Summary
Homeodomain transcription factors play important roles in the specification and differentiation of neuronal subpopulations. In the cerebral cortex, the expression patterns of Cux-1 and Cux-2 in the medial ganglionic eminence (MGE) suggest a role for these transcription factors in the development of interneurons, a heterogeneous neuronal population. In this report, we describe expression of Cux-1 and Cux-2 proteins in Reelin-secreting interneurons of the cortical plate, but not in calretinin or parvalbumin subpopulations. The role of Cux genes in the development of Reelin positive neurons was studied using Cux-1 and Cux-2 knockout mice. These experiments demonstrate that Cux-1−/−; Cux-2−/− double mutation is embryonically lethal. Although this phenotype is highly penetrant, a small proportion of mice develop to birth (P0). Analysis of these animals demonstrate that expression of Reelin is completely absent in layers II–IV of Cux-1−/−; Cux-2−/− double mutant mice, but it is not affected in the cortex of Cux-1−/− or Cux-2−/− single mutants. No Cux-1−/−; Cux-2−/− double-mutant were collected after P0. Since, GABA-ergic populations mature at late postnatal stages, this did not allow us to analyze the expression of subclass specific markers and define the affected interneuron subpopulations. Our analysis of Cux-1−/−; Cux-2−/− double mutant thus demonstrates essential yet redundant roles for Cux-1 and Cux-2 in specifying Reelin expressing cortical interneurons.
Keywords: cerebral cortex, Cux-1, Cux-2, interneurons
Introduction
Inhibitory GABA-ergic interneurons are a heterogeneous population as defined by their morphology, electrophysiological properties and expression of cellular markers. This diversity reflects distinct neuronal molecular identities and precursor origin. However, little is known about the intrinsic factors that define each interneuron subpopulation and direct the differentiation of their specific precursors.
Interneurons originate in the ventral telencephalon during development and migrate tangentially to reach the cortical plate. Most interneurons are born at the medial ganglionic eminence (MGE), and not the lateral ganglionic eminence (LGE), but some subpopulations are born at the caudal ganglionic eminence (CGE) (reviewed in (Flames and Marin, 2005)).
Reelin is a large extracellular matrix glycoprotein secreted by several populations of neocortical neurons (Alcantara et al., 1998). Most known is the expression of Reelin by the Cajal-Retzius neuronal population of layer I during embryonic development (D'Arcangelo et al., 1995; D'Arcangelo et al., 1997). At postnatal stages, expression of Reelin gradually disappears from layer I and appears in subsets of neurons distributed across cortical layers II–VI (Alcantara et al., 1998). The majority of these Reelin positive neurons in the cortical plate express markers of GABA-ergic interneurons such as somatostatin, neuropeptide Y or calretinin (Alcantara et al., 1998). Thus, Reelin is expressed by GABA-ergic interneurons but does not mark a specific homogeneous interneuron population.
Homeobox transcription factors are involved in embryonic patterning and cell type specification. The transcription factors Cux-1 and Cux-2 are homologous to the Drosophila homeobox gene Cut. In the adult brain, expression of Cux-1 and Cux-2 selectively marks the upper cortical layers (II–IV) of the cerebral cortex, with only a few scattered neurons in the lower layers (V–VI) and the hippocampus expressing Cux-1 and Cux-2 (Nieto et al., 2004). During development, Cux-1 and Cux-2 genes are early markers of neuronal differentiation and are expressed in neural precursors in the telencephalon (Nieto et al., 2004; Zimmer et al., 2004). In the ventral telencephalon, Cux-1 is expressed both in the ventricular zone (VZ) and the subventricular zone (SVZ) of the LGE, MGE and CGE (Nieto et al., 2004). In contrast, Cux-2 marks the SVZ of the MGE and is not expressed in the LGE or the CGE (Nieto et al., 2004; Zimmer et al., 2004). The expression of Cux genes in these ventral telencephalon regions thus suggests possible roles in interneuron differentiation. Moreover, the overlapping expression of Cux-1 and Cux-2 in the MGE indicates possible redundant functions for Cux proteins in neurons originating in this region. A previous report showed that Cux-1 knockout (ko) mice (Cux-1−/−) die shortly after birth and show abnormal growth, but have no phenotype specifically related to the development of the nervous system (Luong et al., 2002). We have generated and analyzed Cux-2 ko mice (Cux-2−/−), which survive normally but present excessive production of upper layer neurons, that packed at abnormally high cellular densities (Cubelos et al., 2007).
Here we report the expression of Cux-1 and Cux-2 by a subpopulation of Reelin expressing interneurons (Alcantara et al., 1998) that occur throughout the cortical plate (layers II–VI) of perinatal wild-type (WT) animals. To investigate the roles of Cux-1 and Cux-2 in the specification of these neuronal subpopulations we set out to analyze Reelin expression in the brains of Cux-1−/− and Cux-2−/− single mutant mice as well as in Cux-1−/−; Cux-2−/− double mutant animals. In the course of these experiments we found that Cux-1−/−; Cux-2−/− double mutation is embryonically lethal, suggesting a function for Cux genes early in embryonic development. However, although this phenotype is highly penetrant, a small proportion of mice develop to birth. Analysis of the expression of upper and lower cortical layer markers, such as Brn-1 and Foxp-1 (Sugitani et al., 2002; Ferland et al., 2003), suggests that the majority of upper and lower pyramidal neurons of the Cux-1−/− and Cux-2−/− single mutants, and of Cux-1−/−; Cux-2−/− double mutant mice, correctly acquire their early laminar identity. In contrast, the development of cortical interneurons was impaired by the loss of Cux function: while Reelin expression in the cortical plate of Cux-1−/− or Cux-2−/− single mutants was not affected, it was absent from cortical layers II–VI of Cux-1−/−; Cux-2−/− double mutant mice. In conclusion, our data indicate novel and important roles for Cux genes in interneuron differentiation.
Methods
Animals
All animal procedures were approved by the Centro Nacional de Biotecnología Animal Care and Use Committee in compliance with National and European Legislation. The generation of Cux-2 null allele (Cux-2−/−) is reported elsewhere (Cubelos et al. 2007). Briefly, a targeting construct was designed to conditionally eliminate exons 22 and 23, which encode the third Cut repeat and part of the homeodomain. Mice carrying the conditional allele (Cux2loxP) were mated with mice expressing CRE recombinase under the human beta actin promoter (Tg (ACTB-CRE)2Mrt deleter mice; Jackson Laboratories) to obtain mice giving germline transmission of the floxed null allele. Cux-2+/− animals were mated to obtain Cux-2 homozygous mutant mice (Cux-2−/−). Cux-1−/− mice have been described previously (Luong et al., 2002)), and were obtained from A.J. van Wijnen (Umass. MA. USA). Animals were maintained on a C57BL6: Swiss Webster background. Morning of the day of the appearance of the vaginal plug was defined as embryonic day (E) 0.5.
Antibodies, immunohistochemistry and histology
Mice were perfused transcardially with 0.1 M phosphate-buffered saline (PBS; pH 7.4) followed by cold 4% paraformaldehyde in PBS. The perfused brains were removed and post-fixed in 4% paraformaldehyde at 4 °C. Brains were embedded in parafin and sectioned (5µm) or were cryoprotected in 30% sucrose in PBS and sectioned on a cryostat to produce either 10–20 µm cryosections on Superfrost plus microscope slides (Fisher Scientific, Pittsburgh, PA) or 50–100 µM floating cryosections. Sections were blocked for 1 h at room temperature (r.t.) with 5% horse serum in PBST (PBS containing 0.5% Triton-X 100; blocking solution) and then incubated for 1 h at r.t. or overnight at 4 °C with primary antibodies diluted in blocking solution. Fluorescent-tagged secondary antibodies (in PBS, 5% horse serum) were applied for 1 hour at r.t., and sections were counterstained with Hoechst 33342 (Molecular probes, Eugene,OR) and mounted in Aqua-polymount mounting medium (Poly-Labo). Peroxidase/diaminobenzidine immunohistochemical (IHC) staining was performed as described (Cubelos et al., 2005). Briefly, after incubation with primary antibody, sections were incubated with biotinylated donkey anti-rabbit IgG (Sigma) for 1 h. Sections were then washed 3 times in PBS, incubated with streptavidin-biotinylated horseradish peroxidase complex, washed, and incubated with 0.1 mg/ml H2O2, 0.5 mg/ml diaminobenzidine in PBS. Sections were mounted in glycerol-gelatin.
The following primary antibodies were used at the dilutions indicated: rabbit polyclonal anti-Cux-1 (clone M222) (1:10) and anti-Brn-1 (1:50) (Santa Cruz Biotechnologies, inc. CA); rabbit polyclonal anti-phospho-histone H3 (pH3) (1:500) (Upstate, Spartanburg, SC); rabbit polyclonal anti-Cux-2 (antibody 356, a gift from Dr. Alex Nepveu of McGill University Health Centre, Canada). Mouse anti-Reelin CR50 (1:50) was kindly provided by M. Ogawa, (RIKEN Brain Science Institute, Wako, Japan) and rabbit anti-Reelin (1:500) and anti-parvalbumin (clone PARV-19) were from Sigma (St. Louis, Missouri). Alexa 488- and 594-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies (Molecular Probes) were applied at 1:500. Before staining for Cux-1, Cux-2, sections were boiled for 30 min in the antigen retrieval vector solution (Vector, CA). This was followed, in the case of staining for BrdU, CldU, IdU and pH3, by 30 min treatment with 2N HCl.
Confocal microscopy and imaging
Confocal microscopy was performed with a Radiance 2100 (Bio-Rad) Laser Scanning System on a Zeiss Axiovert 200 microscope. For fluorescence excitation, an argon ion laser (488 nm), a Krypton-Neon laser (543 nm) and a red diode (637 nm) were employed. The combination of filters used was as follows: a 560 DCLPXR beam splitter and an HQ 515/30 emission filter for detection of Alexa 488; and a 650 DCLPXR beam splitter with an HQ 590/70 for Alexa 594. Sequential images were taken with LaserSharp v5.0 software (Bio-Rad) and analyzed using LaserPix v.4 image software (Bio-Rad).
Results
Cux-1 and Cux-2 label populations of Reelin expressing neurons in the cortical plate
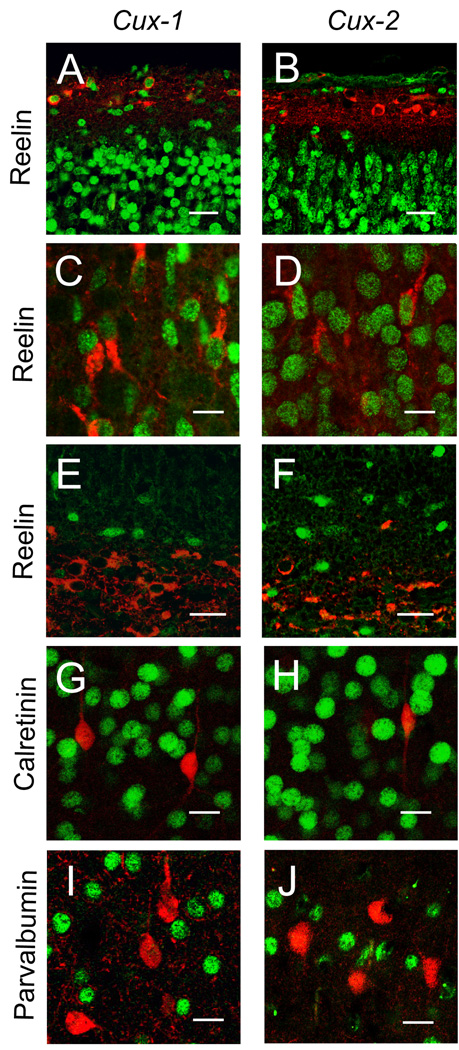
Cux-1 and Cux-2 label the majority of neurons of the upper layers of the cerebral cortex and also scattered neurons distributed throughout the deep layers (Nieto et al., 2004). The expression pattern in the deep layers overlaps with the expression of Reelin in the cortical plate (Alcantara et al., 1998). Reelin is secreted by Cajal-Retzius cells in layer I, which are eliminated during postnatal development, and by cortical neurons that appear at late embryonic and early postnatal stages. To observe both populations of Reelin positive cells, we performed double labeling immunofluorescence studies at P0 to determine whether neurons in this region coexpress Reelin and Cux-1 and Cux-2 proteins. This analysis showed that Cux-1, but not Cux-2, is expressed in the Cajal-Reztius neurons of layer I (Fig 1, panels A, B), and that both Cux-1 and Cux-2 proteins are expressed in Reelin expressing neurons (Fig 1, panels C, D) distributed throughout the layers II–VI. These Cux-positive neurons display a bipolar morphology characteristic of some interneuron populations. Cux-1 and Cux-2 immunoreactivity was also detected in scattered cells in the marginal zone of the hippocampus, but neither Cux protein was localized in Reelin positive cells in this region (Fig 1, panels E, F).
Figure 1. Expression of Cux-1, Cux-2 and Reelin in the mouse cortex.
Panels A and B show double-staining of Cux-1 (A), Cux-2 (B) and Reelin in layer I in the dorsal cortex of P0 animals. Scale bar, 25 µm. Panels C and D show colocalization of Cux-1(C) or Cux-2 (D) and Reelin in neurons of layers II–VI Scale bar, 10 µm. Panels (E and F) show expression of Cux-1 (E) or Cux-2 (F) and Reelin in the marginal zone of the developing P0 hippocampus. Scale bar 20 µm. Double-staining of Cux-1 (G, I), Cux-2 (H, J) and calretinin (G, H) or parvalbumin (I, J) in P21brain. Scale bar, 15 µm
Most of the markers for specific interneuron subpopulations are expressed only in the late postnatal and adult cortex in mature neurons. Double labeling experiments with Cux-1 and Cux-2 rabbit polyclonal antiserums are confined to the availability of antibodies generated in different species. With the aim to define the subpopulation of interneuron that expresses Cux proteins we performed double staining with two markers of interneuron subpopulations, parvoalbumin and calretinin proteins. Neither of these two markers colocalized with Cux-1 or Cux-2 protein (Fig 1, G–J). Thus, we concluded that Cux-1 and Cux-2 are expressed in a subset of cortical interneurons.
Cux-1−/−; Cux-2−/− double mutation is lethal during embryonic development
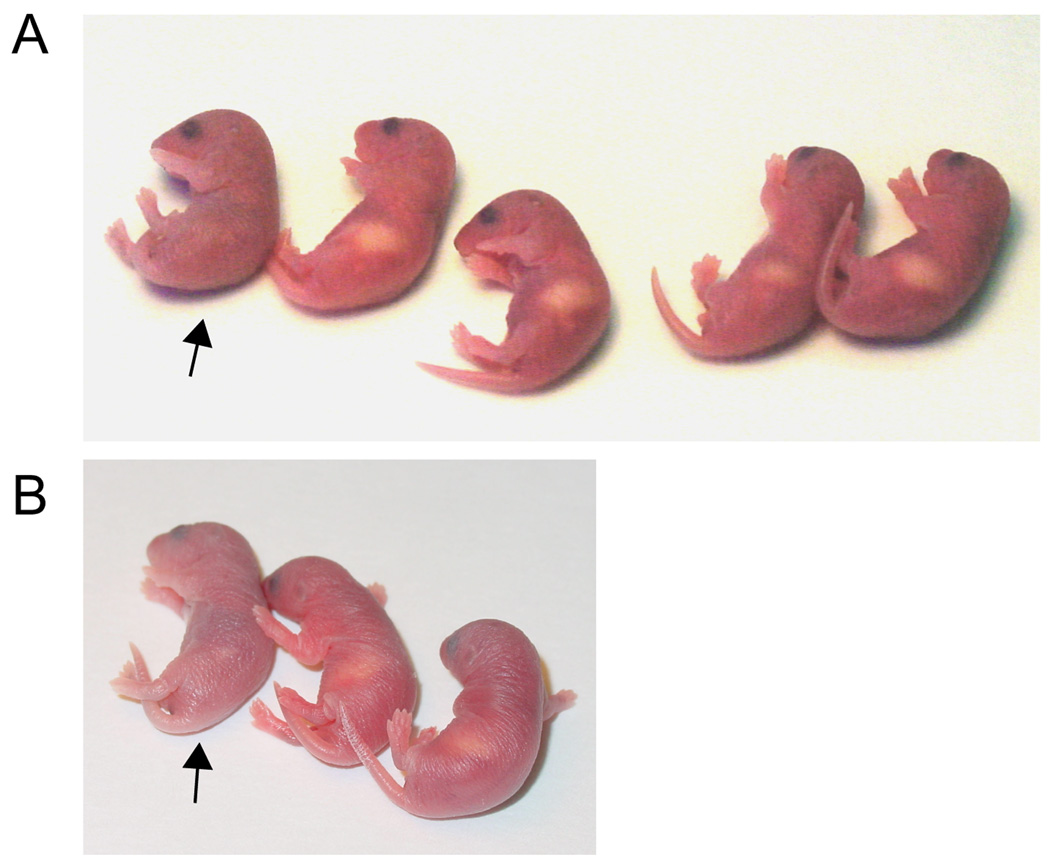
We next set out to analyze the development of Reelin expressing neurons of the dorsal telencephalon in WT, Cux-1−/− and Cux-2 −/− single knockouts and in Cux-1−/−; Cux-2−/− double ko animals. Mice carrying null mutations of one or both Cux genes were generated by crossing Cux-1 and Cux-2 heterozygous mice, and progeny were collected at embryonic and postnatal stages for analysis. Table I shows the numbers of animals collected for each genotype and the expected proportion of animals that would be recovered according to a Mendelian transmission of both alleles. As shown in table I, Cux-1−/− and Cux-2−/− animals were collected at the expected Mendelian ratios at embryonic and postnatal (P0) stages. Only 1.3% of total animals collected were Cux-1−/−; Cux-2−/−, which represents an 80% reduction from the predicted Mendelian proportion (Table I). The lethality of Cux-1−/−; Cux-2−/− double mutation was observed at embryonic stages (E9 to E19) as well as P0. This indicates that Cux-1−/−; Cux-2−/− embryos do not develop normally and die before E19. A few Cux-1−/−; Cux-2−/− were collected at P0 but none after the first postnatal day (Table I). A previous report showed that Cux-1 −/− mice are born normally but that 70% of the animals die within a week (Luong et al., 2002). Accordingly, we observed that Cux-1 mice survive the first postnatal days with no overt phenotype but die subsequently (not shown). In addition, we detected that most Cux-1−/− mice had no milk in their stomachs during the first 8 to 12 hours after birth. In contrast, their wild-type (WT) and Cux-2−/− littermates fed promptly after birth, with milk observed in their stomachs within the first hours (Fig 2, panel A). This feeding problem is overcome after the first postnatal day, and milk was observed as normal in those Cux-1−/− animals that survived beyond P1 (not shown). We reason that Cux-1−/− animals are less able to compete with their littermates for nursing initially, but are eventually able to feed. None of the animals carrying the Cux-1−/−; Cux-2−/− double mutation had milk in their stomachs, and these animals appeared dehydrated, cyanotic, and in a generally bad state (Fig 2 C). Although we did not identify any Cux-1−/−; Cux-2−/− double mutant mice among the animals analyzed after P0 (Table I), the number of animals analyzed at P1–P5 is insufficient to allow determination of survival rates at these stages. Nonetheless, the general bad state of the Cux-1−/−; Cux-2−/− double mutant mice indicates that the very few animals that survive to birth would be unable to survive beyond the first hours.
Table I.
Early embryonic lethality of Cux-1−/−; Cux-2−/− double mutation
|
Cux-1+/?; Cux2+/? |
Cux-1−/−; Cux2+/? |
Cux-2−/−; Cux-1+/? |
Cux-1−/−; Cux2−/− |
Total | |
|---|---|---|---|---|---|
| E9 | 2 | 3 | 2 | 0 | 7 |
| E11–E13 | 10 | 3 | 4 | 0 | 17 |
| E15–E16 | 25 | 5 | 6 | 1 | 37 |
| E19 | 46 | 13 | 15 | 0 | 74 |
| E9–E19 | 83 | 24 | 27 | 1* | 135 |
| P0 | 121 | 49 | 48 | 4** | 222 |
| P1–P5 | 11 | 5 | 4 | 0 | 20 |
| Total | 215 | 78 | 79 | 5*** | 377 |
| Total % | 57% | 20,7% | 21% | 1,3% | 100% |
| Expected | 56% | 18,75% | 18,75% | 6,25% | 100% |
Genotypes and numbers of progeny, collected at different embryonic and postnatal stages, from crosses between Cux-1+/− and Cux-2+/− heterozygotes. Total %: the percentages of each genotype recovered. Expected: the predicted percentages of progeny genotypes according to Mendelian allelic transmission.
p<0.1,
p<0.05,
p<0.01 as compared to the expected Mendelian distribution using a chi square test.
Figure 2. Early embryonic lethality of Cux-1−/−; Cux-2−/− double mutation and nursing defects associated to the Cux-1 phenotype.
(A and B) Photographs showing P0 animals of two litters obtained from breeding Cux-1+/− and Cux-2+/− heterozygous animals. Arrows indicate animals with little or no detectable milk in their stomachs. Subsequent genotyping demonstrated that the animals marked with arrows bear the Cux-1−/− Cux-2+/− null mutation (A) and the Cux-1−/−; Cux-2−/− double mutation (B).
Pyramidal neurons of the cerebral cortex of Cux-1−/−; Cux-2−/− animals normally express markers of their appropriate cortical layers
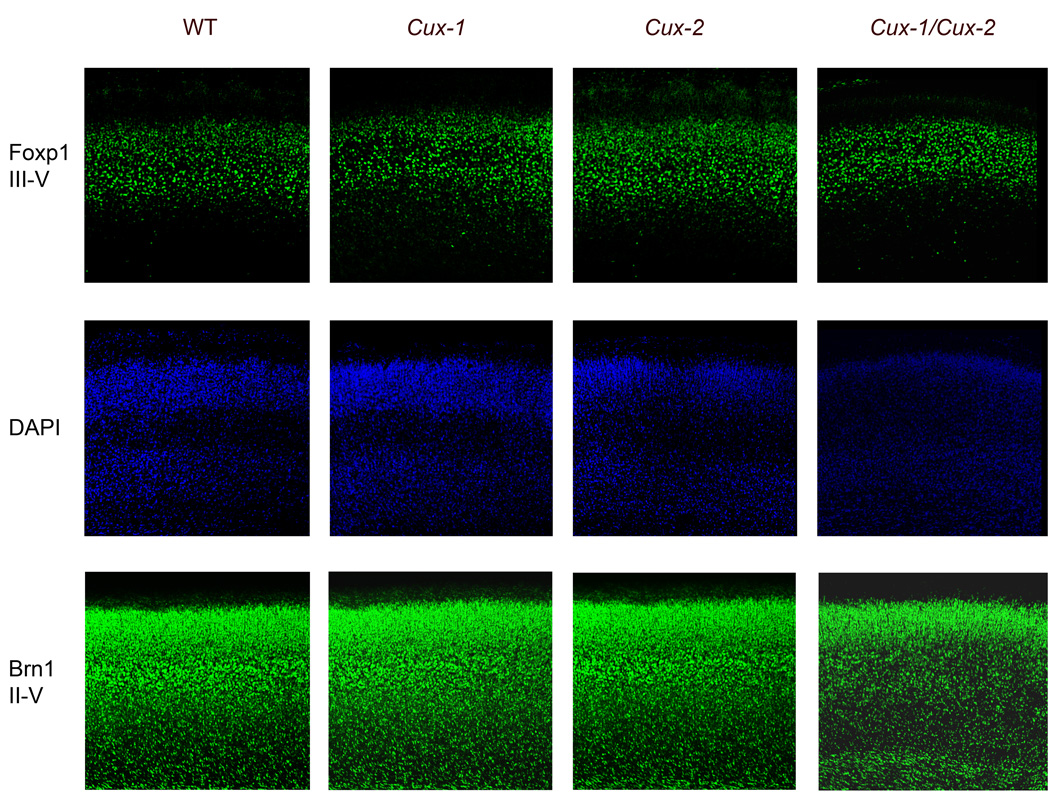
The overlapping expression patterns of Cux-1 and Cux-2 in the cerebral cortex suggest redundancy in their functions during the specification of neuronal populations, and predict that these functions should be revealed in the cerebral cortex of Cux-1−/−; Cux-2−/− double mutants. We therefore investigated possible defects in cortical lamination and in the molecular identity of cortical neurons of the Cux-1−/−; Cux-2−/− mice that escape the lethal phenotype. We analyzed the expression of neuronal markers that are restricted to specific cortical layers. Brn-1 (layers II, III, IV and V) and Foxp-1 (layers III, IV and V) were correctly expressed in WT, Cux-1−/−, Cux-2−/− P0 animals. Expression of Foxp1 and Brn1 was also observed in Cux-1−/−; Cux-2−/− upper layers (Fig 3). We have reported increased cellular density of the upper layer of Cux-2−/− animals (Cubelos et al., 2007). Foxp1 and Brn1 staining revealed defects in fine lamination also in Cux-1−/− and Cux-1−/−; Cux-2−/− double mutants (Fig 3). Increased cellular density, as estimated by the number of Foxp1 positive cells per area, was observed in the upper layers of Cux-1−/− (3521± 356 cells/mm2); Cux-2−/− (4155 ±361 cells/mm2) and Cux-1−/−; Cux-2−/− (3973± 250 cells/mm2) mutants compared to WT (2998 ±135 cells/mm2) (Supplementary Fig 1). Double mutant mice show also reduced thickens of the upper layers (Fig 3). Id-2 (layers II, III, and V) and Tbr-1 (layer VI) were also observed in the expected neuronal subpopulations in all genotypes (data not shown). These results strongly suggest that inside-outside migration of most cortical pyramidal neurons proceeds normally and that the majority of pyramidal neurons acquire their correct molecular identity in Cux-1−/−, Cux-2−/− and Cux-1−/−; Cux-2 −/− mice.
Figure 3. Expression of cortical layer-specific markers in Cux mutant mice.
Immunfluorescence staining showing the expression patterns of Foxp-1 and Brn-1 in the cortical plates of P0 WT and Cux-1−/−, Cux-2−/− and Cux-1−/−; Cux-2−/− mutant mice. Middle panels show nuclear staining corresponding to sections shown in the upper panels.
Reelin expression is absent from neurons of the cortical plate of Cux-1−/−; Cux-2−/− P0 mice
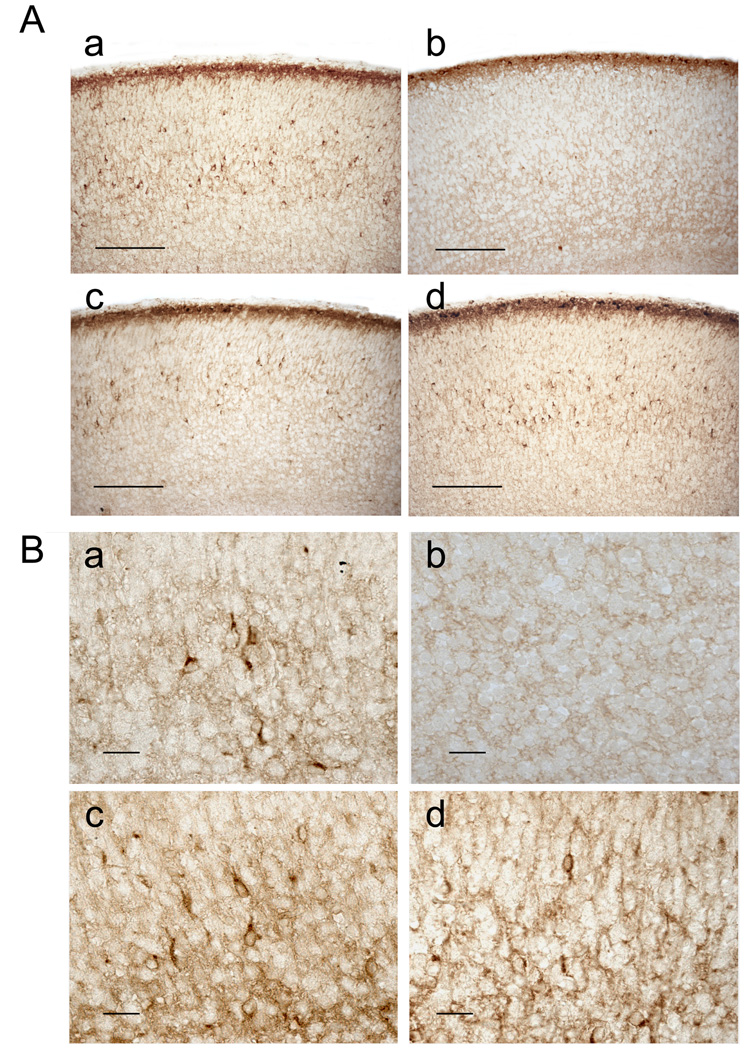
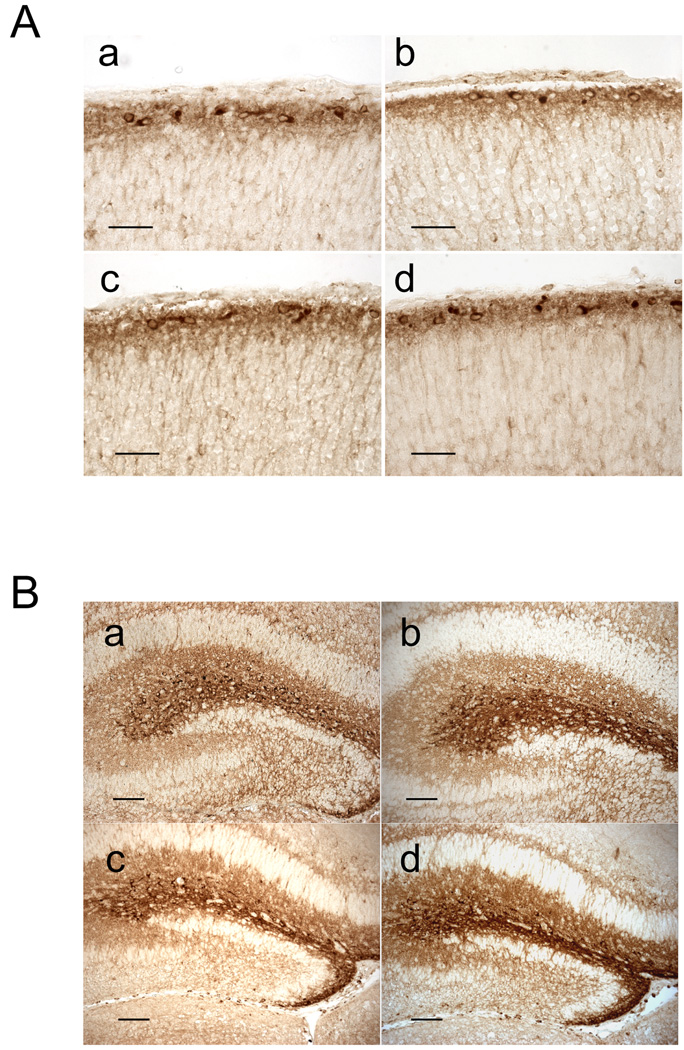
Since Cux-1 and Cux-2 label subpopulations of Reelin expressing neurons, we next analyzed the differentiation of these neurons in the absence of Cux genes. In WT animals, Reelin is abundantly expressed in layer I of the cortical plate, where it is secreted by the Cajal-Retzius neurons, which are strongly immunoreactive for this protein (Fig 4A and 5A, panel a). Reelin is also observed in scattered interneurons distributed throughout layers II–III, IV, V and VI, being more abundant in the deep layers (V and VI) (Fig 4A and B, panel a). In Cux-1−/− and Cux-2−/− single knockout mice, the expression of Reelin is normal both in layer I and in layers II–VI (Fig 4A and B, panels c and d). Reelin is also correctly expressed by Cajal-Retzius neurons of layer I in Cux-1−/−; Cux-2−/− double mutant animals (Fig 4A, panel b and Fig 5A, panel b). But surprisingly Reelin-expressing cells were completely absent from layers II–VI of Cux-1−/−; Cux-2−/− animals (Fig 4A and B, panel b). This phenotype is observed in all regions of the cerebral cortex and at all rostral, caudal, medial and lateral levels (not shown).
Figure 4. Expression of Reelin in P0 Cux mutant mice.
(A and B) Micrographs showing the expression of Reelin in the cortical plate of WT (a), Cux-1−/−; Cux-2−/− (b), Cux-1−/− (c), and Cux-2−/− (d) P0 mice. Panel A shows low magnification images of P0 cortical sections. In all genotypes, Reelin inmunoreactivity is detected in the marginal zone, where it strongly labels Cajal-Reztius cells. Reelin immunoreactivity is also detected in scattered neurons in layers II–VI in WT (a), Cux-1−/− (c) and Cux-2 (d) animals, but is completely absent in layers II–VI of Cux-1−/−; Cux-2−/− double mutant mice (b). Scale bars = 100 µm. (B) High power micrographs showing Reelin expressing cells in the cortical plate of WT (a), Cux-1−/− (c), and Cux-2−/− (d) animals but not in Cux-1−/−; Cux-2−/− double mutant mice (b). Scale bars = 10 µm.
Figure 5. Expression of Reelin in cortical layer I and hippocampus of Cux mutant mice.
(A) Micrographs showing Reelin immunoreactivity in the cortical plate of WT (a), Cux-1−/−; Cux-2−/− (b), Cux-1−/− (c), and Cux-2−/− (d) P0 mice. Scale bars = 50 µm. (B) Micrographs showing Reelin immunoreactivity in the hippocampus of WT (a), Cux-1−/−; Cux-2−/− (b), Cux-1−/− (c), and Cux-2−/− (d) P0 brains. Reelin is strongly expressed in the marginal zone of the hippocampus in mice of all genotypes. Scale bars = 100 µm.
On the other hand, analysis of the hippocampus of single and double Cux knockouts showed that Reelin-expression was present in cells of the hippocampal marginal zone and was indistinguishable across all genotypes (Fig 5B, panels a to d). All together, these results indicate that Cux-1 and Cux-2 genes are involved in the differentiation and/or proliferation of Reelin expressing interneurons of the cortical plate but do not regulate the expression of Reelin in other neuronal populations such as the Cajal-Retzius and hippocampal cells. These findings support the distinct anatomical and molecular origins of the different interneuron subpopulations and situate Cux genes among the players regulating their specific identities.
Discussion
Expression of Cux-1 and Cux-2 homeobox in the developing and adult brain suggests possible functions in the development of the nervous system. Such a role is supported by the reported functions of their invertebrate counterpart Drosophila Cut in neuronal specification (Boulder-Committe, 1970; Bodmer et al., 1987; Brewster et al., 2001; Grueber et al., 2003). In the ventral telencephalon, the expression of Cux-1 and Cux-2 in the MGE suggests a role in interneuron differentiation. However, this possibility has not previously been explored, and the expression of Cux genes in GABA-ergic interneurons has not been reported (Nieto et al., 2004; Zimmer et al., 2004; Butt et al., 2005). Here, we provide the first description of the expression of Cux-1 and Cux-2 in a subpopulation of cortical interneurons. These interneurons are characterized by their expression of Reelin, and we demonstrate that their development depends on redundant functions of the two Cux genes. Unfortunately, most of the specific markers defining GABA-ergic subpopulations appeared only in the late postnatal brain and the lethality of the double mutation did not allow us to further define the specific interneuron population affected.
Our studies demonstrate that Cux-1 and Cux-2 double gene deletion is embryonically lethal as a result of embryonic defects manifest at E19 or earlier. This suggests an earlier function of Cux genes in other tissues, and underlines their importance and the redundancy of their functions in cell fate specification. The cause of this lethality is currently unknown, and the early expression patterns of Cux-1 and Cux-2 from E0 to E8 have not been reported. The reported embryonic expression patterns at subsequent stages do not suggest obvious reasons for the observed embryonic lethality (Quaggin et al., 1996; Vanden Heuvel et al., 1996; Nepveu, 2001; Iulianella et al., 2003; Sharma et al., 2005). Further studies are therefore required to identify the affected tissues and processes in which Cux functions are indispensable for the development of a viable embryo.
Our results provide the first demonstration that Cux-1 and Cux-2 proteins are expressed in neurons of the cortical plate that also express Reelin at P0. These data indicate that Cux transcription factors are expressed in a population of inhibitory interneurons. Previous reports have provided detailed descriptions of the expression of markers of GABA-ergic interneurons in Reelin expressing cells of the cortical plate (Alcantara et al., 1998; Flames and Marin, 2005), whereas Reelin is not secreted by pyramidal neurons of layers II–IV. Hence, this is the first description of the expression of Cux-1 and Cux-2 in GABA-ergic interneurons.
Inhibitory interneurons originate in the ventral portion of the developing telencephalon and subsequently migrate tangentially to the dorsal cerebral cortex. Most of the interneurons are thought to be born from precursors in the MGE. However, strong evidence support that calretinin-expressing interneurons are born in the CGE (Flames and Marin, 2005). We show that parvalbumin and calretinin-expressing interneurons do not express Cux-1 or Cux-2 proteins. In the ventral portion of the developing telencephalon, Cux-2 expression is restricted to the SVZ of the MGE. This expression pattern is clearly suggestive of possible roles in the early specification or proliferation of an interneuronal subpopulation. In contrast, Cux-1 is broadly expressed in the VZ and SVZ of the MGE, LGE and most of the ventral telencephalon (Nieto et al., 2004). While not precluding similar roles, this pattern does not suggest the same level of specificity. The evidence is suggestive of Cux genes functioning specifically in certain MGE derived interneurons, and not in GABA-ergic neurons derived from other regions, although it should be consider the possibility that expression of Cux genes shuts off in the postmitotic interneuron. They also support the different anatomical origin of calretinin-expressing interneurons. Having found evidence for Cux-1 and Cux-2 expression in Reelin interneurons, we analyzed the expression Reelin in the telencephalon of Cux mutants. We found that expression of Reelin in the telencephalon of Cux-1−/− and Cux-2−/− single mutant animals is not affected in layer I, layers II–VI, or in the hippocampus. In contrast, Reelin expression is specifically and completely lost in layers II–VI in Cux-1−/−; Cux-2−/− double mutant P0 animals, but not in the hippocampus or the Cajal-Retzius cells of layer I. Thus, loss of either Cux-1 or Cux-2 can be compensated for by the corresponding homolog in Reelin expressing interneurons, but these neurons require Cux function for their correct development.
Our data thus indicate redundant and essential functions for Cux-1 and Cux-2 in the development of an interneuron subpopulation. At present, it is not clear if the absence of Reelin-positive interneurons from the cortical plate in Cux-1−/−; Cux-2−/− double mutant animals is due to a failure of these cells to develop or to later differentiation defects. Failure of these interneurons to develop would indicate early specification problems, proliferation defects or alterations to migration routes. Regarding migration, it is not known whether GABA-ergic interneurons express Reelin in the course of their migration through layer I, and the SVZ, but we did not observe any accumulation of Reelin expressing cells in any regions of the ventral telencephalon. Alternatively, our data could be interpreted as indicating that Cux-1 and Cux-2 regulate later aspects of neuronal differentiation and control expression of Reelin. In summary, although it remains to be determined whether Cux genes control early or late aspects of neuronal differentiation, the current report provides the first unequivocal demonstration that Cux-1 and Cux-2 play an important role in interneuron development.
Supplementary Material
number of Foxp1 positive cells per area in layers II–III as estimated by the analysis of histological sections of the cerebral cortex of WT, Cux-1−/−, Cux-2−/− and Cux-1−/−; Cux-2−/− animals. *p<0,05; **<0,01 compared to WT.
Abbreviations
- E
embryonic
- Ko
knockout
- LGE
lateral ganglionic eminence
- MGE
medial ganglionic eminence
- P
postnatal
- SVZ
subventricular zone
References
- Alcantara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R, Barbel S, Sheperd S, Jack JW, Jan LY, Jan YN. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell. 1987;51:293–307. doi: 10.1016/0092-8674(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Boulder-Committe. Embryonic vertebrate central nervous system: revised terminology. The Boulder Committee. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Brewster R, Hardiman K, Deo M, Khan S, Bodmer R. The selector gene cut represses a neural cell fate that is specified independently of the Achaete-Scute-Complex and atonal. Mech Dev. 2001;105:57–68. doi: 10.1016/s0925-4773(01)00375-6. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Sebastian-Serrano A, Kim S, Moreno-Ortiz C, Redondo JM, Walsh CA, Nieto M. Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cereb Cortex. 2007 Nov 21; doi: 10.1093/cercor/bhm199. doi 10. 1093. CerCor/bhm199. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- Flames N, Marin O. Developmental mechanisms underlying the generation of cortical interneuron diversity. Neuron. 2005;46:377–381. doi: 10.1016/j.neuron.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Iulianella A, Vanden Heuvel G, Trainor P. Dynamic expression of murine Cux2 in craniofacial, limb, urogenital and neuronal primordia. Gene Expr Patterns. 2003;3:571–577. doi: 10.1016/s1567-133x(03)00123-6. [DOI] [PubMed] [Google Scholar]
- Luong MX, van der Meijden CM, Xing D, Hesselton R, Monuki ES, Jones SN, Lian JB, Stein JL, Stein GS, Neufeld EJ, van Wijnen AJ. Genetic ablation of the CDP/Cux protein C terminus results in hair cycle defects and reduced male fertility. Mol Cell Biol. 2002;22:1424–1437. doi: 10.1128/mcb.22.5.1424-1437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu A. Role of the multifunctional CDP/Cut/Cux homeodomain transcription factor in regulating differentiation, cell growth and development. Gene. 2001;270:1–15. doi: 10.1016/s0378-1119(01)00485-1. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Quaggin SE, Heuvel GB, Golden K, Bodmer R, Igarashi P. Primary structure, neural-specific expression, and chromosomal localization of Cux-2, a second murine homeobox gene related to Drosophila cut. J Biol Chem. 1996;271:22624–22634. doi: 10.1074/jbc.271.37.22624. [DOI] [PubMed] [Google Scholar]
- Sharma M, Brantley JG, Alcalay NI, Zhou J, Heystek E, Maser RL, Vanden Heuvel GB. Differential expression of Cux-1 and p21 in polycystic kidneys from Pkd1 null and cpk mice. Kidney Int. 2005;67:432–442. doi: 10.1111/j.1523-1755.2005.67099.x. [DOI] [PubMed] [Google Scholar]
- Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–1765. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel GB, Bodmer R, McConnell KR, Nagami GT, Igarashi P. Expression of a cut-related homeobox gene in developing and polycystic mouse kidney. Kidney Int. 1996;50:453–461. doi: 10.1038/ki.1996.336. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
number of Foxp1 positive cells per area in layers II–III as estimated by the analysis of histological sections of the cerebral cortex of WT, Cux-1−/−, Cux-2−/− and Cux-1−/−; Cux-2−/− animals. *p<0,05; **<0,01 compared to WT.