Abstract
Background:
Successive elderly birth cohorts improved in cognitive performance during the 20th century. It is not clear whether this influences cognitive predictors of dementia and mortality.
Objective:
In 2 longitudinal population studies, representing 2 cohorts of 70-year-olds examined 30 years apart, we investigated the relation between baseline cognitive function and 5-year occurrence of dementia and mortality.
Methods:
Two representative cohorts of 70-year-olds initially free from dementia born in 1901–1902 (cohort 1901–1902: n = 381) and 1930 (cohort 1930: n = 551) from Gothenburg, Sweden, were examined in 1971–1972 and 2000–2001 and after 5 years for the outcome of dementia and death. Recent memory was evaluated during psychiatric examinations, and nonmemory domains using psychometric tests.
Results:
At age 70, cohort 1930 performed better on psychometric tests, and had fewer recent memory problems compared to cohort 1901–1902. During 5-year follow-up, 5.0% in cohort 1901–1902 and 4.4% in cohort 1930 (p = 0.742) developed dementia, and 15.7% in cohort 1901–1902 and 4.4% in cohort 1930 died (p < 0.001). Recent memory was associated with incident dementia in both cohorts. Low scores in nonmemory tests were associated with incident dementia in cohort 1901–1902, but not in cohort 1930. Recent memory problems and lower scores in nonmemory tests were associated with 5-year mortality in cohort 1901–1902, but not in cohort 1930.
Conclusions:
Secular changes in cognitive performance may influence cognitive predictors of dementia and mortality, despite similar incidence of dementia. The findings should be taken cautiously due to differences between cohorts in refusal rates, quality of education, and dementia recognition in medical records.
GLOSSARY
- CI
= confidence interval;
- DSM-III-R
= Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised;
- OR
= odds ratio.
Life expectancy increases worldwide have led to a dramatic increase in the number of elderly. Mild cognitive impairment is common in the elderly, and is associated with increased risk of dementia1–7 and death.8–12 During the 20th century, later-born cohorts performed better on cognitive tests than earlier-born cohorts.13–15 Later-born cohorts may thus have better cognitive reserve that protects against or delays dementia onset,15,16 and decreases the influence of impending death on cognitive function. It remains to be further elucidated whether secular changes in cognitive functions influence whether cognitive ability predicts dementia and mortality.
Our objective was to examine the incidence of dementia and death, and the relation between cognitive function at baseline and 5-year occurrence of dementia and death in 2 cohorts of 70-year-olds examined in 1971–1972 and 2000–2001.
METHODS
The Longitudinal Gerontological and Geriatric Population Studies in Gothenburg (H70) started in 1971–1972 with an examination of a representative population sample of 70-year-olds born in 1901–1902. In 2000–2001, another population sample of 70-year-olds born in 1930 was examined with identical instruments to study secular trends in health and health-related factors. Both samples included people living in private households and in institutions, and were systematically obtained from the Swedish Population Register, which contains names and addresses of all residents in Sweden. Both samples were followed up at age 75.
Cohort 1901–1902.
Seventy-year-olds born between July 1, 1901, and June 30, 1902, on dates ending with 2, 5, or 8 were invited to health examinations in 1971–1972.17 The individuals received consecutively a number from 1 to 5. Those with numbers 1 and 2 (n = 460) were invited to take part in psychiatric examinations. Of these, 392 (response rate 85.2%) participated (226 women and 166 men). The sample has been described in detail previously.18 Ten participants were excluded due to dementia, and 1 due to dysphasia, leaving 381 70-year-olds without dementia (221 women and 160 men). At the follow-up at age 75 years (average follow-up 5.0 ± 0.2 years), 60 individuals (15.7%) had died and 25 (6.6%) refused new examinations, leaving 296 participants (183 women and 113 men).
Cohort 1930.
Seventy-year-olds born between January 1 and December 31, 1930, on day 3, 6, 12, 18, 21, 24, or 30, were invited to a health examination in 2000–2001. Of 850 invited, 579 (response rate 68.1%) participated in the psychiatric examination (350 women and 229 men). Fourteen participants were excluded due to dementia and 1 due to language difficulties, leaving 564 70-year-olds without dementia (337 women and 227 men). Thirteen individuals (2.3%) had moved from Gothenburg, and lacked follow-up data. These were excluded, leaving 551 individuals (331 women and 220 men) at baseline. At the follow-up at age 75 years (average follow-up 4.9 ± 0.3 years), 24 individuals (4.4%) had died and 91 (16.5%) refused a new examination, leaving 436 participants (274 women and 162 men).
Those lost to follow-up (deceased and refusals) were traced for dementia in medical records and in the Swedish Hospital Discharge Register.
Participants and nonparticipants at baseline in both cohorts were similar regarding gender, marital status, and 3-year mortality rate.19 Participants and nonparticipants in cohort 1901–1902 were further compared regarding income, municipal rent allowance, previous outpatient or inpatient psychiatric care, and registration for alcohol abuse. There were no significant differences.18,20 Participants and nonparticipants in cohort 1930 were compared with regard to inpatient psychiatric care during the past 2 years according to the Swedish Hospital Discharge Register. No differences were found.19
Participants were examined at a geriatric outpatient clinic in Gothenburg. Similar or identical instruments were used at all examinations. Those who declined examination at the outpatient clinic were offered home visits.
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained from participants or their relatives. The study was approved by the Ethics Committee for Medical Research at the University of Gothenburg.
Mental and cognitive assessments.
Recent memory was evaluated during psychiatric examinations, and short-term memory/attention and nonmemory domains (verbal ability, visuospatial ability, perceptual speed, and logical reasoning) in psychometric testing.
Psychiatric examinations included the Comprehensive Psychopathological Rating Scale,21 a structured rating scale including 40 reported and 27 observed psychiatric symptoms, and ratings of cognitive functions. Observed recent memory was rated on a 7-step scale, with 0 score assigned to unimpaired performance and 1 to 6 reflecting increasing levels of impaired performance. It was performed by psychiatrists at the examinations in 1971–1972 and 1976–1977, and by experienced psychiatric nurses at examinations in 2000–2001 and 2005–2006. Interrater reliability was high between psychiatrists18,20,22 and between psychiatrists and psychiatric nurses.23 The psychiatric nurses were supervised and trained by a psychiatrist (I.S.), who was trained by the psychiatrists who examined cohort 1901–1902 in 1971–1972 and 1976–1977.
Psychometric tests were administered by psychologists in 1971–1972 and in 2000–2001. The psychometric tests have been described previously.24–26 Briefly, a battery of 6 cognitive tests was used:
Digit Span Forward measures short-term memory and attention. Tasks require immediate recall (forward) of increasingly longer lists of digits (maximum score 9).
Digit Span Backward measures short-term memory and attention. Tasks require immediate recall (backward) of increasingly longer lists of digits (maximum score 8).
Synonyms measures verbal ability. Participants identify 1 synonym among 5 given alternatives from a list of 30 words (maximum score 30).
Block Design measures spatial ability. Participants are asked to organize wooden cubes in accordance with 7 patterns presented on cards (maximum score 42).
Figure Classification measures inductive reasoning ability. From a set of 5 figures, participants identify the figure constructed on a principle not shared by the other 4 (maximum score 30).
Identical Forms measures perceptual speed, which also is a facet of executive function. Participants are instructed to identify the pattern identical to the stimulus pattern (maximum score 60).
In cohort 1901–1902, examined in 1971–1972, the Synonym and Figure Classification tests were administered to all participants, while the Digit Span tests (Forward and Backward), Block Design, and Identical Forms were systematically administered to every second participant.24 In cohort 1930, examined in 2000–2001, all psychometric tests were administered to every second participant. There were no differences in demographics or incident dementia, or in the results on Synonym and Figure Classification tests in cohort 1901–1902, between those administered the psychometric battery and the rest of the sample.
Dementia.
Dementia was diagnosed according to the historical criteria described by Kay et al.,27 which were widely used at the time of the examinations of cohort 1901–1902 in 1971–1972 and 1976–1977.18,20,28 These criteria required the presence of severe disorientation for time or place or severe memory impairment as assessed during the psychiatric examination. In cohort 1930, dementia was diagnosed according to both historical and DSM-III-R criteria. Observed agreement between historical and DSM-III-R criteria in cohort 1930 was high (0.807).29
For those lost to follow-up (deceased and refusals), psychiatrists examined medical records from all major hospitals, geriatric and psychiatric institutions, and outpatient services in Gothenburg. Swedish Hospital Discharge Register and death certificates were also used. The diagnosis of dementia was made if medical records revealed impairments of memory and other cognitive functions producing significant difficulties in activities of daily living. Information regarding dementia diagnoses was available for all participants since almost all people in Sweden have access to public health services and therefore have equal chances to have medical records or to be in the hospital discharge register.
Mortality.
Dates of death were obtained from the Swedish Population Register, which is a national register covering all people living in Sweden and Swedish citizens living abroad.
Statistical analyses.
Differences in raw mean scores of psychometric tests were tested using univariate analyses of covariance with sex and education as covariates. Using similar methods as previously,6,30 low performance in recent memory during the psychiatric examination was defined as the presence of any symptoms or failures (scores >0). Differences in proportions were evaluated using Fisher exact test and logistic regression models adjusted for sex and education. Similar logistic regression analyses estimated odds of dementia (odds ratio [OR]) over the 5-year follow-up within each cohort (OR with a 95% confidence interval [CI]). Educational level was dichotomized as compulsory education (6 years if born 1901–1902, and 7 years if born in 1930) vs more than compulsory education. There was no interaction between cohort and education. A 2-tailed level of significance, p < 0.05, was used for all tests. Participants were included in the analyses for which they provided valid data; no data were imputed.
RESULTS
Baseline data.
Ten (2.6%) 70-year-olds had dementia in cohort 1901–1902 and 14 (2.4%) in cohort 1930 (p = 1.000). These individuals were excluded from further analyses. Demographic characteristics and results of psychometric testing at age 70 without dementia for each cohort are presented in table 1. There were no differences regarding sex or auditory and visual disabilities between the cohorts. A larger proportion of cohort 1930 had higher education and a lower proportion self-reported memory problems than in cohort 1901–1902.
Table 1 Demographic characteristics and cognitive performance in 70-year-olds without dementia representing 2 birth cohorts
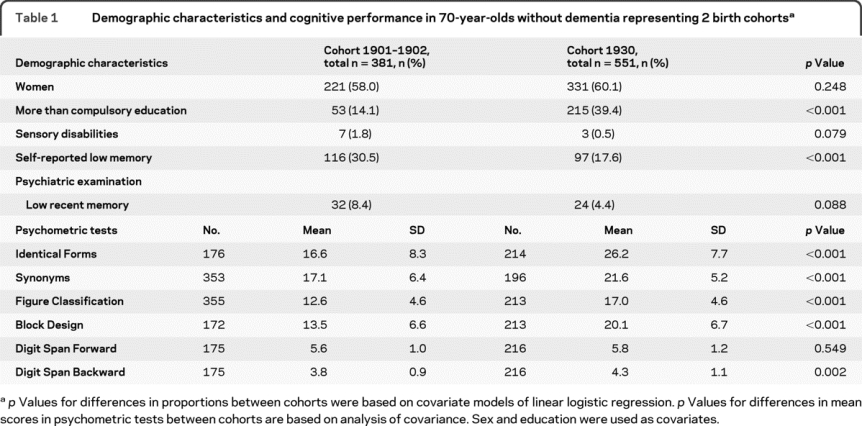
Low performance in recent memory observed in the psychiatric examination tended to be more common in cohort 1901–1902 than in cohort 1930 (table 1). Mean scores on all psychometric tests (except Digit Span Forward) were higher in cohort 1930 than in cohort 1901–1902 among 70-year-olds without dementia, independent of educational level. There was no interaction of education by cohort regarding psychometric test results, and stratification by education revealed similar differences between cohort 1901–1902 and cohort 1930 among those with low and high education (data not shown).
Follow-up data.
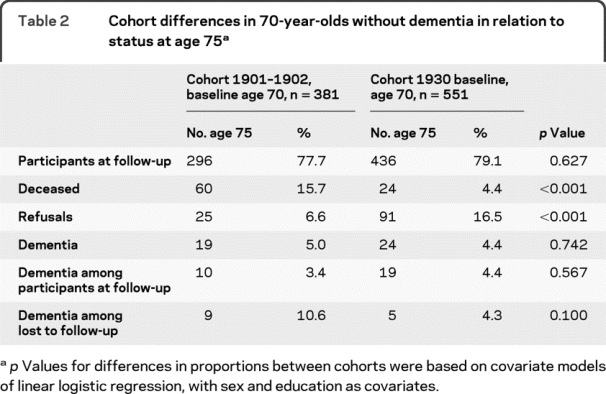
From age 70 to 75, mortality rate was higher and refusal rate lower in cohort 1901–1902 compared to cohort 1930 (table 2). There were no cohort differences regarding incidence of dementia. In cohort 1901–1902, 10 cases were diagnosed with dementia at the examination at age 75, and 9 from medical records or registry data. In cohort 1930, 19 cases were diagnosed at examination at age 75, and 5 from medical records or registry data.
Table 2 Cohort differences in 70-year-olds without dementia in relation to status at age 75
Secular trends in cognitive performance in relation to mortality and refusal at follow-up.
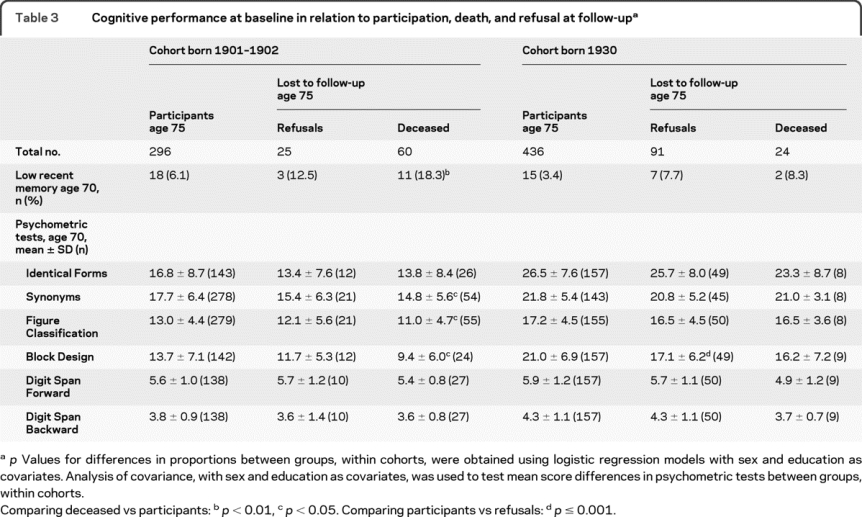
Those who died between age 70 and 75 in cohort 1901–1902 more often had observed recent memory problems and performed worse on Synonyms, Figure Classification, and Block Design at baseline, while no such differences were observed in cohort 1930 (table 3). These results did not change if patients with dementia were excluded. The only difference between refusals and participants was that refusals in cohort 1930 performed worse on Block Design.
Table 3 Cognitive performance at baseline in relation to participation, death, and refusal at follow-up
Secular trends in demographic characteristics in relation to incident dementia.
In cohort 1901–1902, men were more likely to develop dementia than women (8.1% vs 2.7%, OR 3.1, 95% CI 1.2–8.5), while male sex was not related to dementia occurrence in cohort 1930 (4.5% vs 4.2%, OR 1.0, 95% CI 0.4–2.4). Education was not related to dementia development in either cohort.
Secular trends in cognitive performance in relation to incident dementia.
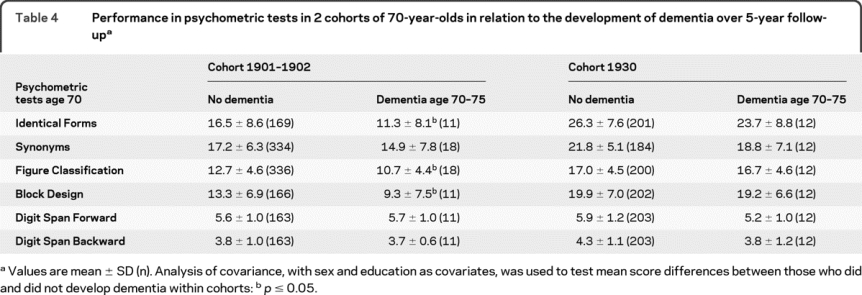
Seventy-year-olds with low performance in recent memory at the psychiatric examination were more likely to develop dementia between age 70 and 75 years than those who were unimpaired, both in cohort 1901–1902 (21.1% vs 7.8%; OR 3.5, 95% CI 1.1–11.8) and in cohort 1930 (20.8% vs 3.6%; OR 6.7, 95% CI 2.2–20.3), after adjustment for sex and education. In cohort 1901–1902, mean scores in Identical Forms, Figure Classification, and Block Design were lower among those who developed dementia compared to those who did not. The mean scores in Synonyms showed the same tendency. No such differences were observed in cohort 1930 (table 4).
Table 4 Performance in psychometric tests in 2 cohorts of 70-year-olds in relation to the development of dementia over 5-year follow-up
DISCUSSION
Dementia incidence between age 70 and 75 years did not differ between 2 birth cohorts examined in 1971–1972 and 2000–2001, while mortality rate decreased substantially. Mild disturbance in recent memory was related to dementia development over 5 years among 70-year-olds in both cohorts. However, while several nonmemory psychometric tests were associated with dementia development in 70-year-olds examined in 1971–1972, this association was not observed in 70-year-olds examined in 2000–2001. Similarly, disturbance in memory and low performance in nonmemory psychometric tests were associated with mortality in those examined in 1971–1972, while no such associations were observed in 2000–2001. It remains to be elucidated whether specific nonmemory tests are becoming less important as early signs of dementia and whether cognitive symptoms to a lesser extent predict mortality in later-born cohorts. The findings should be interpreted cautiously due to the considerable differences between cohorts, and the complex interplay between higher mortality and the possibility of lower dementia recognition by practitioners in the earlier cohort, together with higher refusal rates in the later cohort. Key limitations also include differences in quality of education and quality of the medical records regarding information on dementia between the 1970s and 2000s. The neuropsychological test scores and their predictive value may be especially subject to these biases.
Before discussing the findings in more detail, limitations need further consideration. First, response rate in the 1970s was higher than in the examinations 30 years later. It is thus possible that some of the differences between the birth cohorts may be due to selective participation. However, participants and refusals across cohorts were similar regarding most background factors, including performance on all tests except Block Design. Second, neuropsychological test results are related to educational level. In cohort 1901–1902, information on education was collected as a dichotomous variable, i.e., compulsory education vs more than compulsory, not as actual years of education. However, none had less than compulsory education in either cohort. Thus, years of education do not vary in the majority of cases. Furthermore, in addition to different educational levels, education quality may have changed over time. Third, longitudinal studies suffer from attrition to follow-up. We used medical records and registry data to diagnose dementia in those lost to follow-up. Although insensitive for detection of dementia, the use of this information gives better estimates of dementia incidence than if only participants at follow-up were diagnosed. At the same time, the recognition of dementia in medical records may have changed over the 30-year period. Fourth, some negative results may be due to low statistical power, as only systematic subsamples were tested psychometrically and dementia incidence at age 70–75 years is low. However, Identical Forms and Block Design were administered to more persons in cohort 1930 than in cohort 1901–1902. Therefore, low statistical power does not explain the lack of association with dementia and mortality in these tests in cohort 1930. Fifth, cognitive predictors of dementia6,30 and death11 may differ by age. Thus, our results cannot be generalized to predictors of dementia and mortality at older ages. Sixth, to compare the 2 cohorts, we used dementia criteria from the early 1970s. However, agreement between DSM-III-R and historical criteria is high.29 Seventh, assessment of memory performance was based on clinical judgment. However, this variable predicted dementia in both cohorts, and we have previously reported that the positive predictive values for predicting dementia are similar for memory observed at psychiatric examinations as for memory assessed with psychometric testing.30 Eighth, psychiatric examinations were performed by psychiatrists in the 1970s and by psychiatric research nurses 30 years later. However, interrater reliability between nurses and psychiatrists was high.29 Strengths of this study are extensive examinations performed with identical methods over 30 years in representative population samples, and the completeness of the Swedish Population Register for measuring mortality.
It is noteworthy that while mortality decreased substantially, the incidence of dementia was similar over 30 years, in agreement with a recent study comparing prevalence of dementia in 1992 and 2001.31 At the same time, cognitive performance improved dramatically over 30 years, as also reported by others.15,16,32,33 Furthermore, the later-born cohort had less memory impairment observed at psychiatric examinations and reported fewer memory problems. The later-born cohort also evidenced a considerably higher proportion with higher education, but this did not explain the results. Perhaps later-born cohorts have better cognitive function also due to other factors, such as better prenatal and perinatal care, nutrition, treatment of vascular and other diseases, and because they experienced a consistently higher mental effort and more mental stimulation throughout life.15,32,34 It has also been suggested that maternal education influences cognitive function in later life,35 and this influence may be more accentuated in cohort 1901–1902. Thus, even in cases of pending dementia or death, the brain's capacity to adapt might have increased in later-born cohorts.
Many studies report that individuals developing dementia perform worse on psychometric tests more than 10 years before disease onset.36–39 We have previously reported that only isolated low memory performance predicts dementia in the long term,6 while a global pattern of low performance predicts dementia in the short term,30 which is in line with the theoretical model of a slowly progressive course starting with memory impairments and followed by impairments in other cognitive domains. It has been suggested that individuals with better cognitive function develop dementia later, but decline faster when they develop dementia.40 Thus, later-born cohorts may exhibit a shorter prodromal phase of dementia. This may explain why only memory was a predictor of dementia in cohort 1930, while a global pattern predicted dementia in cohort 1901–1902. Decline in abstract reasoning36,37 and visuospatial ability37 many years before dementia onset have been reported. Participants in these studies were born in the beginning of the 20th century, and results are in line with our findings in cohort 1901–1902. It is conceivable that cutoff scores useful for defining low cognitive performance may change over time.
Dementia incidence remained the same, although later-born generations performed better on cognitive tests than earlier-born generations. Our results further suggest that impending death and dementia may have less effect on cognitive function in later generations of elderly. This may have implications for selection of preclinical tests of dementia. Low performance in memory was related to dementia in both cohorts. Why specific nonmemory tests seem to be less sensitive as early signs of dementia in later-born cohorts remains to be elucidated. The findings need to be taken cautiously due to a number of limitations, such as differences in refusal rates, and changes in quality of education, dementia recognition, and medical records between the 1970s and 2000s.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. S. Sacuiu.
ACKNOWLEDGMENT
The authors thank Valter Sundh and Thomas Marlow for statistical assistance, both affiliated with the University of Gothenburg and Sahlgrenska University Hospital, Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry, Unit of Psychiatric Epidemiology, Mölndal, Sweden.
DISCLOSURE
Dr. Sacuiu reports no disclosures. Dr. Gustafson has served as a consultant for the Albuquerque Area Indian Health Board, Columbia University, and SUNY-Downstate Medical Center; has served on the speakers' bureau for Shire plc; has received speaker honoraria from the Albuquerque Area Indian Health Board and Shire plc; has received research support from the NIH/NIA (5R03AG026098- 02 [PI]) and receives research support from the Swedish Research Council. Dr. Sjögren is employed as Director of Translational Medicine by Merck Research Laboratories; serves as Vice President of Global Exploratory Development for UCB; and serves on the editorial board of the Open Aging Journal. Dr. Guo reports no disclosures. Dr. Östling has received research support from Söderström Königska. Dr. Johansson serves on scientific advisory boards for the Aging Research Center, Stockholm, the Danish Aging Research Center, Denmark, and the Kavli Research Center for Ageing and Dementia, Norway; and serves on the editorial boards of Aging and Mental Health, the European Journal of Ageing, the Journal of Gerontopsychology and Geriatric Psychiatry, the Journal of Aging Research, and the Journal of European Psychology Students. Dr. Skoog serves on speakers' bureaus for and has received speaker honoraria from Shire plc, Janssen, Eisai Inc., Pfizer Inc., and GE Healthcare; has served on scientific advisory boards for Pfizer Inc. and AstraZeneca; has served/serves on the editorial boards of International Psychogeriatrics, the American Journal of Geriatric Psychiatry, and the European Journal of Psychiatry; receives royalties from the publication of Alzheimers sjukdom och andra kognitiva sjukdomar (English title: Alzheimer's Disease and Other Cognitive Disorders) (Liber, 2003); and has received research support from the Swedish Research Council, the Swedish Council for Working Life and Social Research, the Alzheimer's Association, and the Bank of Sweden Tercentenary Foundation.
Address correspondence and reprint requests to Dr. Simona Sacuiu, University of Gothenburg and Sahlgrenska University Hospital, Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry, Unit of Psychiatric Epidemiology, Wallinsgatan 6, 431 41 Mölndal, Sweden simona.sacuiu@neuro.gu.se
See page 786
Study funding: Supported by the Swedish Research Council (11267, 2005-8460, 825-2007-7462), the Swedish Council for Working Life and Social Research (2001-2835, 2001-2646, 2003-0234, 2004-0150, 2006-0020, 2008-1229, 2004-0145, 2006-0596, 2008-1111, 2006-1506), the Alzheimer's Association Zenith Award (ZEN-01-3151), the Alzheimer's Association Stephanie B. Overstreet Scholars (IIRG-00-2159), the NIH/NIA 5R03AG026098-02 (D.G.), the Bank of Sweden Tercentenary Foundation, Axel Linder's Foundation, Makarna Sylvan's Foundation, Handlanden Hjalmar Svensson's Foundation, Stiftelsen Professor Bror Gadelius' Minnesfond, and Demensfonden.
Disclosure: Author disclosures are provided at the end of the article.
Received November 26, 2009. Accepted in final form April 28, 2010.
REFERENCES
- 1.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 2.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006;67:2176–2185. [DOI] [PubMed] [Google Scholar]
- 3.Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology 2007;68:1909–1916. [DOI] [PubMed] [Google Scholar]
- 4.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007;68:288–291. [DOI] [PubMed] [Google Scholar]
- 5.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002;59:1594–1599. [DOI] [PubMed] [Google Scholar]
- 6.Sacuiu S, Gustafson D, Johansson B, et al. The pattern of cognitive symptoms predicts time to dementia onset. Alzheimers Dementia 2009;5:199–206. [DOI] [PubMed] [Google Scholar]
- 7.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology 2006;67:229–234. [DOI] [PubMed] [Google Scholar]
- 8.Backman L, Laukka EJ, Wahlin A, Small BJ, Fratiglioni L. Influences of preclinical dementia and impending death on the magnitude of age-related cognitive deficits. Psychol Aging 2002;17:435–442. [DOI] [PubMed] [Google Scholar]
- 9.Johansson B, Berg S. The robustness of the terminal decline phenomenon: longitudinal data from the Digit-Span Memory Test. J Gerontol 1989;44:184–186. [DOI] [PubMed] [Google Scholar]
- 10.Laukka EJ, MacDonald SW, Backman L. Terminal-decline effects for select cognitive tasks after controlling for preclinical dementia. Am J Geriatr Psychiatry 2008;16:355–365. [DOI] [PubMed] [Google Scholar]
- 11.Lavery LL, Dodge HH, Snitz B, Ganguli M. Cognitive decline and mortality in a community-based cohort: the Monongahela Valley Independent Elders Survey. J Am Geriatr Soc 2009;57:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorvaldsson V, Hofer SM, Berg S, Skoog I, Sacuiu S, Johansson B. Onset of terminal decline in cognitive abilities in individuals without dementia. Neurology 2008;71:882–887. [DOI] [PubMed] [Google Scholar]
- 13.Flynn JR. The discovery of IQ gains over time. Am Psychol 1999;54:5–20. [Google Scholar]
- 14.Schaie KW, Labouvie-Vief G. Generational versus ontogenetic components of change in adult cognitive behaviour: a fourteen-year cross-sequential study. Dev Psychol 1974;10:305–320. [Google Scholar]
- 15.Schaie KW, Willis SL, Pennak S. An historical framework for cohort differences in intelligence. Res Hum Dev 2005;2:43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llewellyn DJ, Matthews FE. Increasing levels of semantic verbal fluency in elderly English adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2009;16:433–445. [DOI] [PubMed] [Google Scholar]
- 17.Rinder L, Roupe S, Steen B, Svanborg A. Seventy-year-old people in Gothenburg: a population study in an industrialized Swedish city. Acta Med Scand 1975;198:397–407. [DOI] [PubMed] [Google Scholar]
- 18.Persson G. Prevalence of mental disorders in a 70-year-old urban population. Acta Psychiatr Scand 1980;62:119–139. [DOI] [PubMed] [Google Scholar]
- 19.Beckman N, Waern M, Gustafson D, Skoog I. Secular trends in self reported sexual activity and satisfaction in Swedish 70 year olds: cross sectional survey of four populations, 1971–2001. BMJ 2008;337:a279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson L. Prevalence of mental disorders in a 70-year-old urban sample: a cohort comparison. J Clin Experimental Gerontol 1983;5:101–120. [Google Scholar]
- 21.Asberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl 1978;271:5–27. [DOI] [PubMed] [Google Scholar]
- 22.Skoog I. Mental Disorders in The Elderly: A Population Study in 85-Year-Olds [PhD Thesis]. Göteborg: University of Göteborg; 1993. [Google Scholar]
- 23.Guo X, Waern M, Sjogren K, et al. Midlife respiratory function and Incidence of Alzheimer's disease: a 29-year longitudinal study in women. Neurobiol Aging 2007;28:343–350. [DOI] [PubMed] [Google Scholar]
- 24.Andersson E, Berg S, Lawenius M, Svanborg A. Intellectual functioning in a 70-year-old urban population. Acta Psychiatr Scand 1978;57:59–66. [DOI] [PubMed] [Google Scholar]
- 25.Berg S. Psychological functioning in 70- and 75-year-old people: a study in an industrialized city. Acta Psychiatr Scand Suppl 1980;288:1–47. [PubMed] [Google Scholar]
- 26.Skoog I, Berg S, Johansson B, Palmertz B, Andreasson LA. The influence of white matter lesions on neuropsychological functioning in demented and non-demented 85-year-olds. Acta Neurol Scand 1996;93:142–148. [DOI] [PubMed] [Google Scholar]
- 27.Kay DW, Roth M, Beamish P. Old age mental disorders in Newcastle Upon Tyne: II: a study of possible social and medical causes. Br J Psychiatry 1964;110:668–682. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson LV, Persson G. Prevalence of mental disorders in an urban sample examined at 70, 75 and 79 years of age. Acta Psychiatr Scand 1984;69:519–527. [DOI] [PubMed] [Google Scholar]
- 29.Wancata J, Borjesson-Hanson A, Ostling S, Sjogren K, Skoog I. Diagnostic criteria influence dementia prevalence. Am J Geriatr Psychiatry 2007;15:1034–1045. [DOI] [PubMed] [Google Scholar]
- 30.Sacuiu S, Sjogren M, Johansson B, Gustafson D, Skoog I. Prodromal cognitive signs of dementia in 85-year-olds using four sources of information. Neurology 2005;65:1894–1900. [DOI] [PubMed] [Google Scholar]
- 31.Hall KS, Gao S, Baiyewu O, et al. Prevalence rates for dementia and Alzheimer's disease in African Americans: 1992 versus 2001. Alzheimers Dement 2009;5:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steen G, Berg S, Steen B. Cognitive function in 70-year-old men and women: a 16-year cohort difference population study. Aging 1998;10:120–126. [DOI] [PubMed] [Google Scholar]
- 33.Zelinski EM, Kennison RF. The Long Beach Longitudinal Study: evaluation of longitudinal effects of aging on memory and cognition. Home Health Care Serv Q 2001;19:45–55. [DOI] [PubMed] [Google Scholar]
- 34.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–1010. [PubMed] [Google Scholar]
- 35.Rogers MA, Plassman BL, Kabeto M, et al. Parental education and late-life dementia in the United States. J Geriatr Psychiatry Neurol 2009;22:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol 2000;57:808–813. [DOI] [PubMed] [Google Scholar]
- 37.La Rue A, Jarvik LF. Cognitive function and prediction of dementia in old age. Int J Aging Hum Dev 1987;25:79–89. [DOI] [PubMed] [Google Scholar]
- 38.Linn RT, Wolf PA, Bachman DL, et al. The ‘preclinical phase’ of probable Alzheimer's disease: a 13-year prospective study of the Framingham cohort. Arch Neurol 1995;52:485–490. [DOI] [PubMed] [Google Scholar]
- 39.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology 2005;64:1853–1859. [DOI] [PubMed] [Google Scholar]
- 40.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 1999;53:1942–1947. [DOI] [PubMed] [Google Scholar]


