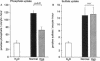Abstract
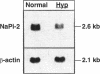
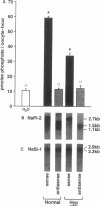
The X-linked Hyp mouse is characterized by a specific defect in proximal tubular phosphate (Pi) reabsorption that is associated with a decrease in Vmax of the high affinity Na(+)-Pi cotransport system in the renal brush border membrane. To understand the mechanism for Vmax reduction, we examined the effect of the Hyp mutation on renal expression of Na(+)-Pi cotransporter mRNA and protein. Northern hybridization of renal RNA with a rat, renal-specific Na(+)-Pi cotransporter cDNA probe (NaPi-2) (Magagnin et al. 1993. Proc. Natl. Acad. Sci. USA. 90:5979-5983.) demonstrated a reduction in a 2.6-kb transcript in kidneys of Hyp mice relative to normal littermates (NaPi-2/beta-actin mRNA = 57 +/- 6% of normal in Hyp mice, n = 6, P < 0.01). Na(+)-Pi cotransport, but not Na(+)-sulfate cotransport, was approximately 50% lower in Xenopus oocytes injected with renal mRNA extracted from Hyp mice when compared with that from normal mice. Hybrid depletion experiments documented that the mRNA-dependent expression of Na(+)-Pi cotransport in oocytes was related to NaPi-2. Western analysis demonstrated that NaPi-2 protein is also significantly reduced in brush border membranes of Hyp mice when compared to normals. The present data demonstrate that the specific reduction in renal Na(+)-Pi cotransport in brush border membranes of Hyp mice can be ascribed to a proportionate decrease in the abundance of Na(+)-Pi cotransporter mRNA and protein.
Full text
PDF

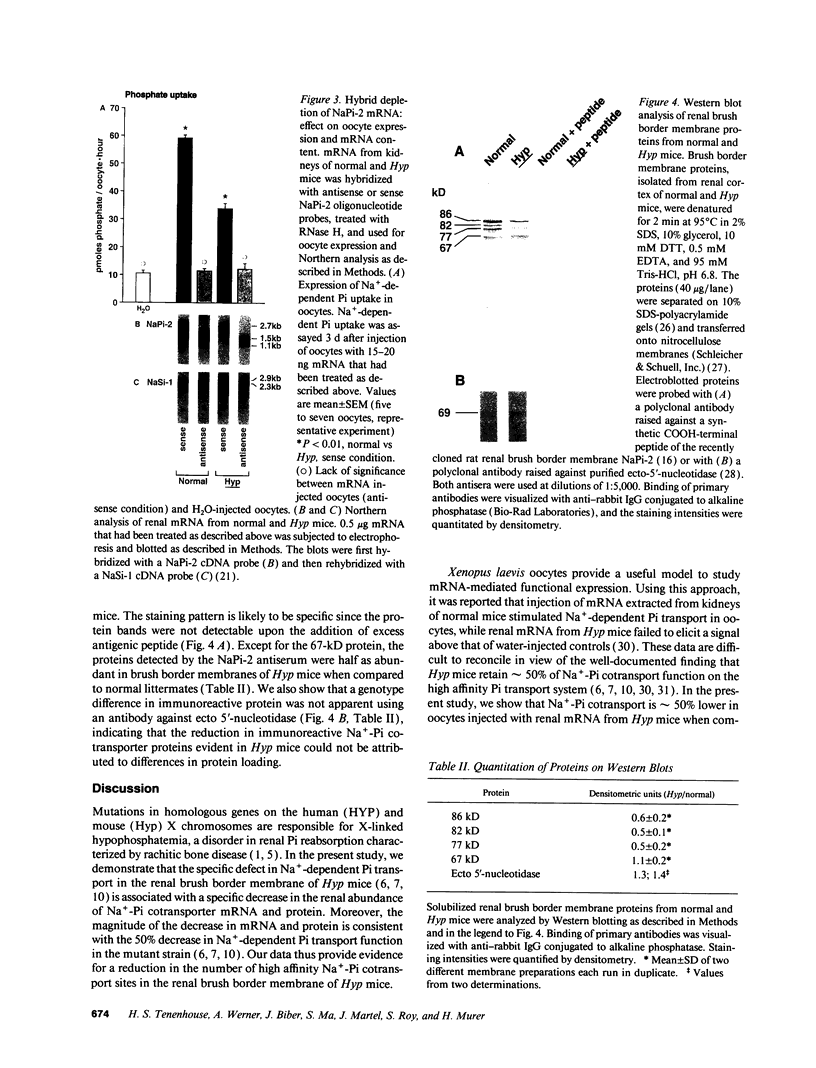


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. L., Tenenhouse H. S., Scriver C. R. Primary cultures of renal epithelial cells from X-linked hypophosphatemic (Hyp) mice express defects in phosphate transport and vitamin D metabolism. Am J Hum Genet. 1988 Sep;43(3):293–303. [PMC free article] [PubMed] [Google Scholar]
- Biber J., Custer M., Werner A., Kaissling B., Murer H. Localization of NaPi-1, a Na/Pi cotransporter, in rabbit kidney proximal tubules. II. Localization by immunohistochemistry. Pflugers Arch. 1993 Aug;424(3-4):210–215. doi: 10.1007/BF00384344. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cowgill L. D., Goldfarb S., Lau K., Slatopolsky E., Agus Z. S. Evidence for an intrinsic renal tubular defect in mice with genetic hypophosphatemic rickets. J Clin Invest. 1979 Jun;63(6):1203–1210. doi: 10.1172/JCI109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer M., Meier F., Schlatter E., Greger R., Garcia-Perez A., Biber J., Murer H. Localization of NaPi-1, a Na-Pi cotransporter, in rabbit kidney proximal tubules. I. mRNA localization by reverse transcription/polymerase chain reaction. Pflugers Arch. 1993 Aug;424(3-4):203–209. doi: 10.1007/BF00384343. [DOI] [PubMed] [Google Scholar]
- Dawson T. P., Gandhi R., Le Hir M., Kaissling B. Ecto-5'-nucleotidase: localization in rat kidney by light microscopic histochemical and immunohistochemical methods. J Histochem Cytochem. 1989 Jan;37(1):39–47. doi: 10.1177/37.1.2535703. [DOI] [PubMed] [Google Scholar]
- Econs M. J., Barker D. F., Speer M. C., Pericak-Vance M. A., Fain P. R., Drezner M. K. Multilocus mapping of the X-linked hypophosphatemic rickets gene. J Clin Endocrinol Metab. 1992 Jul;75(1):201–206. doi: 10.1210/jcem.75.1.1352307. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Southard J. L., Scriver C. R., Glorieux F. H. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. M., Molitoris B. A. Abnormal proximal tubule apical membrane protein composition in X-linked hypophosphatemic mice. Am J Physiol. 1991 Mar;260(3 Pt 2):F317–F322. doi: 10.1152/ajprenal.1991.260.3.F317. [DOI] [PubMed] [Google Scholar]
- Giasson S. D., Brunette M. G., Danan G., Vigneault N., Carriere S. Micropuncture study of renal phosphorus transport in hypophosphatemic vitamin D resistant rickets mice. Pflugers Arch. 1977 Oct 19;371(1-2):33–38. doi: 10.1007/BF00580769. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Chase L. R. Pi transport, phosphorylation, and dephosphorylation in renal membranes from HYP/Y mice. Am J Physiol. 1983 Dec;245(6):F701–F706. doi: 10.1152/ajprenal.1983.245.6.F701. [DOI] [PubMed] [Google Scholar]
- Harvey N., Tenenhouse H. S. Renal Na(+)-phosphate cotransport in X-linked Hyp mice responds appropriately to Na+ gradient, membrane potential, and pH. J Bone Miner Res. 1992 May;7(5):563–571. doi: 10.1002/jbmr.5650070513. [DOI] [PubMed] [Google Scholar]
- Kos C. H., Tihy F., Econs M. J., Murer H., Lemieux N., Tenenhouse H. S. Localization of a renal sodium-phosphate cotransporter gene to human chromosome 5q35. Genomics. 1994 Jan 1;19(1):176–177. doi: 10.1006/geno.1994.1034. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magagnin S., Werner A., Markovich D., Sorribas V., Stange G., Biber J., Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovich D., Forgo J., Stange G., Biber J., Murer H. Expression cloning of rat renal Na+/SO4(2-) cotransport. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8073–8077. doi: 10.1073/pnas.90.17.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Meyer M. H., Gray R. W. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res. 1989 Aug;4(4):493–500. doi: 10.1002/jbmr.5650040407. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Tenenhouse H. S., Meyer M. H., Klugerman A. H. The renal phosphate transport defect in normal mice parabiosed to X-linked hypophosphatemic mice persists after parathyroidectomy. J Bone Miner Res. 1989 Aug;4(4):523–532. doi: 10.1002/jbmr.5650040411. [DOI] [PubMed] [Google Scholar]
- Meyerhof W., Richter D. Identification of G protein-coupled receptors by RNase H-mediated hybrid depletion using Xenopus laevis oocytes as expression system. FEBS Lett. 1990 Jun 18;266(1-2):192–194. doi: 10.1016/0014-5793(90)81537-x. [DOI] [PubMed] [Google Scholar]
- Nakagawa N., Arab N., Ghishan F. K. Characterization of the defect in the Na(+)-phosphate transporter in vitamin D-resistant hypophosphatemic mice. J Biol Chem. 1991 Jul 25;266(21):13616–13620. [PubMed] [Google Scholar]
- Nesbitt T., Coffman T. M., Griffiths R., Drezner M. K. Crosstransplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic renal defect. J Clin Invest. 1992 May;89(5):1453–1459. doi: 10.1172/JCI115735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z. Q., Tenenhouse H. S., Scriver C. R. Parental origin of mutant allele does not explain absence of gene dose in X-linked Hyp mice. Genet Res. 1993 Aug;62(1):39–43. doi: 10.1017/s0016672300031542. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Tenenhouse H. S. Conserved loci on the X chromosome confer phosphate homeostasis in mice and humans. Genet Res. 1990 Oct-Dec;56(2-3):141–152. doi: 10.1017/s0016672300035229. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Fast D. K., Scriver C. R., Koltay M. Intestinal transport of phosphate anion is not impaired in the Hyp (hypophosphatemic) mouse. Biochem Biophys Res Commun. 1981 May 29;100(2):537–543. doi: 10.1016/s0006-291x(81)80210-0. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Klugerman A. H., Neal J. L. Effect of phosphonoformic acid, dietary phosphate and the Hyp mutation on kinetically distinct phosphate transport processes in mouse kidney. Biochim Biophys Acta. 1989 Sep 4;984(2):207–213. doi: 10.1016/0005-2736(89)90218-6. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Lee J., Harvey N., Potier M., Jette M., Beliveau R. Normal molecular size of the Na(+)-phosphate cotransporter and normal Na(+)-dependent binding of phosphonoformic acid in renal brush border membranes of X-linked Hyp mice. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1288–1293. doi: 10.1016/0006-291x(90)90533-s. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Lee J., Harvey N. Renal brush-border membrane Na(+)-sulfate cotransport: stimulation by thyroid hormone. Am J Physiol. 1991 Sep;261(3 Pt 2):F420–F426. doi: 10.1152/ajprenal.1991.261.3.F420. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Lee J. Sulfate inhibits [14C]phosphonoformic acid binding to renal brush-border membranes. Am J Physiol. 1990 Aug;259(2 Pt 2):F286–F292. doi: 10.1152/ajprenal.1990.259.2.F286. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Martel J. Renal adaptation to phosphate deprivation: lessons from the X-linked Hyp mouse. Pediatr Nephrol. 1993 Jun;7(3):312–318. doi: 10.1007/BF00853232. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R., McInnes R. R., Glorieux F. H. Renal handling of phosphate in vivo and in vitro by the X-linked hypophosphatemic male mouse: evidence for a defect in the brush border membrane. Kidney Int. 1978 Sep;14(3):236–244. doi: 10.1038/ki.1978.115. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. The defect in transcellular transport of phosphate in the nephron is located in brush-border membranes in X-linked hypophosphatemia (Hyp mouse model). Can J Biochem. 1978 Jun;56(6):640–646. doi: 10.1139/o78-096. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. X-linked hypophosphatemia. A phenotype in search of a cause. Int J Biochem. 1992 May;24(5):685–691. doi: 10.1016/0020-711x(92)90001-h. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Biber J., Forgo J., Palacin M., Murer H. Expression of renal transport systems for inorganic phosphate and sulfate in Xenopus laevis oocytes. J Biol Chem. 1990 Jul 25;265(21):12331–12336. [PubMed] [Google Scholar]
- Werner A., Moore M. L., Mantei N., Biber J., Semenza G., Murer H. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]