Abstract
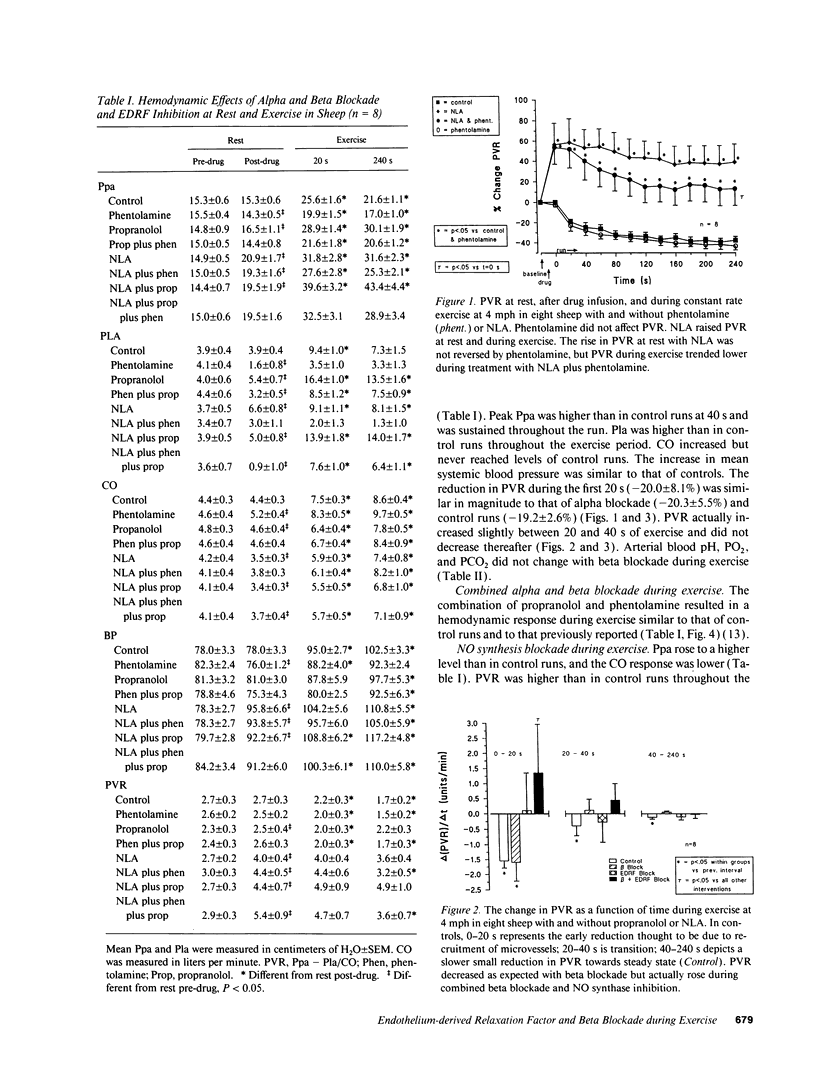
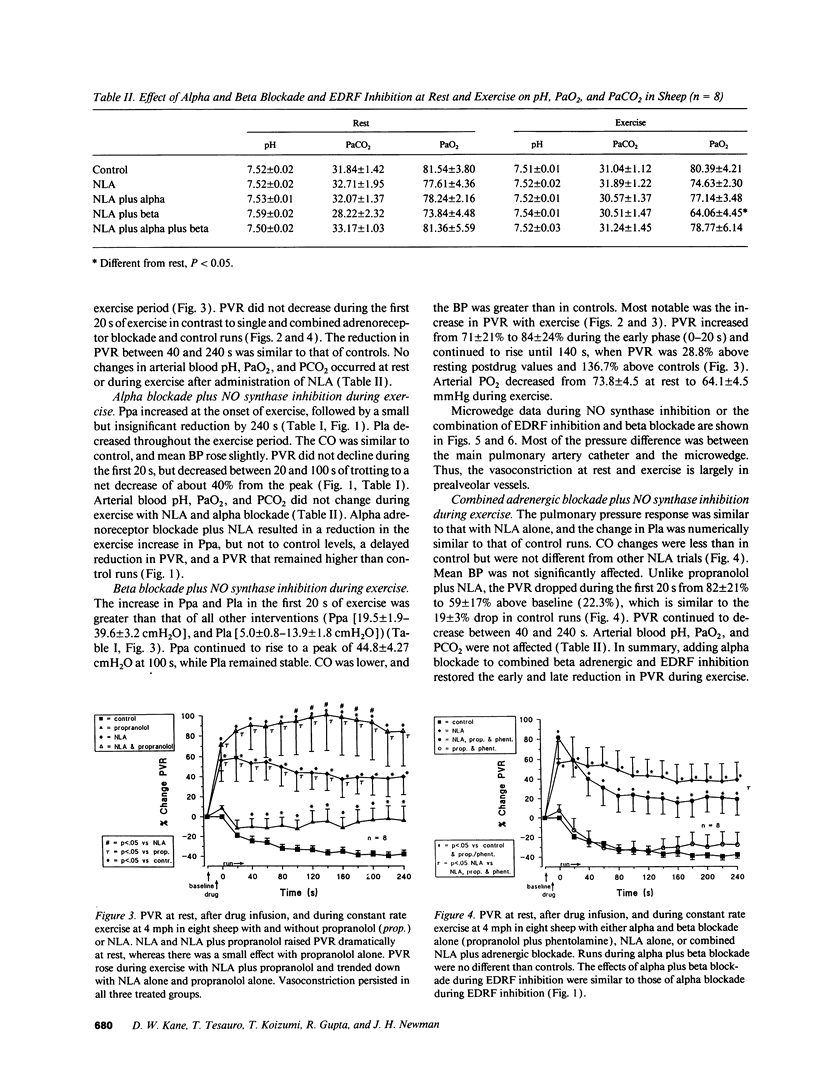
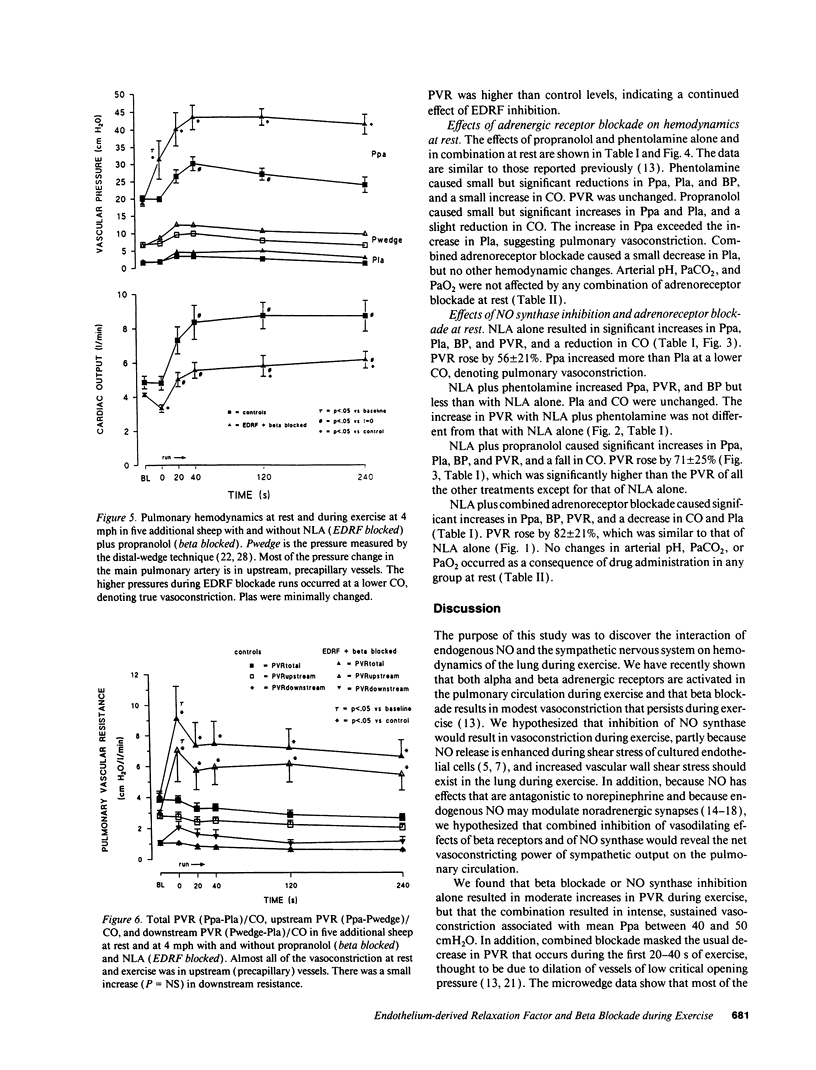
We studied the effects of inhibition of nitric oxide (NO) (endothelium-derived relaxation factor) synthase in combination with alpha and beta adrenergic receptor blockade on pulmonary vascular tone during exercise. In paired studies, we exercised sheep on a treadmill at a speed of 4 mph, and measured blood flow and pressures across the pulmonary circulation with and without inhibition of NO synthase (N omega-nitro-L-arginine 20 mg/kg intravenous [i.v.]), alpha receptor blockade (phentolamine 5 mg i.v.), beta receptor blockade (propranolol 1 mg i.v.), and combined alpha and beta receptor blockade. Activation of both types of adrenergic receptors occurs with exercise, and because increased release in NO is hypothesized to occur during exercise, these studies were designed to determine the magnitude of effect and interactions of these competing dilator and constrictor influences. We found that inhibition of NO synthase raised pulmonary vascular resistance (PVR) at rest and that, although a reduction in PVR occurred with exercise from this new baseline, vasoconstriction persisted. Combined beta blockade and NO synthase inhibition unmasked unopposed alpha vasoconstriction; PVR rose at rest and continued to rise with exercise; and mean pulmonary arterial pressures approached very high levels, 43.8 +/- 4.4 cmH2O. Using a distal wedged pulmonary artery catheter technique, most of the vasoconstriction was found to be in vessels upstream from small pulmonary veins. During exercise in sheep there appears to be a high degree of alpha and beta adrenergic-mediated tone in the pulmonary circulation. Endogenous production of NO actively dilates pulmonary vessels at rest and opposes potent alpha-mediated pulmonary vasoconstriction during exercise.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. L., Rist K., Nelson D. P., DeMaster E. G., Cowan N., Weir E. K. Comparison of the hemodynamic effects of nitric oxide and endothelium-dependent vasodilators in intact lungs. J Appl Physiol (1985) 1990 Feb;68(2):735–747. doi: 10.1152/jappl.1990.68.2.735. [DOI] [PubMed] [Google Scholar]
- Brashers V. L., Peach M. J., Rose C. E., Jr Augmentation of hypoxic pulmonary vasoconstriction in the isolated perfused rat lung by in vitro antagonists of endothelium-dependent relaxation. J Clin Invest. 1988 Nov;82(5):1495–1502. doi: 10.1172/JCI113757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga G. M., Gold M. E., Fukuto J. M., Ignarro L. J. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991 Feb;17(2):187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Dinh-Xuan A. T., Higenbottam T. W., Clelland C. A., Pepke-Zaba J., Cremona G., Butt A. Y., Large S. R., Wells F. C., Wallwork J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991 May 30;324(22):1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- Drayer D. E. Lipophilicity, hydrophilicity, and the central nervous system side effects of beta blockers. Pharmacotherapy. 1987;7(4):87–91. doi: 10.1002/j.1875-9114.1987.tb04029.x. [DOI] [PubMed] [Google Scholar]
- Frostell C., Fratacci M. D., Wain J. C., Jones R., Zapol W. M. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991 Jun;83(6):2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Greenberg S. S., Diecke F. P., Peevy K., Tanaka T. P. Release of norepinephrine from adrenergic nerve endings of blood vessels is modulated by endothelium-derived relaxing factor. Am J Hypertens. 1990 Mar;3(3):211–218. doi: 10.1093/ajh/3.3.211. [DOI] [PubMed] [Google Scholar]
- Greenberg S. S., Peevy K., Tanaka T. P. Endothelium-derived and intraneuronal nitric oxide-dependent inhibition of norepinephrine efflux from sympathetic nerves by bradykinin. Am J Hypertens. 1991 May;4(5 Pt 1):464–467. doi: 10.1093/ajh/4.5.464. [DOI] [PubMed] [Google Scholar]
- Holtz J., Förstermann U., Pohl U., Giesler M., Bassenge E. Flow-dependent, endothelium-mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1161–1169. [PubMed] [Google Scholar]
- Kane D. W., Tesauro T., Newman J. H. Adrenergic modulation of the pulmonary circulation during strenuous exercise in sheep. Am Rev Respir Dis. 1993 May;147(5):1233–1238. doi: 10.1164/ajrccm/147.5.1233. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Lodato R. F., Michael J. R. Neural antagonists modulate pulmonary vascular pressure-flow plots in conscious dogs. J Appl Physiol (1985) 1986 Jun;60(6):1900–1907. doi: 10.1152/jappl.1986.60.6.1900. [DOI] [PubMed] [Google Scholar]
- Nava E., Palmer R. M., Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991 Dec 21;338(8782-8783):1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- Newman J. H., Butka B. J., Parker R. E., Roselli R. J. Effect of progressive exercise on lung fluid balance in sheep. J Appl Physiol (1985) 1988 May;64(5):2125–2131. doi: 10.1152/jappl.1988.64.5.2125. [DOI] [PubMed] [Google Scholar]
- Newman J. H., Cochran C. P., Roselli R. J., Parker R. E., King L. S. Pressure and flow changes in the pulmonary circulation in exercising sheep: evidence for elevated microvascular pressure. Am Rev Respir Dis. 1993 Apr;147(4):921–926. doi: 10.1164/ajrccm/147.4.921. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Parker R. E., Brigham K. L. Effects of endotoxemia on pulmonary vascular resistances in unanesthetized sheep. J Appl Physiol (1985) 1987 Sep;63(3):1058–1062. doi: 10.1152/jappl.1987.63.3.1058. [DOI] [PubMed] [Google Scholar]
- Perrella M. A., Edell E. S., Krowka M. J., Cortese D. A., Burnett J. C., Jr Endothelium-derived relaxing factor in pulmonary and renal circulations during hypoxia. Am J Physiol. 1992 Jul;263(1 Pt 2):R45–R50. doi: 10.1152/ajpregu.1992.263.1.R45. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossaint R., Falke K. J., López F., Slama K., Pison U., Zapol W. M. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993 Feb 11;328(6):399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Togashi H., Yoshioka M., Saito H., Yanagida M., Tamura M., Kobayashi T., Yasuda H., Gross S. S., Levi R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res. 1992 Mar;70(3):607–611. doi: 10.1161/01.res.70.3.607. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Wylam M. E., Samsel R. W., Umans J. G., Mitchell R. W., Leff A. R., Schumacker P. T. Endotoxin in vivo impairs endothelium-dependent relaxation of canine arteries in vitro. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1263–1267. doi: 10.1164/ajrccm/142.6_Pt_1.1263. [DOI] [PubMed] [Google Scholar]



