigelow, 1983, 1984).
Although more participants in the descending groups produced criterion breath CO levels throughout the 1st week of the contingent incentive phase, abstinence was usually not differentially sustained thereafter (Figure 1). The median number of consecutive abstinent visits was two and zero for the descending and escalating groups, respectively. Thus, participants in the descending groups had the opportunity to maintain abstinence for longer periods of time by virtue of initiating abstinence. However, after those participants who never initiated abstinence were eliminated from the analysis, participants in the escalating groups were better able to maintain abstinence (median = 4.5 consecutive abstinent visits) throughout the contingent incentive phase than were those in the descending groups (median = 3 consecutive abstinent visits). This supports the previous finding that escalating schedules are better at maintaining abstinence once abstinence is initiated (Higgins et al., 1993; Roll & Higgins, 2000; Roll et al., 1996; Silverman et al., 1996), even without a reset contingency. However, in either case, the median duration of abstinence is far from the 2-week rule used to predict long-term abstinence, as described in the introduction.
Several participants in both conditions were able to maintain abstinence after the incentives were withdrawn (four participants in the LI and three participants in the SI). These results may be meaningful, despite the relatively short follow-up phase, because we specifically recruited participants without plans to quit smoking in the next 6 months. Inducing and maintaining abstinence in this population is an important goal, because the majority of smokers will not make even one quit attempt per year (Eisenberg, Stitzer, & Henningfield, 1999). Those that do try to quit on their own typically have success rates between 3% and 8% at 12 months after the initial quit attempt (Cohen, Lichtenstein, & Prochaska, 1989). Greater motivation to quit smoking appears to increase outcomes in smoking cessation programs (Perkins, Stitzer, & Lerman, 2006; Prochaska & DiClemente, 1992; Tang, Law, & Wald, 1994). Thus, it is plausible that the outcomes of the current study may have been enhanced by using smokers with a higher motivation to quit smoking.
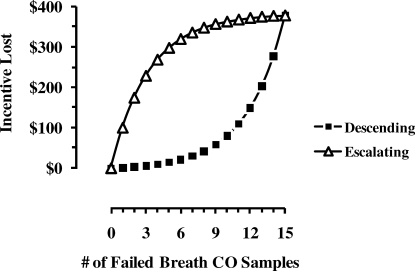
It is also noteworthy that only one participant from either descending group withdrew from the study during the contingent incentive phase, whereas seven participants from the escalating groups withdrew during the contingent incentive phase (Figure 1). Kirby et al. (1998) reported similar results with cocaine-dependent adults. This result may have been a function of a subtle difference in contingencies between the two groups. Figure 2 shows the contingency between failed breath CO samples or unexcused absences and the amount of incentive lost during the 15-visit contingent incentive phase for the LI. Because contingent incentives were limited to the first 15 visits, the last scheduled incentive in the progression was terminated after the first failed breath CO sample or unexcused absence. This was only $1 in the case of the descending contingency (filled squares). However, for the escalating contingency (open triangles), this was $100 ($32 in the SI). Thus, participants in the escalating group automatically forfeited $100 ($32 in the SI) for the first failed breath CO sample or unexcused absence. However, at the beginning of the contingent incentive phase, when this negative reinforcing contingency to avoid incentive losses would have been the greatest, participants in the escalating group submitted fewer criterion breath CO samples than participants in the descending group. Perhaps as a consequence of the mounting losses (relative to small immediate incentives that were available), some participants chose to withdraw from the study. The idea that smoking in contingency management studies may also be sensitive to punishment or negative reinforcement contingencies other than a reset contingency has been mentioned previously (Roll et al., 1996) and tested with some success in a standard escalating incentive schedule (Roll & Howard, 2008).
Figure 2.
Amount of incentive lost as a function of an increasing number of visits not meeting the breath CO criterion during the larger incentive condition (LI). Open triangles represent the escalating incentive contingency. Filled squares represent the descending incentive contingency.
As mentioned above, our experimental design did not allow us to disentangle effects of the schedule from effects of the incentive magnitude. A study by Silverman et al. (1998) showed that the addition of a large start-up bonus incentive did not increase the rate of participants initiating or maintaining cocaine abstinence in a contingency management procedure using an escalating incentive schedule. Future studies will be necessary to disentangle these two factors in descending schedules. For example, it might be informative to include a control condition in which participants receive the same incentive values as in the descending condition but in a random order. If a large proportion of participants met the breath CO criterion when the random incentive was large (i.e., $100), then the current results could be explained more easily by the magnitude of the incentive, instead of the schedule of the incentive.
There are also a few procedural differences between the current experiment and previous research on schedules of reinforcement in smokers. First, the current participants provided only one breath CO sample per day, whereas some of the previous studies scheduled breath CO tests and incentives multiple times per day (Roll & Higgins, 2000; Roll et al., 1996, 1998; Stitzer, Rand, Bigelow, & Mead, 1986). It is plausible that more frequent incentives result in higher rates of abstinence. Second, we used a lower breath CO criterion than most other published research. This breath CO level was based on a prior analysis of breath CO levels and number of cigarettes smoked over a 24-hr period (Javors et al., 2005). Thus, participants in the current experiment would have a chance of receiving incentives after smoking during the previous 24 hr. To insure abstinence and avoid unintended reinforcement of low levels of smoking, it may be beneficial for future contingency management programs to combine more frequent measurements of breath CO levels together with other biological measures, such as cotinine. Recently, a Web-based smoking cessation program (Dallery & Glenn, 2005; Dallery, Glenn, & Raiff, 2007; Reynolds, Dallery, Shroff, Patak, & Leraas, 2008) has produced impressive results by combining frequent breath CO measures with a lower abstinence criterion (<4 ppm). Third, as mentioned in the introduction, the current experiment did not use a reset contingency during the escalating schedules. Because there has been no prior research done with descending schedules and reset contingencies, it is not clear how a reset contingency during a descending schedule would affect behavior. Thus, to eliminate this potential confounding effect, we decided not to use a reset contingency in either schedule.
In conclusion, the current experiment showed that the initiation of abstinence from cigarette smoking can be achieved using an incentive schedule with high-magnitude incentives at the beginning of the intervention. However, as these incentive magnitudes decreased, so did the proportion of abstinent participants. Future investigation should attempt to disentangle the roles that incentive schedule and magnitude play in abstinence initiation. Clear identification of the variables responsible for abstinence initiation should help researchers to build contingency management procedures to promote long-term abstinence.
Acknowledgments
The research reported in this paper was supported by Grant DA013304 to R. J. Lamb. We thank Floyd Jones and Gilbert Holguin for their expert technical assistance.
REFERENCES
- Annual smoking-attributable mortality, years of potential life lost, productivity losses—United States 1997–2001. (2005, July 1) Morbidity and Mortality Weekly Report. 54:625–628. [PubMed] [Google Scholar]
- Benowitz N.L, Jacob P, Ahijevych K, Jarvis M.F, Hall S, et al. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E, Prochaska J.O. Debunking myths about self-quitting. American Psychologist. 1989;11:1355–1365. doi: 10.1037//0003-066x.44.11.1355. [DOI] [PubMed] [Google Scholar]
- Corby E.A, Roll J.M, Ledgerwood D.M, Schuster C.R. Contingency management interventions for treating adolescents: A feasibility study. Experimental and Clinical Psychopharmacology. 2000;8:371–376. doi: 10.1037//1064-1297.8.3.371. [DOI] [PubMed] [Google Scholar]
- Correia C.J, Benson T.A. The use of contingency management to reduce cigarette smoking among college students. Experimental and Clinical Psychopharmacology. 2006;14:171–179. doi: 10.1037/1064-1297.14.2.171. [DOI] [PubMed] [Google Scholar]
- Dallery J, Glenn I.M. Effects of an Internet-based voucher reinforcement program for smoking abstinence: A feasibility study. Journal of Applied Behavior Analysis. 2005;38:349–357. doi: 10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Glenn I.M, Raiff B.R. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence. 2007;86:230–238. doi: 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Donny E.C, Bigelow G.E, Walsh S.L. Choosing to take cocaine in the human laboratory: Effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug and Alcohol Dependence. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Donny E.C, Bigelow G.E, Walsh S.L. Assessing the initiation of cocaine self-administration in humans during abstinence: Effects of dose, alternative reinforcement, and priming. Psychopharmacology. 2004;172:316–323. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Stitzer M.L, Henningfield J.E. Current issues in nicotine replacement. In: Seidman D.F, Covey L.S, editors. Helping the hard-core smoker: A clinician's guide. Mahwah, NJ: Erlbaum; 1999. pp. 137–158. (Eds.) [Google Scholar]
- Garvey A.J, Bliss R.E, Hitchcock J.L, Heinold J.W, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gourlay S.G, Forbes A, Marriner T, Pethica D, McNeil J.J. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. British Medical Journal. 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S.T, Budney A.J, Bickel W.K, Hughes J.R, Foerg F, Badger G. Achieving cocaine abstinence with a behavioral approach. American Journal of Psychiatry. 1993;150:763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Heil S.H, Solomon L.J, Lussier J.P, Abel R.L, Lynch M.E, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine and Tobacco Research. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Silverman K, editors. Motivating behavior change among illicit drug abusers. Washington, DC: American Psychological Association; 1999. (Eds.) [Google Scholar]
- Higgins S.T, Silverman K, Heil S.H, editors. Contingency management in substance abuse. New York: Guilford; 2008. (Eds.) [Google Scholar]
- Javors M.A, Hatch J.P, Lamb R.J. Evaluation of cut-off levels for breath carbon monoxide as a marker for cigarette smoking over the past 24 hours. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Kenford S.L, Fiore M.C, Jorenby D.E, Smith S.S, Wetter D, Baker T.B. Predicting smoking cessation: Who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kirby K.C, Marlowe D.B, Festinger D.S, Lamb R.J, Platt J.J. Schedule of voucher delivery influences initiation of cocaine abstinence. Journal of Consulting and Clinical Psychology. 1998;66:761–767. doi: 10.1037//0022-006x.66.5.761. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Kirby K.C, Morral A.R, Galbicka G, Iguchi M.Y. Improving contingency management programs for addiction. Addictive Behaviors. 2004;29:507–523. doi: 10.1016/j.addbeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Morral A.R, Galbicka G, Kirby K.C, Iguchi M.Y. Shaping reduced smoking in smokers without cessation plans. Experimental and Clinical Psychopharmacology. 2005;13:83–92. doi: 10.1037/1064-1297.13.2.83. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Morral A.R, Kirby K.C, Iguchi M.Y, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Morral A.R, Kirby K.C, Javors M.A, Galbicka G, Iguchi M.Y. Contingencies for change in complacent smokers. Experimental and Clinical Psychopharmacology. 2007;15:245–255. doi: 10.1037/1064-1297.15.3.245. [DOI] [PubMed] [Google Scholar]
- Paxton R. Deposit contracts with smokers: Varying frequency and amount of repayments. Behavior Research and Therapy. 1981;19:117–123. doi: 10.1016/0005-7967(81)90035-8. [DOI] [PubMed] [Google Scholar]
- Perkins K.A, Stitzer M, Lerman C. Medication screening for smoking cessation: A proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Prochaska J.O, DiClemente C.C. Stages of change in the modification of problem behaviors. In: Hersen M, Eisler R.M, Miller P.M, editors. Progress in behavior modification. Sycamore, IL: Sycamore; 1992. pp. 184–214. (Eds.) [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A Web-based contingency management program with adolescent smokers. Journal of Applied Behavior Analysis. 2008;41:597–601. doi: 10.1901/jaba.2008.41-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T. A within-participant comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T, Badger G.J. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T, Steingard S, McGinley M. Use of monetary reinforcement to reduce cigarette smoking of persons with schizophrenia: A feasibility study. Experimental and Clinical Psychopharmacology. 1998;6:157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Roll J.M, Howard J.T. The relative contribution of economic valence to contingency management efficacy: A pilot study. Journal of Applied Behavior Analysis. 2008;41:629–633. doi: 10.1901/jaba.2008.41-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon S.C, Lamb R.J, Dallery J. Tobacco. In: Higgins S.T, Silverman K, Heil S.H, editors. Contingency management in substance abuse. New York: Guilford; 2008. pp. 99–119. (Eds.) [Google Scholar]
- Silverman K, Higgins S.T, Brooner R.K, Montoya I.D, Cone E.J, Schuster C.R, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong C.J, Umbricht-Schneiter A, Montoya I.D, Schuster C.R, Preston K.L. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. Journal of Consulting and Clinical Psychology. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Stitzer M.L, Bigelow G.E. Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addictive Behaviors. 1982;7:403–412. doi: 10.1016/0306-4603(82)90010-7. [DOI] [PubMed] [Google Scholar]
- Stitzer M.L, Bigelow G.E. Contingent payment for carbon monoxide reduction: Effects of pay amount. Behavior Therapy. 1983;14:647–656. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer M.L, Bigelow G.E. Contingent reinforcement for carbon monoxide reduction: Within-participant effects of pay amount. Journal of Applied Behavior Analysis. 1984;17:477–483. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer M.L, Rand C.S, Bigelow G, Mead A.M. Contingent payment procedures for smoking reduction and cessation. Journal of Applied Behavior Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.L, Law M, Wald N. How effective is nicotine replacement therapy in helping people to stop smoking. British Medical Journal. 1994;308:21–26. doi: 10.1136/bmj.308.6920.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey J.W, O'Neill S.C, Higgins S.T. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology. 2002;10:241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Yudkin P.L, Jones L, Lancaster T, Fowler G.H. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. British Journal of General Practice. 1996;46:145–148. [PMC free article] [PubMed] [Google Scholar]