Abstract
Objective:
To determine factors associated with baseline neurocognitive performance in HIV-infected participants enrolled in the Strategies for Management of Antiretroviral Therapy (SMART) neurology substudy.
Methods:
Participants from Australia, North America, Brazil, and Thailand were administered a 5-test neurocognitive battery. Z scores and the neurocognitive performance outcome measure, the quantitative neurocognitive performance z score (QNPZ-5), were calculated using US norms. Neurocognitive impairment was defined as z scores <−2 in two or more cognitive domains. Associations of test scores, the QNPZ-5, and impairment with baseline factors including demographics and risk factors for HIV-associated dementia (HAD) and cardiovascular disease (CVD) were determined in multiple regression.
Results:
The 292 participants had a median CD4 cell count of 536 cells/mm3, 88% had an HIV viral load ≤400 copies/mL, and 92% were taking antiretrovirals. Demographics, HIV, and clinical factors differed between locations. The mean QNPZ-5 score was −0.72; 14% of participants had neurocognitive impairment. For most tests, scores and z scores differed significantly between locations, with and without adjustment for age, sex, education, and race. Prior CVD was associated with neurocognitive impairment. Prior CVD, hypercholesterolemia, and hypertension were associated with poorer neurocognitive performance but conventional HAD risk factors and the CNS penetration effectiveness rank of antiretroviral regimens were not.
Conclusions:
In this HIV-positive population with high CD4 cell counts, neurocognitive impairment was associated with prior CVD. Lower neurocognitive performance was associated with prior CVD, hypertension, and hypercholesterolemia, but not conventional HAD risk factors. The contribution of CVD and cardiovascular risk factors to the neurocognition of HIV-positive populations warrants further investigation.
GLOSSARY
- AD
= Alzheimer disease;
- ART
= antiretroviral therapy;
- BP
= blood pressure;
- CES-D
= Center for Epidemiologic Studies-Depression scale;
- CT
= Color Trails;
- CVD
= cardiovascular disease;
- FTT
= Finger Tapping Test;
- GPB
= Grooved Pegboard;
- HAD
= HIV-associated dementia;
- NCI
= neurocognitive impairment;
- QNPZ-5
= quantitative neurocognitive performance z score;
- SMART
= Strategies for Management of Antiretroviral Therapy;
- TG
= Timed Gait.
In advanced untreated HIV disease, HIV-associated dementia (HAD) develops in approximately 15% of patients1 and combination antiretroviral therapy (ART) has effectively reduced the incidence of HAD.2 The Strategies for Management of Antiretroviral Therapy (SMART) study randomized participants to intermittent, CD4-guided ART or continuous ART.3 In a neurology substudy, a neurocognitive test battery was administered. We hypothesized that neurocognitive performance would be superior in patients receiving continuous ART via its attendant benefits upon both peripheral and CNS immunity.
We present a cross-sectional analysis of 292 HIV-infected persons coenrolled in the SMART neurology substudy at sites in Australia, North America, Brazil, and Thailand. We sought to explore factors associated with neurocognitive performance. These included demographics, ART, HAD, and cardiovascular risk factors and cardiovascular disease (CVD). In HIV-negative populations, smoking,4 hypertension,5 high cholesterol,6 obesity,5 and diabetes5 are the cardiovascular risk factors associated with increased risk of poor cognitive function, vascular dementia, and Alzheimer disease (AD). Prior myocardial infarction,7 coronary artery bypass grafting,8 and stroke5 are also associated with poor cognitive function. In HIV-positive populations, diabetes has been associated with HAD,9 and increased carotid intima media thickening10 has been associated with poorer neurocognitive performance. CVD risk factors were common in the SMART study3: we hypothesized that they would be associated with lower baseline neurocognitive performance. We sought to describe and compare neuropsychological test results obtained in Australia, North America, Brazil, and Thailand and hypothesized that test scores, but not the standardized z scores, would differ across locations.
METHODS
Study design.
The SMART study was an international randomized trial comparing continuous ART with CD4-cell count guided, intermittent ART in HIV-infected persons with CD4 cell count >350 cells/mm3. The neurocognitive component of the SMART neurology substudy aimed to compare the 2 study arms for changes in neurocognitive function through follow-up. Baseline, month 6, and annual assessment of neurocognitive functioning in 600 participants would have provided 80% power to detect a difference of 0.27 in the primary outcome (change in the aggregate quantitative neurocognitive performance z score, QNPZ-5, described below). The neurology substudy commenced enrollment in July 2005. In January 2006, enrollment and the intermittent CD4-guided ART strategy of the SMART study were stopped due to increased risk of AIDS and serious non-AIDS complications. The primary results of the SMART study are published elsewhere.3 At the time of this protocol change, only 292 of the 600 planned substudy participants were enrolled, and minimal follow-up data were available.
Forty-seven SMART study sites in Australia, North America, Brazil, and Thailand participated in the substudy. All eligible SMART participants were offered substudy coenrollment. Substudy eligibility criteria included age ≥18 years, and the ability to perform the study's neurocognitive tests in the site clinician's judgment.
Standard protocol approvals, registrations, and patient consents.
Substudy approval was obtained by each site's institutional review board. Patient information and consent forms were translated into Thai, Portuguese, and Spanish, and back-translated into English. All participants provided written informed consent. The study was registered at clinical trials.gov: NCT00432003.
Outcome measures.
The study's neurocognitive test battery comprised the Grooved Pegboard (GPB) (dominant hand),11 Color Trails (CT) 1 and CT2,12 Timed Gait (TG),13 and Finger Tapping Test (FTT) (nondominant hand).14 Details of these tests can be found in appendix e-1 on the Neurology® Web site at www.neurology.org. This test battery was chosen because it is sensitive to changes observed in HIV-associated neurocognitive disorders, brief, easy to administer and teach, and can be used internationally where little formal Western education exists15; our tests closely resemble the battery used in a large, randomized ART HIV treatment study.16
For all 5 tests, the raw scores of each participant were standardized to z scores by subtracting the mean test scores of matched, HIV-negative reference populations, and dividing by the reference SD. Therefore, z scores estimate how many SDs a test score is above or below the average score of the reference population; negative z scores denote below-average performance. For the TG, raw scores were standardized to z scores using previously published reference distributions,13 matched by education level. For the other tests, z scores were calculated using scoring software by Psychological Assessment Resources, Inc., Odessa, FL.17 Reference distributions were matched by education level for all 5 tests, and additionally by age, sex, and race/ethnicity for GPB and FTT,18 and by age for CT. All reference populations were obtained in the United States.
Our primary neurocognitive performance outcome measure was the QNPZ-5, calculated as the average of the 5 z scores from the individual tests in the battery. A QNPZ-5 score below 0 denotes below-average neurocognitive performance. For the purpose of this study, we defined neurocognitive impairment (NCI) as z scores <−2 in at least 2 cognitive ability domains. Ability domains assessed were 1) speed/fine motor skills (GPB and FTT), 2) attention/speed of processing (CT1), 3) abstraction/executive function (CT2), and 4) gross motor skills (TG). The cutoff was chosen to reflect criteria for abnormal neurocognitive performance that are a required component for the diagnosis of HAD.19
We administered the Center for Epidemiologic Studies-Depression scale (CES-D)20 to screen for depression at baseline. The CES-D has been used in international studies in HIV-infected15 populations. We used the recommended cutoff score of ≥16 to define depression (sensitivity 86%–100%, specificity 53%–84%).21
Neuropsychological tests and the CES-D were administered to participants by site staff, including medical practitioners, specialists, and nurses. Site staff underwent centralized training and certification in the United States and Australia. Training was led by a neuropsychologist or neurologist and an infectious disease physician. Each trainee was required to administer the full test battery during training, and at least 3 times afterwards to nonstudy participants before administering it to study participants. Test instructions and training materials were available for site staff on the study Web site. Neuropsychological test instructions were translated into Portuguese, Spanish, and Thai. We used published translations of the Spanish22 and Brazilian Portuguese23 CES-D and an online Thai24 translation.
Baseline data collected within the parent SMART study included demography, HIV history, general medical history, and laboratory values (summarized in table 1 and table e-1); additionally, alcohol and drug use were collected within the substudy. We summarized risk factors for CVD into a modified Framingham score; since blood pressure (BP) was not available, we assigned a systolic BP of 140 mm Hg to participants using antihypertensive drugs, and 120 mm Hg otherwise.25 We calculated the CNS penetration effectiveness rank26 of patients' ART regimens.
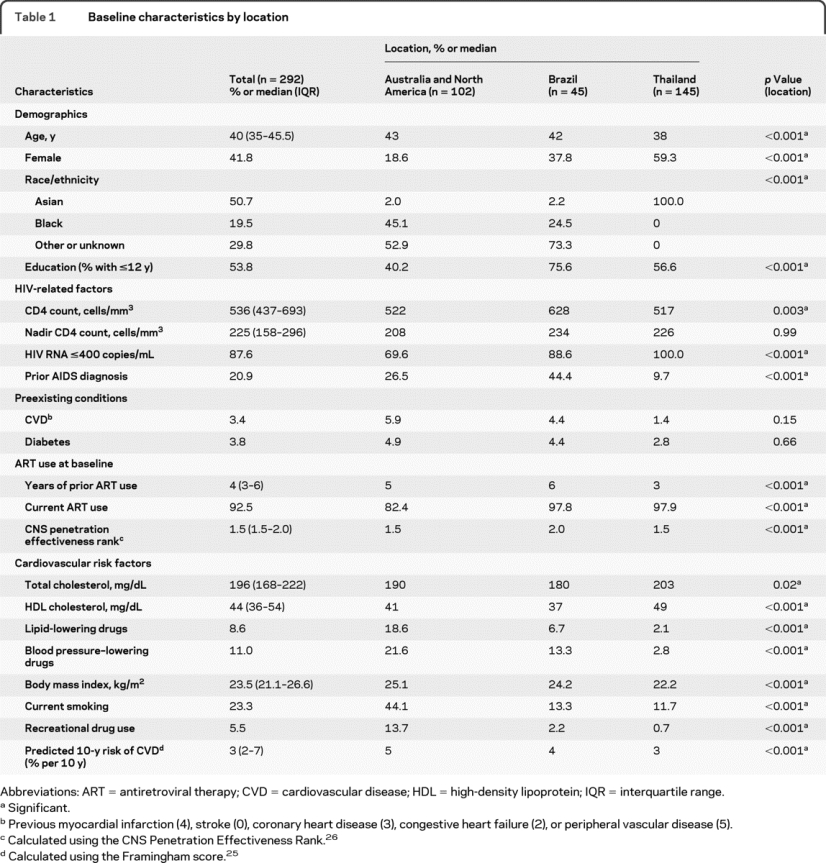
Table 1 Baseline characteristics by location
Statistical methods.
Baseline characteristics, neuropsychological test scores, and CES-D scores were summarized by location of enrollment. The Kruskal-Wallis rank sum test was used to compare median values across locations, χ2 tests to compare percentages. Mean test scores (raw scores and z scores) were compared across locations using analysis of variance and the Tukey Honest Significant Difference pairwise comparison method. In order to account for variability between clinical sites within countries, we also compared locations in hierarchical mixed effects models with sites nested in location.
Associations of baseline factors with raw test scores, z scores, the QNPZ-5 score, and having z scores <−2 in 2 or more cognitive domains (a total of 12 outcomes) were determined by multiple linear or logistic regression. In a first step, the following factors were included: HAD risk factors (age, current and nadir CD4 cell counts, prior AIDS, diabetes); sex; race/ethnicity; location of enrollment; education; body mass index; smoking; alcohol abuse; recreational drug use; history of hepatitis B, hepatitis C, or CVD; viral load (HIV RNA ≤400 copies/mL); CNS penetration effectiveness rank; use of BP-lowering and lipid-lowering drugs; total cholesterol, high-density lipoprotein, and low-density lipoprotein; and CES-D score ≥16. Factors were then eliminated using backwards selection with the Akaike information criterion. We retained age, gender, race, and education in all models, because these factors were associated with neuropsychological test results in noninfected populations, and reference norms for calculating z scores are usually matched by these factors. We also retained location to consistently assess the location effect. Coefficients and p values from the final models were presented. In a separate model, lipids and use of BP-lowering drugs were replaced by Framingham risk estimates for CVD (4 categories),25 extrapolating BP from use of BP-lowering drugs.
Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.9. All tests were 2-sided. p Values ≤0.05 were considered significant.
RESULTS
Baseline characteristics.
Baseline characteristics of the 292 study participants by their location of enrollment are shown in table 1 and table e-1. Participant characteristics differed across locations, including their demographics, HIV, and general medical history.
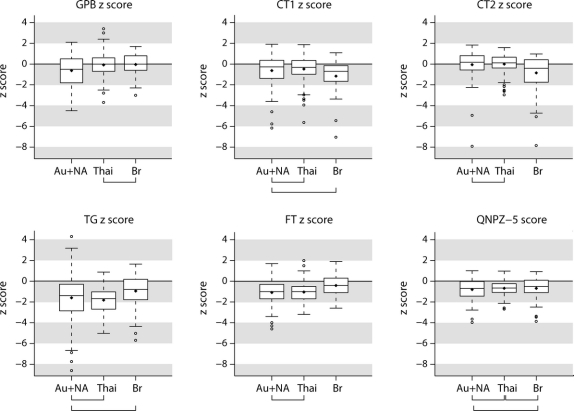
Baseline neurocognitive test scores are summarized by location in table 2 and the figure. Overall, the mean QNPZ-5 score was −0.72; in each location, the mean z score was below 0 (p < 0.001), denoting below-average performance compared to a healthy, matched population. Fourteen percent of patients met the study definition of NCI (z scores <−2 in 2 or more cognitive domains); 51% of participants had z scores <−1 in 2 or more cognitive domains. For each of the 5 tests, mean z scores differed between locations (all p ≤ 0.02). There was no evidence, however, for regional differences in mean QNPZ-5 scores (p = 0.53), and no one region performed consistently higher or lower across all tests. In pairwise comparisons, there were no significant differences in test scores between Australia/North America and Thailand, except for the GPB (figure). Table 2 also shows the variability in test scores between clinical sites (between-site SD) in relation to the between-patient SD, estimated in hierarchical mixed models. For all tests except GPB, the between-site SD was about one-third to one-half of the between-patient SD, and remaining differences between locations (countries) were not statistically significant relative to the differences between sites (p > 0.05 in the hierarchical models).
Table 2 Neuropsychological test performance and CES-D scores by location
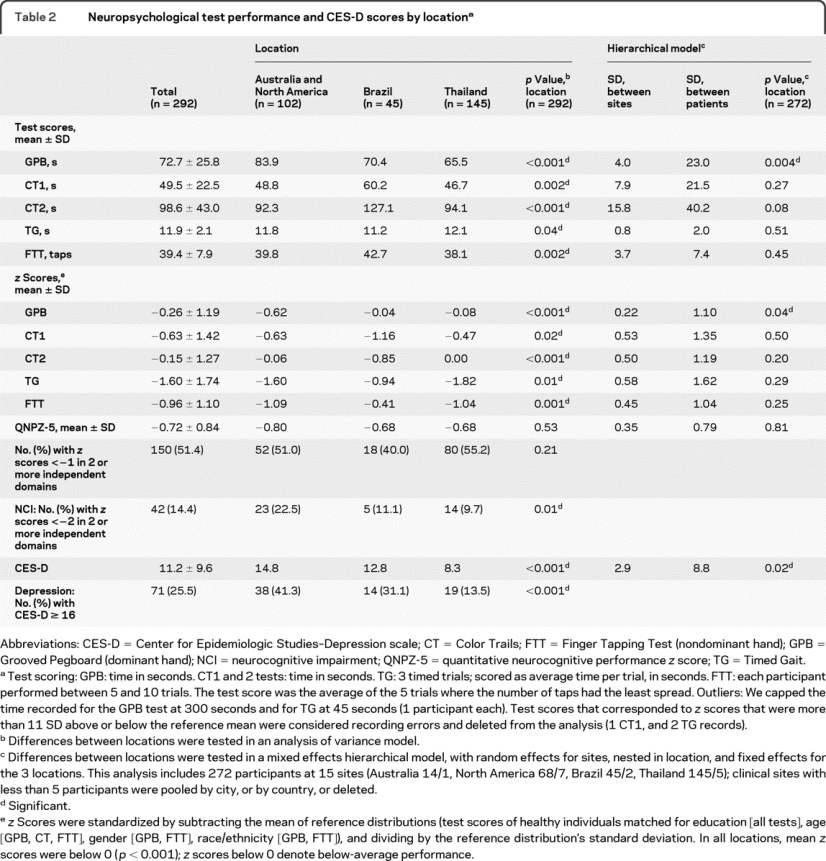
Figure z Scores and summary QNPZ-5 for 5 neuropsychological tests, by location
Box plots show the distributions of baseline z scores of 292 participants; 102 participants were enrolled in Australia and North America, 45 in Brazil, and 145 in Thailand. Brackets below the location labels connect pairs of locations where mean scores are not significantly different. Boxes show the interquartile range; the horizonta line in the box denotes the median; the diamond denotes the mean. Whiskers extend 1.5 times the interquartile range above and below the z score quartiles. Circles denote outliers. Au = Australia; Br = Brazil; CT = Color Trails Test; FT = Finger Tapping Test; GPB = Grooved Pegboard Test; NA = North America; QNPZ-5 = quantitative neurocognitive performance z score; TG = Timed Gait Test; Thai = Thailand.
The mean CES-D score was 11.2. Overall, 71 participants (26%) met the study definition of depression (table 2). The proportion of participants with depression was highest in Australia/North America (41%) and lowest in Thailand (14%).
Association between baseline factors and neurocognitive performance.
The z scores for all 5 tests, but not the QNPZ-5 scores, differed by location after adjustment for age, gender, race/ethnicity, education, and selected other factors (table 3). Older age was associated with worse test performance in raw test scores except for TG, but not with any of the z scores, which are standardized by age. Women and black participants had lower estimated mean QNPZ-5 scores (by 0.21 and 0.48) after adjustment for the other covariates.
Table 3 Association of participants' characteristics with neurocognitive performance: Regression coefficients and p values in multivariate linear (QNPZ-5) and logistic (NCI) regression
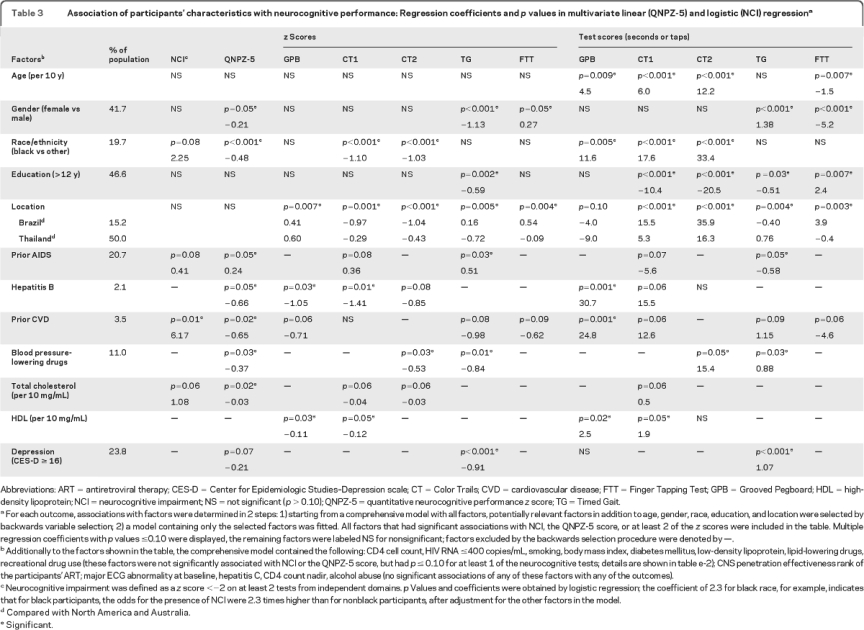
Patients with preexisting CVD had 6.2-fold higher odds of having NCI (p = 0.01, 95% CI 1.4–26.4), after adjustment for age, gender, race/ethnicity, education, location, prior AIDS, and total cholesterol (table 3). Prior CVD was also associated with lower QNPZ-5 scores (by −0.7; p = 0.02), as were use of antihypertensive agents (by −0.4, p = 0.03), higher total cholesterol (by −0.03 per 10 mg/mL, p = 0.02), and hepatitis B (by −0.7, p = 0.05); estimated mean QNPZ-5 scores were lower for women, black participants, and, borderline, for those with depression (p = 0.07) (table 3). Smoking, diabetes, higher body mass index, higher low-density lipoprotein, and use of lipid-lowering drugs were not independently associated with NCI or lower QNPZ-5 scores but were associated with lower z scores or worse test scores for some of the tests (table 3 and table e-2). Higher high-density lipoprotein was associated with lower z scores on GPB and CT1, but not with NCI or QNPZ-5 scores. In a sensitivity analysis, we included the Framingham CVD risk score as a factor instead of fitting cholesterol and BP-lowering drugs separately: there was no evidence for an association of the Framingham score with either the QNPZ-5 or NCI (data not shown). There was no evidence that major abnormalities on the baseline ECG, hepatitis C, or alcohol abuse were independently associated with any of the neurocognitive performance measures (table 3, footnote).
There was no evidence for an association of baseline or nadir CD4 cell counts, viral load, diabetes, or the CNS penetration effectiveness rank of ART regimens with QNPZ-5 scores or NCI.
DISCUSSION
Our cross-sectional study of baseline neurocognitive performance included 292 SMART study participants from Australia, North America, Brazil, and Thailand with CD4 cell counts >350 cells/mm3. At baseline, 14% of participants had NCI and prior CVD was the single associated factor. Participants with prior CVD, higher total cholesterol, and those using antihypertensive drugs had lower estimated mean baseline neurocognitive performance, but neither HIV-related risk factors commonly associated with HAD nor the CNS penetration effectiveness rank of ART were associated with NCI or neurocognitive performance measured by the QNPZ-5. The 10 participants (3.4%) with prior CVD in our study had experienced myocardial infarction, coronary heart disease, congestive heart failure, or peripheral vascular disease, but not stroke.
Our findings suggest that cardiovascular risk factors and disease may be key drivers of impairment in HIV-positive persons with high CD4 cell counts, more so than HIV-related HAD risk factors. These findings are important because HIV-positive populations have a high number of CVD risk factors including smoking,3 hypertension,3 diabetes,3 hyperlipidemia,3 metabolic syndrome,27 insulin resistance,28 and antiretroviral use.29
The mechanisms that might link lower neurocognitive performance with CVD and cardiovascular risk factors in HIV-positive populations are likely multifactorial. In HIV-negative populations increasing evidence suggests that cardiovascular risk factors contribute to the pathogenesis of both vascular dementia and AD.4–6,30 Theoretically the pathogenesis of these 2 dementing illnesses may overlap. Recently it was proposed that insulin resistance, which underlies several of the abovementioned cardiovascular risk factors, may be one of the primary convergent mechanisms that links these risk factors to both vascular dementia and AD.31
Raised inflammatory markers, including IL-6 and C-reactive protein, are risk factors for dementia32 and are common to CVD,33 the metabolic syndrome,34 aging, and HIV, including treated HIV infection.35 However, we do not have inflammatory markers for study analysis.
The changes in the adaptive immune system of HIV-infected patients receiving long-term antiretroviral therapy resemble those seen in the elderly.36 Key factors that may contribute to immune senescence in HIV-infected patients include persistent immune activation, inflammation, and poor CD4 cell response to antiretroviral treatment.36 A recent study of HIV-infected patients measured coronary artery calcium accumulation and found an increased vascular age in over 40% of patients, with a mean increase of 15 years over their chronological age.37 Increased vascular aging would help to explain why we found an association between lower neurocognitive performance and cardiovascular risk factors/CVD in a relatively young study population whereas cognitive decline and dementia associated with these factors in HIV-negative populations usually do not occur until at least the sixth decade. Similarly, the early expression of Parkinson disease in HIV infection has been reported recently.38
None of the conventional HIV-related HAD risk factors was associated with NCI or lower neurocognitive performance and the reason for this is unclear. The median CD4 cell count was high (536 cells/mm3) but there was also no association of NCI with nadir CD4 counts. There was no evidence for an association of diabetes with impairment or poorer neurocognitive performance, although the lack of evidence may have been due to the low number of participants with diabetes. There was also no evidence for an association of the ART CNS penetration effectiveness rank with any of the neurocognitive performance scores. ART regimens with CNS penetration effectiveness ranks ≥2 have been associated with improved neurocognitive performance26; in our study, 25% of participants had CNS penetration effectiveness ranks ≥2. Contrary to expectations, participants with prior AIDS had a slightly increased estimated mean QNPZ-5 score (by 0.24, p = 0.05, table 3). This may be a statistical artifact due to confounding; in univariate analysis, those with and without prior AIDS had similar QNPZ-5 scores (p = 0.60 for difference), but those with prior AIDS had more CVD risk factors, including a higher body mass index (mean 25 vs 23) and more use of BP-lowering drugs (18% vs 9%).
Mean z scores differed significantly between locations for each of the 5 neurocognitive tests; moreover, differences between locations persisted after adjusting for differences in age, sex, race, and education and other factors (table 3). At first glance, this leads to the hypothesis that the standard US norms were not appropriate for Brazil or Thailand. Within countries, however, z scores also varied considerably between clinical sites (table 2, hierarchical model). Further adjustment for age, race, sex, and education decreased between-site variability in raw test scores, but had only minimal effect on the between-site variability in z scores (data not shown). The latter suggests that our US norm z scores reasonably standardized the test scores for these 4 demographic factors, and that the remaining variability between countries may be largely due to other unmeasured factors such as nutritional status, urban vs rural residency, cultural/ethnic factors, and test administration. It may be that if we had been able to more accurately measure and adjust for the education of participants, for example by assessing their reading level39 instead of using years of education, we may have seen less between-country variability.
Twenty-four percent of participants met the study definition of depression; this proportion is commensurate with some studies but lower than others.15 Antidepressant use was not assessed.
One should interpret our study definition of NCI (z scores <−2 in 2 or more domains) with caution because 1) NCI was not confirmed clinically; 2) we used US norms in Brazilian and Thai populations; and 3) we assessed 4 cognitive domains only.19 Moreover, NCI may not always represent HAD or other HIV-associated neurocognitive disorders because there is an overlap between the neurocognitive impairment associated with cerebrovascular disease40 and HIV-associated CNS disease.
Study limitations include a moderate sample size, which limits the power to detect associations; a modest test battery; study-trained staff rather than neuropsychologists administering the neurocognitive tests; and the lack of local reference norms in Brazil and Thailand. Also, hypertension was not assessed directly, but extrapolated from the use of BP-lowering drugs. Finally, we evaluated multiple outcomes; some of the observed associations might be false-positives, in particular where statistical significance was borderline.
Our findings suggest that, in HIV-infected persons with high CD4 cell counts, cardiovascular-related insults may be more detrimental to neurocognitive functioning than factors more directly related to HIV. Given the high prevalence of cardiovascular risk factors in HIV-infected populations, this finding is important and warrants further investigation.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Grund and M. Roediger.
ACKNOWLEDGMENT
The authors thank the participants, site investigators, and staff for their contributions. Details of the clinical site investigators for the SMART Neurology substudy by site and country are presented in appendix e-2.
DISCLOSURE
Dr. Wright has served on a scientific advisory board for GlaxoSmithKline and receives research support from Gilead Sciences, Inc., Boehringer Ingelheim, Abbott, the National Health and Medical Research Council of Australia, and the NIH (NIMH/NINDS 1U01-AI068641 [Study Chair]). Dr. Grund has received research support from the NIH/NIAID (U01-AI068641 [Statistician], U01-AI042170 [Statistician], and U01-AI46362 [Statistician]). Dr. Robertson has received speaker honoraria from GlaxoSmithKline, Abbott, Boehringer Ingelheim, and Clinical Care Options, and receives research support from the NIH (NIAID, supplement to 1U01AI068636-01 [PI], NIMH MH067751 [coinvestigator], NIAID U01-AI068641 [Co-PI], and NINDS R21NS0692 [coinvestigator]). Prof. Brew serves on scientific advisory boards for GlaxoSmithKline, ViiV Healthcare, Biogen Idec, and Merck Serono; has received funding for travel from Abbott; serves on the editorial boards of Open Virology, the International Journal for Tryptophan Research, Faculty of 1000 Medicine, the Journal of Neurovirology, and Neurobehavioral HIV Medicine; receives royalties from the publication of HIV Neurology (Oxford University Press, 2001) and Palliative Neurology (Cambridge University Press, 2006); has received speaker honoraria from GlaxoSmithKline, ViiV Healthcare, Boehringer Ingelheim, Abbott, and Biogen Idec; and receives/has received research support from Eli Lilly and Company, GlaxoSmithKline, ViiV Healthcare, Merck Serono, the National Health and Medical Research Council (NHMRC) of Australia, the NIH (R01 NS43103, co-PI), the University of New South Wales, and from St. Vincent's Clinic Research Foundation. Ms. Roediger has received research support from the NIH (NIAID U01-AI068641 [Statistician], NIAID U01-AI042170 [Statistician], and NIAID U01-AI46362 [Statistician]). Ms. Bain has received speaker honoraria from GlaxoSmithKline. Dr. Drummond and Dr. Vjecha report no disclosures. Prof. Hoy serves on scientific advisory boards for Gilead Sciences, Inc., Tibotec Therapeutics (Janssen), Merck Sharp & Dohme, and Bristol-Myers Squibb; has received speaker honoraria from Gilead Sciences, Inc.; and receives research support from the National Health and Medical Research Council of Australia, Gilead Sciences, Inc., Merck Sharp & Dohme, Tibotec Therapeutics, Bristol-Myers Squibb, and Boehringer Ingelheim. Ms. Miller and Dr. Penalva de Oliveira report no disclosures. Dr. Pumpradit has received funding for travel from Pfizer Inc. and has received research support from the Ministry of Public Health, Thailand. Dr. Shlay reports no disclosures. Dr. El-Sadr receives research support from the NIH/NIAID (5 U01 AI69466-04 [PI], 5 U01 AI69466-03S1 [PI], and R01AI083038-01 [PI]). Dr. Price has received speaker honoraria from the International AIDS Society and receives research support from Merck and Co, and from the NIH (NIDA P01DA026134 [Project PI], NIMH R01-MH62701 [PI], NIMH R21-MH083520 [PI],. NIMH U01-MH083545 [Consultant], NIMH R01MH081772 [coinvestigator], NIMH/NIAIDU01-AI068641 [Co-Chair Neurology substudy Protocol], and NIMH/NIAID U01-AI38858 [Co-Chair AACTG study]).
Supplementary Material
Address correspondence and reprint requests to Dr. Edwina Wright, Infectious Diseases Unit, Alfred Hospital, Commercial Road, Melbourne, Victoria 3004, Australia e.wright@alfred.org.au
Supplemental data at www.neurology.org
e-Pub ahead of print on August 11, 2010, at www.neurology.org.
Study funding: Supported by the NIH (NIMH/NINDS U01-AI4636, NIAID U01AI042170, and NIAID U01AI46362).
Disclosure: Author disclosures are provided at the end of the article.
Received January 30, 2010. Accepted in final form May 12, 2010.
REFERENCES
- 1. McArthur JC Hoover DR Bacellar H et al. Dementia in AIDS patients: incidence and risk factors: Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 2. Bhaskaran K Mussini C Antinori A et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 3. El-Sadr WM Lundgren JD Neaton JD et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 4. Anstey KJ von Sanden C Salim A O'Kearney R Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 5. Hayden KM Zandi PP Lyketsos CG et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 6. Anstey KJ Lipnicki DM Low LF Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 7. Silbert BS Scott DA Evered LA Lewis MS Maruff PT Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg. 2007;104:1023–1028. doi: 10.1213/01.ane.0000263285.03361.3a. [DOI] [PubMed] [Google Scholar]
- 8. Newman MF Kirchner JL Phillips-Bute B et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 9. Valcour VG Shikuma CM Shiramizu BT et al. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;38:31–36. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Becker JT Kingsley L Mullen J et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klove H Clinical neuropsychology. Med Clin North Am. 1963;47:1647–1658. [PubMed] [Google Scholar]
- 12.D'Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- 13. Robertson KR Parsons TD Sidtis JJ et al. Timed Gait test: normative data for the assessment of the AIDS dementia complex. J Clin Exp Neuropsychol. 2006;28:1053–1064. doi: 10.1080/13803390500205684. [DOI] [PubMed] [Google Scholar]
- 14.Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Indianapolis: Reitan RM; 1969. [Google Scholar]
- 15. Wright E Brew B Arayawichanont A et al. Neurologic disorders are prevalent in HIV-positive outpatients in the Asia-Pacific region. Neurology. 2008;71:50–56. doi: 10.1212/01.wnl.0000316390.17248.65. [DOI] [PubMed] [Google Scholar]
- 16. Price RW Yiannoutsos CT Clifford DB et al. Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS. 1999;13:1677–1685. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- 17.PAR Psychological Assessment Resources, Inc. PAI® Software Portfolio, 2006. Available at: www3.parinc.com. Accessed March 30, 2009.
- 18.Heaton RK, Miller SW, Taylor JJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African Americans and Caucasian adults. Lutz, FL: PAR Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- 19. Antinori A Arendt G Becker JT et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radloff L The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21. Van Voorhees BW Fogel J Houston TK Cooper LA Wang NY Ford DE Beliefs and attitudes associated with the intention to not accept the diagnosis of depression among young adults. Ann Fam Med. 2005;3:38–46. doi: 10.1370/afm.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perczek R Carver CS Price AA Pozo-Kaderman C Coping, mood, and aspects of personality in Spanish translation and evidence of convergence with English versions. J Pers Assess. 2000;74:63–87. doi: 10.1207/S15327752JPA740105. [DOI] [PubMed] [Google Scholar]
- 23. Fleck MP Lima AF Louzada S et al. [Association of depressive symptoms and social functioning in primary care service, Brazil.] Rev Saude Publica. 2002;36:431–438. doi: 10.1590/s0034-89102002000400008. [DOI] [PubMed] [Google Scholar]
- 24.Center for Epidemiologic Studies-Depression Scale (CES-D). Available at: http://www.dmh.go.th/test/cesd/cesd/. Accessed July 30, 2010.
- 25. Anderson KM Odell PM Wilson PW Kannel WB Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 26. Letendre S Marquie-Beck J Capparelli E et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samaras K Wand H Law M Emery S Cooper D Carr A Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30:113–119. doi: 10.2337/dc06-1075. [DOI] [PubMed] [Google Scholar]
- 28. Brown TT Li X Cole SR et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–1383. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 29. SMART/INSIGHT and D:A:D Study Groups. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kivipelto M Helkala EL Laakso MP et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craft S The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Engelhart MJ Geerlings MI Meijer J et al. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam Study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 33. Sattar N Murray HM Welsh P et al. Are markers of inflammation more strongly associated with risk for fatal than for nonfatal vascular events? PLoS Med. 2009;6:e1000099. doi: 10.1371/journal.pmed.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kowalska I Straczkowski M Nikolajuk A et al. Insulin resistance, serum adiponectin, and proinflammatory markers in young subjects with the metabolic syndrome. Metabolism. 2008;57:1539–1544. doi: 10.1016/j.metabol.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 35. Kuller LH Tracy R Belloso W et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deeks SG Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. [PubMed] [Google Scholar]
- 37. Guaraldi G Zona S Alexopoulos N et al. Coronary aging in HIV-infected patients. Clin Infect Dis. 2009;49:1756–1762. doi: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- 38. Tisch S Brew B Parkinsonism in HIV-infected patients on highly active antiretroviral therapy. Neurology. 2009;73:401–403. doi: 10.1212/WNL.0b013e3181b04b0d. [DOI] [PubMed] [Google Scholar]
- 39. Manly JJ Echemendia RJ Race-specific norms: using the model of hypertension to understand issues of race, culture, and education in neuropsychology. Arch Clin Neuropsychol. 2007;22:319–325. doi: 10.1016/j.acn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 40. Roman GC Erkinjuntti T Wallin A Pantoni L Chui HC Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.