Abstract
Hyperglycemia and insulin resistance often occur following injury and/or critical illness. While intensive insulin treatment reduces hyperglycemia, mortality and morbidity in certain patients, little is known regarding the pathophysiology of acute insulin resistance following injury and infection. Studies suggest that acute insulin resistance is complex and may differ in a tissue-specific manner, involving multiple causative factors and intracellular signaling pathways. Therefore, the advantages of intensive insulin therapy may not be uniform to all injuries or critical illnesses. Clearly, the increased incidence of hypoglycemic incidents following intensive insulin therapy indicates a need to understand the underlying molecular mechanisms of the acute development of insulin resistance, which will allow a more targeted approach to treating altered glucose metabolism of critically ill patients.
Historical perspectives on the link between hyperglycemia and intensive insulin therapy
Claude Bernard first described the development of hyperglycemia following hemorrhagic shock in 1877 [1], and it is now accepted that many acute illnesses or injuries result in hyperglycemia, glucose intolerance and insulin resistance [2-8]. This ‘diabetes of injury’, now more commonly referred to as ‘critical illness diabetes’, can occur in patients without a previous history of Type 2 diabetes. Patients in the intensive care unit (ICU) with multiple injuries, extensive burns, major surgical trauma, or infections often present with ‘critical illness diabetes’. Many survive the initial injury, but then succumb to multiple organ failure [9]. Although extensive research efforts have focused on strategies to prevent or reverse multiple organ failure, few results are encouraging and the mechanisms remain unclear [10-12]. However, intensive insulin therapy may result in substantial improvements [1,13], suggesting that perturbations of glucose metabolism contributes to multiple organ failure, as well as to other problems that occur in injured and critically ill individuals [14-16]. It is well known that insulin can have anti-inflammatory actions, but how intensive insulin therapy can potentially decrease morbidity and mortality in the ICU is still poorly understood. This review focusees on the development of hyperglycemia and insulin resistance following injury or critical illness, the controversy over how to treat these patients in the ICU, and the varying mechanisms by which insulin resistance develops in different tissues.
Acute insulin resistance, hyperglycemia, and intensive insulin therapy in critically ill patients
One practical definition of insulin resistance is the inability of insulin to adequately stimulate glucose uptake, mainly into skeletal muscle, or to inhibit gluconeogenesis in the liver [17,18]. Insulin resistance that occurs in chronic diseases, such as Type 2 diabetes, obesity and hypertension, normally takes months, years, or even decades to develop [17,18]. Hyperglycemia and insulin resistance in critically ill patients is characterized by rapid onset, developing in minutes, hours, or days, therefore termed acute insulin resistance.
Hyperglycemia is considered an independent risk factor for adverse outcomes in critically ill patients because it directly or indirectly confers a predisposition to a variety of complications including severe infections, polyneuropathy, multiple-organ failure and death. Previously, a moderate level of hyperglycemia, below 215 mg/dl, was not thought to be problematic. However, the Leuven study, conducted in critically ill patients in a surgical ICU, demonstrated that intensive insulin therapy to control blood glucose levels between 80-110 mg/dl (4.4-6.1 mmol/l) significantly reduces mortality compared with conventional insulin therapy, which has a target blood glucose level of 180-200 mg/dl (10.0-11.1 mmol/l) [1]. The benefit of intensive insulin therapy is particularly apparent in those who require intensive care for more than 5 days, with mortality reduced by 34-50% [1]. The benefits of intensive insulin therapy are measurable in either medical or mixed medical and surgical ICUs [19-21], and intensive insulin therapy reduces numerous complications common in the ICU, decreasing infections, acute renal failure, liver dysfunction, muscle weakness, and anemia, and it shortens the length of time of mechanical ventilation and time in the hospital [19-21].
However, much of the early work in this field was from a single center or small studies at other centers and, thus, a large, multi-center randomized trial, named NICE-SUGAR (Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation), was designed to further evaluate the role of intensive insulin therapy in the ICU [22]. This is an ongoing trial which to date indicates little alteration of mortality following intensive insulin therapy. However, the NICE-SUGAR patients received less insulin to achieve approximately the same blood glucose values as in the Leuven study, suggesting that their initial level of insulin resistance was less in NICE-SUGAR. Meta-analysis of 26 studies (including Leuven and NICE-SUGAR) suggests that intensive insulin therapy may be beneficial to patients admitted to surgical ICUs [23]. This is clearly an ongoing question that needs further evaluation; thus, it is still too early to determine the ideal blood glucose target for critically ill patients.
There may be groups of patients that do not benefit or are even harmed by intensive insulin therapy [24]. For instance, high dose insulin may be harmful in elderly patients and neonates [25-27] and in critically ill patients with longstanding diabetes [28,29]. In addition, intensive insulin therapy increases the incidence of hypoglycemic episodes with the potential for negative outcomes [1,22,24,30-32] and in some cases, the complications due to hypoglycemia may negate the positive effects of tight glucose control. Therefore, further studies of intensive insulin therapy need to be performed to accurately define the patient population most likely to benefit. However, it is our opinion that in many instances the increase in hypoglycemic incidents may have more to do with over-treatment with insulin, as opposed to some negative aspect of intensive insulin therapy. Thus, frequent monitoring of glucose is necessary, and intensive insulin therapy must be customized to the patient to achieve tight glucose control. It must be understood that due to variability in the severity of critical illness or injury in the ICU patient population, as well as preexisting illnesses including but not limited to Type 2 diabetes, there are highly varying degrees of insulin resistance in the patient population. Add to this the variation in genetic susceptibility to develop and/or recover from acute insulin resistance, and one must pursue a highly individualized approach to intensive insulin therapy, with frequent monitoring of blood glucose levels.
Although intensive insulin therapy in critically ill patients has become routine in many ICUs, there is little basic understanding of the molecular mechanisms by which acute insulin resistance develops in different tissues, and the specific physiological actions of intensive insulin therapy. One study suggests that intensive insulin therapy improves mitochondrial function by protecting the ultrastructure and function of the liver, but not of skeletal muscle or mitochondria, in critically ill patients [33]. This study is consistent with mitochondrial involvement in the development of chronic insulin resistance in Type 2 diabetes, possibly via increases in superoxide production and oxidative stress [34,35], but this is a novel concept in the poorly studied development of acute insulin resistance of injury. Insulin is believed to have anti-inflammatory actions, and intensive insulin therapy may have many other positive (and potentially negative) effects. Thus, a much greater understanding of the basic defects that occur in insulin signaling and insulin action following injury and infection are necessary for understanding the dysfunction that occurs in glucose metabolism.
The development of acute insulin resistance in animal models following injury or infection
To understand the clinical problems of acute insulin resistance, research has been performed in several animal models of injury and infection. Experimental sepsis results in reduced glucose transporter 4 (GLUT4; the insulin responsive glucose transporter) mRNA and protein levels in rat adipose tissue [36]. In another rat sepsis model, impaired glucose metabolism was characterized by decreased glycogen synthesis and glycogen synthase activity [37]. Following burn injury, glycogen abundance in rat liver can not be increased by exogenous insulin [38], and skeletal muscle glucose and amino acid uptake are decreased [39], suggesting the development of insulin resistance. Development of acute insulin resistance also occurs in human patients following major burn injury [40,41]. Studies investigating potential mechanisms of skeletal muscle insulin resistance in experimental models indicate decreased insulin signaling via the insulin receptor/insulin receptor substrates/phosphotidylinositol 3-kinase/Akt (IR/IRS/PI3K/Akt) pathway [42,43] following burn injury. However, this is not a uniform finding, with variability in the development of insulin resistance following burn injury between laboratories. This is true in both rat and mouse models of burn injury. This may be due, at least in part, to differences in the method of estimating the burn injury surface area from lab to lab. We believe this inconsistency has slowed progress in this area and is vital for developing potential treatments for patients with major burn injury.
Patients in the surgical ICU are often hyperglycemic and, as discussed previously, intensive insulin treatment often decreases mortality and morbidity in this patient population. To experimentally model the effects of injuries or surgeries, another experimental model has recently been adapted for use, the trauma and hemorrhage model. In this animal model of injury, trauma is a midline laparotomy as well as injury of bilateral femoral catheterization. The combination of trauma and hemorrhage includes these surgeries plus rapid blood loss to achieve a mean arterial pressure of 35-40 mmHg. Consistent and rapid insulin resistance occurs in this rat model, which mimics many of the observations of hyperglycemia and insulin resistance in humans following accidental or surgical injury, with the development of acute insulin resistance in the three major target tissues of insulin - liver, skeletal muscle and adipose tissue [4-8]. Trauma alone results in modest insulin resistance, occurring gradually. However, when injury (trauma) is combined with hemorrhage, a disorder in glucose metabolism rapidly develops, occurring in rat liver as soon as 15 minutes following trauma and hemorrhage. This is characterized by severe defects in hepatic insulin signaling, hyperinsulinemia and hyperglycemia [4-7]. In contrast, skeletal muscle insulin resistance is delayed, not detectable until 60 minutes following trauma and hemorrhage [7,8], suggesting a distinct mechanism in response to trauma and hemorrhage in skeletal muscle. The reasons for the tissue-specific differences in the development of insulin resistance are only partially understood. However, even up to a 60 minute delay in skeletal muscle is still an extremely rapid development of insulin resistance.
Impaired insulin signaling in liver and skeletal muscle following injury or critical illness
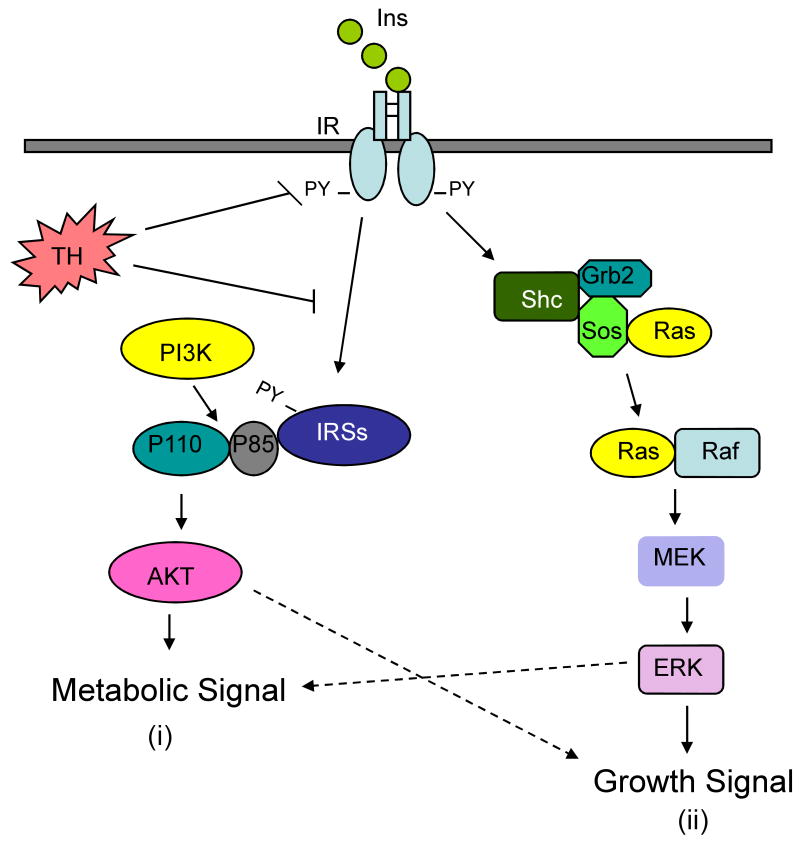
Insulin signaling is initiated by binding of insulin to its receptor, followed by activation of two main intracellular insulin signaling pathways: the IRS/PI3K/Akt pathway, and the Ras/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) pathway (Figure 1) [44,45]. In an experimental model of sepsis, hepatic and muscle insulin signaling is depressed due to decreased activation/tyrosine phosphorylation of IR, IRS-1 and IRS-2 [3]. However, the causative factors and underlying mechanism for the changes in insulin signaling were not investigated.
Figure 1.
Two major insulin signaling pathways. (i) The IRS/PI3K/Akt pathway is initiated by phosphorylation of the insulin receptor (IR). This results in the phosphorylation of the IRSs, increased interaction between IRSs and PI3K, and phosphorylation/activation of Akt. This insulin-stimulated metabolic signaling pathway is severely impaired by trauma and hemorrhage (TH) potentially at several steps along the pathway. (ii) Insulin signaling also activates the MEK/ERK pathway, which is only modestly affected by trauma and hemorrhage. Hashed arrows indicate possible cross signaling/effects of the two different pathways. Ins, insulin; IR, insulin receptor; PY, phospho-tyrosine; TH, trauma and hemorrhage; PI3K, phosphatidylinositol 3-kinase; IRSs, insulin receptor substrates; AKT, protein kinase B; MEK, mitogen activated protein kinase kinase; ERK, extracellular signal-regulated kinase; Grb2, growth factor binding protein 2; Sos, son of sevenless complex; Shc, sequence homology of collagen; Ras, rat sarcoma protein; Raf, Ras binding protein.
In our recent studies, the IRS/PI3K/Akt pathway is severely impaired following trauma and hemorrhage in rat liver and skeletal muscle [4-8]. There was a near complete loss of insulin-activated phosphorylation of IR, IRS-1, IRS-2 and Akt. Since this defect in insulin signaling begins by 15 minutes in liver and by 60 min in skeletal muscle following trauma and hemorrhage, it is not surprising that there is little change in the total protein levels of IR, IRS-1, IRS-2, or Akt (Figure 2). Insulin regulation of the hepatic MEK/ERK pathway is barely affected by the combination of trauma and hemorrhage (Figure 1) [4,5]. This is similar to studies in Type 2 diabetic patients, where there is often little change in insulin regulation of the MEK/ERK pathway, coincident with a dramatic reduction of insulin-stimulated phosphorylation of IR, IRS-1 and insulin-induced PI3K activation [46].
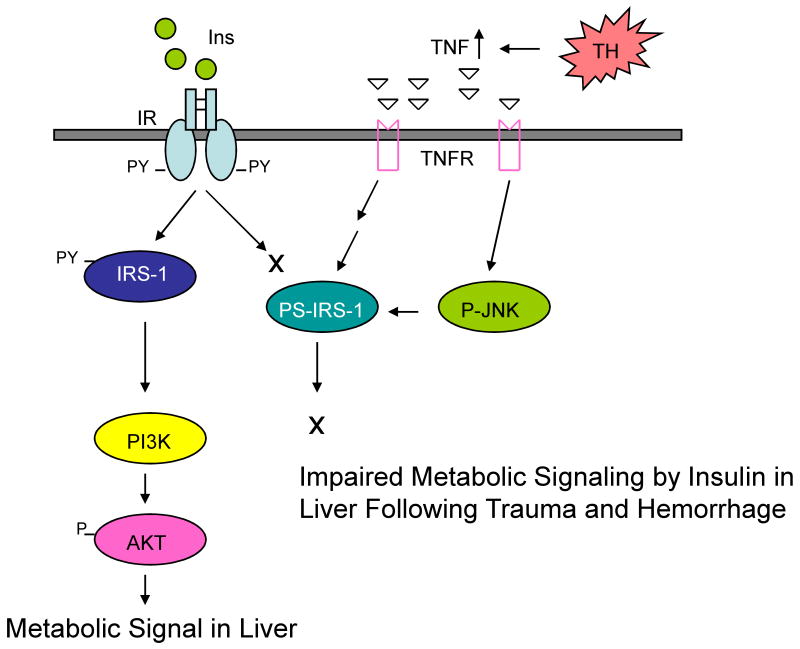
Figure 2.
TNFα-mediated acute hepatic insulin resistance after trauma and hemorrhage (TH). Following trauma and hemorrhage, TNFα levels increase, bind to the TNF receptor (TNFR) on the cell membrane, which activates JNK and serine (rat S307, human S312) phosphorylation of IRS-1. This inhibits tyrosine phosphorylation of IRS-1, which decreases association of PI3K with IRS-1 and impairs Akt phosphorylation, resulting in acute insulin resistance in liver following trauma and hemorrhage. This mechanism may not occur in skeletal muscle. Ins, insulin; IR, insulin receptor; PY, phospho-tyrosine; TH, trauma and hemorrhage; PI3K, phosphatidylinositol 3-kinase; IRSs, insulin receptor substrates; AKT, protein kinase B; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor; PS, phosphor-serine; JNK, c-Jun NH2-Terminal Kinase.
Skeletal muscle fibers can be broadly classified as either slow- or fast-twitch. Slow-twitch muscles are more efficient at using oxygen to generate ATP for continuous, extended muscle contractions before they fatigue. Fast-twitch fibers are better at generating short bursts of strength or speed, fatigue more quickly, and have greater anaerobic capacity. Insulin is known to elicit a greater response in slow-twitch skeletal muscles [47], but severe inhibition of insulin signaling occurs in both slow-twitch (triceps) and fast-twitch (extensor digitorum longus) muscles in the rat following trauma and hemorrhage [7,8]. However, there is only a modest decrease of insulin signaling in the diaphragm (slow-twitch) and little change in cardiac muscle [8]. This can perhaps be explained because the diaphragm (essential for proper ventilation) and the heart (already severely taxed by the decrease in blood volume and pressure) are critical to ensure oxygenation of the remaining blood and perfusion of the brain and heart. It is possible that maintenance of blood flow to the heart and diaphragm protects these tissues from developing severe insulin resistance. However, it is clear from these studies that in skeletal muscles involved in locomotion (such as the triceps and extensor digitorum longus), insulin signaling is rapidly compromised for the overall benefit of the critically injured individuals (Figure 3) [7,8].
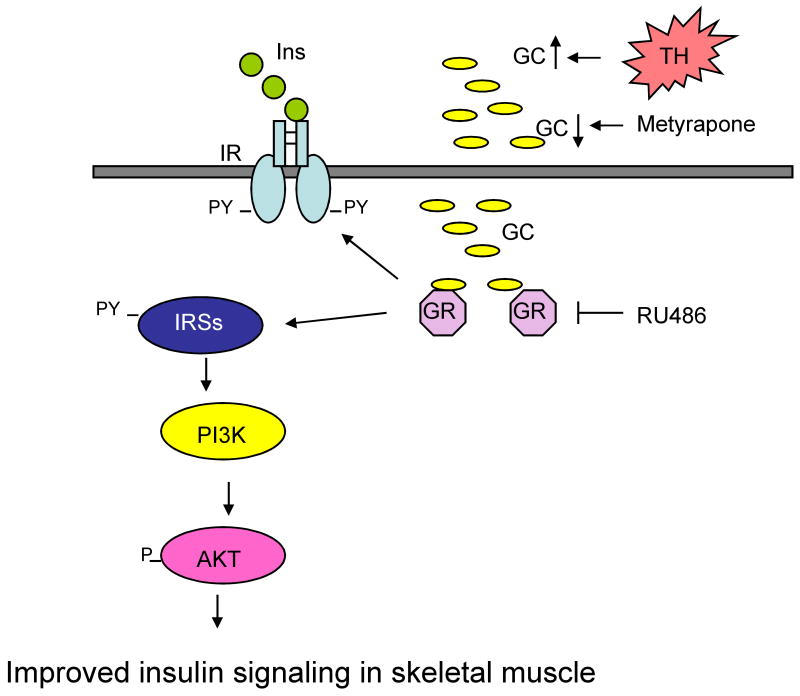
Figure 3.
Glucocorticoid-mediated acute insulin resistance in skeletal muscle after trauma and hemorrhage (TH). Blocking the production of glucocorticoids by metyrapone or their action by RU486 inhibits the development of insulin resistance in skeletal muscle, but not liver, thereby allowing an increase in insulin-induced phosphorylation of IR, IRS-1 and Akt. Ins, insulin; IR, insulin receptor; PY, phospho-tyrosine; TH, trauma and hemorrhage; PI3K, phosphatidylinositol 3-kinase; IRSs, insulin receptor substrates; AKT, protein kinase B; GC, glucocorticoids; GR, glucocorticoid receptor.
Downstream of impaired insulin signaling following trauma and hemorrhage, are changes in insulin action. For example, circulating insulin-like growth factor binding protein (IGF-BP) increases in critical illness in humans, is inversely correlated with the severity of hepatic insulin resistance and survival [48], and hepatic IGF-BP1 mRNA increases rapidly in the acute insulin resistant state obtained in our rodent trauma and hemorrhage model [4]. These data suggest that hepatic insulin resistance can be at least partially overcome by high levels of exogenous insulin. For instance, intensive insulin therapy decreases the elevated triglyceride levels observed in critical illness and increases circulating HDL levels [49]. However, not all hepatic actions of insulin respond to intensive insulin therapy. For example, the increased hepatic expression of phosphoenolpyruvate carboxykinase and glucokinase were unaltered by insulin therapy [48,49]. Thus, a much greater understanding of the causative factors and mechanisms of development of acute insulin resistance in each major target tissue is needed to understand how to counteract injury/critical illness-induced insulin resistance. Furthermore, other tissues normally respond to insulin (e.g. kidney, heart, blood vessels, etc), so we also need to understand the mechanism of development and pathophysiological effects of injury/critical illness insulin resistance in these insulin targets.
Mechanisms of acute insulin resistance following hemorrhage, sepsis and burn
As with chronic forms of insulin resistance, there are multiple potential cellular mechanisms for the acute development of insulin resistance. Based on chronic insulin resistant states, two potential mechanisms for injury-induced insulin resistance are increases in circulating free fatty acids or in cellular levels of suppressors of cytokine signaling (SOCS) proteins which, via increasing inhibitory serine phosphorylation or degradation of IRS proteins, respectively, result in decreased IRS signaling [50-52]. Free fatty acids can be increased by the elevated glucocorticoid levels observed following injury or infection [53], and SOCS proteins are regulated by many cytokines that are increased following injury [54,55]. However, in the trauma and hemorrhage rat model, insulin resistance develops rapidly in the liver with insufficient time for an increase in free fatty acid levels or SOCS proteins [7].
In response to injury or infection, there are increases in counter-regulatory hormones, such as catecholamines and glucocorticoids, which might contribute to the development of insulin resistance [56-58]. Catecholamines are increased following injury and infections, but their direct role(s) in inducing acute insulin resistance have not yet been investigated. Elevated glucocorticoid levels are known to cause insulin resistance within several hours [59-61] or days [62,63]. However, other actions of glucocorticoids can occur more rapidly, sometimes within minutes [64,65]. Elevated corticosterone levels were measured in rats following trauma and hemorrhage [7]. When this increase in corticosterone levels was blocked by the glucocorticoid synthesis inhibitor, metyrapone, the acute development of insulin signaling defects in rat liver still occurs following trauma and hemorrhage. (Figure 3) [7]. In addition, the glucocorticoid receptor antagonist, RU486, is also ineffective in blocking or reversing acute insulin resistance in liver. However, in distinct contrast to the liver, blocking the rise in corticosterone levels by metyrapone, or blocking corticosterone action with RU486, prevents the development of acute skeletal muscle insulin resistance [7]. Thus, there are tissue-specific mechanisms for the development of insulin resistance following trauma and hemorrhage, with glucocorticoids playing a significant role in the acute development of insulin resistance in skeletal muscle, but not in the liver.
Multiple studies indicate a role of the inflammatory response in chronic insulin resistance, and the list of proinflammatory cytokines and chemokines that contribute to the insulin resistant state of Type 2 diabetes and obesity has increased over the years [66,67]. Some proinflammatory cytokines including TNFα activate the JNK and IKKβ/NF-kB pathways [68-70], which can result in the inhibitory serine phosphorylation of IRS-1, decreasing insulin signaling via the IRS-1/PI3K/Akt pathway (Figure 2). Following trauma and hemorrhage in rodents, there are increases in plasma TNFα concentrations and liver TNFα mRNA [5,6]. Administration of a TNFα neutralizing antibody following trauma and hemorrhage reverses the acute insulin resistant state in the liver. This coincides with decreased hepatic JNK activity and reduced hepatic IRS-1 serine phosphorylation [6]. However, in skeletal muscle, there was little measurable increase in JNK activity or IRS-1 serine phosphorylation following trauma and hemorrhage [7]. Thus, from what we know to date, there are dramatically different mechanisms for the acute development of insulin resistance between the liver, which may involve TNFα, JNK activity and IRS-1 serine phosphorylation, and skeletal muscle, which depends on the increase and action of glucocorticoids (Figures 2 and 3). Therefore, it may be possible to independently regulate the insulin resistant state following injury or critical illness in these two major insulin target tissues.
Cellular stresses, including increases in reactive oxygen species (ROS) and endoplasmic reticulum (ER) stress can activate the JNK and IKKβ/NF-kB pathways to impair insulin signaling [71-74]. These and other cell stress pathways may be involved in the development of acute insulin resistance following injury and critical illness and need to be investigated. Also, the insulin resistant state may be caused by an increase in the activity or amounts of proteins that normally inhibit insulin action, including the phosphotyrosine phosphatases PTEN (phosphatase and tensin homolog) and SHIP (Src homology 2 domain-containing inositol 5′-phosphatase) [75,76]. These and other proteins that inhibit insulin signaling following injury and critical illness, especially in a tissue-specific manner, also need to be explored. However, it is already clear that a single mechanism cannot explain the acute insulin resistance that develops following injury or critical illness. Multiple causative factors via different mechanisms may contribute to the development of tissue-specific acute insulin resistance following injury and critical illness (Figures 2 and 3).
Conclusions
As with chronic forms of insulin resistance, the development of insulin resistance following injury and critical illness, what we refer to as acute insulin resistance, is complex and may involve many causative factors and intracellular signaling pathways, most likely in a tissue-specific manner. There are few mechanistic studies outside of the trauma and hemorrhage model. The reports with animal models of burn injury and sepsis give little detail on the causative factors and mechanisms leading to the acute insulin resistant state. Thus, there is the distinct possibility of different mechanisms leading to the development of acute insulin resistance following different injuries, infections or other critical illnesses, which together make up the ICU patient population. Understanding how acute insulin resistance develops is a high priority if we are to properly manage the diverse ICU population. Whether anti-inflammatory strategies and/or glucocorticoid inhibitors are beneficial in critically ill patients has yet to be investigated. The role of preexisting diabetes, obesity, and other preexisting conditions may also radically alter the approach to managing hyperglycemia and insulin resistance in critically ill patients. Intensive insulin therapy has significantly reduced the mortality and morbidity of patients in the ICU. However, due to the increased incidence of hypoglycemic incidents following intensive insulin therapy, it may be preferable to use a more targeted approach, for instance to reverse the acute insulin resistance in either skeletal muscle or liver. In addition, the participation or role of adipose tissue in the acute development of insulin resistance needs to be addressed. This will only be possible when we understand the tissue-specific mechanisms underlying the development of acute insulin resistance and whether those mechanisms differ following different injuries and critical illnesses.
Acknowledgments
This research is supported by grants from the National Institutes of Health Grant (NIDDK), the Department of Defense, and the Veterans Administration Merit Review to J.L.M. We thank Drs. LaWanda H. Thompson, Hyeong T. Kim, Jie Xu, Yuchen Ma, Natalia A. Kokorina, and Ling Zhao, as well as Tatyana Gavrikova, Rachel Martin and J.L. Franklin for their involvement in this work. We also would like to thank Dr. Martin G. Schwacha for his helpful and insightful discussions and suggestions. We would like to acknowledge the members of the UAB Center for Surgical Research for vital assistance in setting up the animal model in our lab. We also thank the UAB Clinical Nutrition Research Unit (P30 DK56336) and Dr. Barbara Gower for glucose, insulin, cytokine, FFA and corticosterone measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Van den Berghe G. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Thorell A, et al. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69–78. doi: 10.1097/00075197-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 3.McCowen KC, et al. Sustained endotoxemia leads to marked down-regulation of early steps in the insulin-signaling cascade. Critical Care Medicine. 2001;29:839–846. doi: 10.1097/00003246-200104000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, et al. Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2003;284:G107–G115. doi: 10.1152/ajpgi.00217.2002. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, et al. Mechanisms of hemorrhage-induced hepatic insulin resistance: role of tumor necrosis factor-alpha. Endocrinology. 2004;145:5168–5176. doi: 10.1210/en.2004-0524. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, et al. Trauma and Hemorrhage-Induced Acute Hepatic Insulin Resistance: Dominant Role of Tumor Necrosis Factor (TNF)-alpha. Endocrinology. 2008;149:2369–2382. doi: 10.1210/en.2007-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, et al. Tissue Specific Difference in the Molecular Mechanisms for the Development of Acute Insulin Resistance Following Injury. Endocrinology. 2009;150:24–32. doi: 10.1210/en.2008-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson LH, et al. Acute muscle-type specific insulin resistance following injury. Mol Med. 2008;11-12:715–723. doi: 10.2119/2008-00081.Thompson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simkova V, et al. Year in review 2006: Critical Care--Multiple organ failure, sepsis, and shock. Crit Care. 2007;11:221. doi: 10.1186/cc5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent JL, et al. Reducing mortality in sepsis: new directions. Crit Care. 2002;6 3:S1–18. doi: 10.1186/cc1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 12.Bernard GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 13.Finney SJ, et al. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL. Metabolic support in sepsis and multiple organ failure: more questions than answers. Crit Care Med. 2007;35:S436–S440. doi: 10.1097/01.CCM.0000278601.93369.72. [DOI] [PubMed] [Google Scholar]
- 15.Herndon DN, Wernerman J. Metabolic support in sepsis and multiple organ failure. Crit Care Med. 2007;35:S435. doi: 10.1097/01.CCM.0000281832.42767.86. [DOI] [PubMed] [Google Scholar]
- 16.Van Cromphaut SJ, et al. Glucose metabolism and insulin resistance in sepsis. Curr Pharm Des. 2008;14:1887–1899. doi: 10.2174/138161208784980563. [DOI] [PubMed] [Google Scholar]
- 17.Desouza C, et al. Management of the insulin resistance syndrome. Curr Diab Rep. 2001;1:140–147. doi: 10.1007/s11892-001-0026-6. [DOI] [PubMed] [Google Scholar]
- 18.Kendall DM, Harmel AP. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: understanding the role of insulin resistance. Am J Manag Care. 2002;8:S635–S653. [PubMed] [Google Scholar]
- 19.Van den Berghe G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 20.Krinsley JS. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2006;18:317–325. doi: 10.1053/j.semtcvs.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 22.Finfer S, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 23.Griesdale DE, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. Can Med Assoc J. 2009;180:821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunkhorst FM, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 25.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 26.Ng SM, et al. Continuous insulin infusion in hyperglycaemic extremely-low- birth-weight neonates. Biol Neonate. 2005;87:269–272. doi: 10.1159/000083863. [DOI] [PubMed] [Google Scholar]
- 27.Beardsall K, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359:1873–1884. doi: 10.1056/NEJMoa0803725. [DOI] [PubMed] [Google Scholar]
- 28.Nasraway SA., Jr Hyperglycemia during critical illness. JPEN J Parenter Enteral Nutr. 2006;30:254–258. doi: 10.1177/0148607106030003254. [DOI] [PubMed] [Google Scholar]
- 29.Egi M, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 30.Arabi YM, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 31.Fatourechi MM, et al. Hypoglycemia with intensive insulin therapy. A systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab. 2008;94:729–740. doi: 10.1210/jc.2008-1415. [DOI] [PubMed] [Google Scholar]
- 32.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35:2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 33.Vanhorebeek I, et al. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 34.bdul-Ghani MA, et al. Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia. 2009;52:574–582. doi: 10.1007/s00125-009-1264-4. [DOI] [PubMed] [Google Scholar]
- 35.Santangelo C, et al. Hepatocyte growth factor protects rat RINm5F cell line against free fatty acid-induced apoptosis by counteracting oxidative stress. J Mol Endocrinol. 2007;38:147–158. doi: 10.1677/jme.1.02133. [DOI] [PubMed] [Google Scholar]
- 36.Stephens JM, et al. Differential regulation of glucose transporter gene expression in adipose tissue or septic rats. Biochem Biophys Res Commun. 1992;183:417–422. doi: 10.1016/0006-291x(92)90497-9. [DOI] [PubMed] [Google Scholar]
- 37.Virkamaki A, Yki-Jarvinen H. Mechanisms of insulin resistance during acute endotoxemia. Endocrinology. 1994;134:2072–2078. doi: 10.1210/endo.134.5.8156907. [DOI] [PubMed] [Google Scholar]
- 38.Hessman Y, Thoren L. Glycogen storage in rat liver and skeletal muscle in thermal trauma. I. Effect of exogenous insulin. Acta Chir Scand. 1975;141:385–392. [PubMed] [Google Scholar]
- 39.Nelson KM, Turinsky J. Effect of insulin on glucose and amino acid uptake by skeletal muscle following burn injury. Studies with 2-deoxyglucose and alpha-aminoisobutyric acid. JPEN J Parenter Enteral Nutr. 1982;6:3–8. doi: 10.1177/014860718200600103. [DOI] [PubMed] [Google Scholar]
- 40.Cree MG, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007;245:214–221. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cree MG, et al. Role of fat metabolism in burn trauma-induced skeletal muscle insulin resistance. Crit Care Med. 2007;35:S476–S483. doi: 10.1097/01.CCM.0000278066.05354.53. [DOI] [PubMed] [Google Scholar]
- 42.Ikezu T, et al. Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J Biol Chem. 1997;272:25289–25295. doi: 10.1074/jbc.272.40.25289. [DOI] [PubMed] [Google Scholar]
- 43.Sugita H, et al. Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E585–E591. doi: 10.1152/ajpendo.00321.2004. [DOI] [PubMed] [Google Scholar]
- 44.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 45.Nakae J, Accili D. The mechanism of insulin action. J Pediatr Endocrinol Metab. 1999;12 3:721–731. [PubMed] [Google Scholar]
- 46.Cusi K, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James DE, et al. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985;248:E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- 48.Mesotten D, et al. Regulation of insulin-like growth factor binding protein-1 during protracted critical illness. J Clin Endocrinol Metab. 2002;87:5516–5523. doi: 10.1210/jc.2002-020664. [DOI] [PubMed] [Google Scholar]
- 49.Mesotten D, et al. Contribution of circulating lipids to the improved outcome of critical illness by glycemic control with intensive insulin therapy. J Clin Endocrinol Metab. 2004;89:219–226. doi: 10.1210/jc.2003-030760. [DOI] [PubMed] [Google Scholar]
- 50.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 51.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 52.Rui L, et al. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 53.Slavin BG, et al. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res. 1994;35:1535–1541. [PubMed] [Google Scholar]
- 54.Fasshauer M, et al. Insulin resistance-inducing cytokines differentially regulate SOCS mRNA expression via growth factor- and Jak/Stat-signaling pathways in 3T3-L1 adipocytes. J Endocrinol. 2004;181:129–138. doi: 10.1677/joe.0.1810129. [DOI] [PubMed] [Google Scholar]
- 55.Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 56.Vaughan GM, et al. Cortisol and corticotrophin in burned patients. J Trauma. 1982;22:263–273. doi: 10.1097/00005373-198204000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Jurney TH, et al. Spectrum of serum cortisol response to ACTH in ICU patients. Correlation with degree of illness and mortality. Chest. 1987;92:292–295. doi: 10.1378/chest.92.2.292. [DOI] [PubMed] [Google Scholar]
- 58.Span LF, et al. Adrenocortical function: an indicator of severity of disease and survival in chronic critically ill patients. Intensive Care Med. 1992;18:93–96. doi: 10.1007/BF01705039. [DOI] [PubMed] [Google Scholar]
- 59.Phillips DI, et al. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- 60.Nosadini R, et al. Insulin resistance in Cushing's syndrome. J Clin Endocrinol Metab. 1983;57:529–536. doi: 10.1210/jcem-57-3-529. [DOI] [PubMed] [Google Scholar]
- 61.Pagano G, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest. 1983;72:1814–1820. doi: 10.1172/JCI111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Binnert C, et al. Dexamethasone-induced insulin resistance shows no gender difference in healthy humans. Diabetes Metab. 2004;30:321–326. doi: 10.1016/s1262-3636(07)70123-4. [DOI] [PubMed] [Google Scholar]
- 63.Ruzzin J, et al. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48:2119–2130. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 64.Atkinson HC, et al. Corticosteroids mediate fast feedback of the rat hypothalamic-pituitary-adrenal axis via the mineralocorticoid receptor. Am J Physiol Endocrinol Metab. 2008;294:E1011–E1022. doi: 10.1152/ajpendo.00721.2007. [DOI] [PubMed] [Google Scholar]
- 65.Hyde GN, et al. Cortisol rapidly suppresses intracellular calcium and voltage-gated calcium channel activity in prolactin cells of the tilapia (Oreochromis mossambicus) Am J Physiol Endocrinol Metab. 2004;286:E626–E633. doi: 10.1152/ajpendo.00088.2003. [DOI] [PubMed] [Google Scholar]
- 66.Hotamisligil GS, et al. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 67.Feinstein R, et al. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 68.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 69.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 70.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 71.Lin Y, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- 72.Eriksson JW. Metabolic stress in insulin's target cells leads to ROS accumulation - a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581:3734–3742. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 73.Kumashiro N, et al. Impact of oxidative stress and peroxisome proliferator-activated receptor gamma coactivator-1alpha in hepatic insulin resistance. Diabetes. 2008;57:2083–2091. doi: 10.2337/db08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clement S, et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature. 2001;409:92–97. doi: 10.1038/35051094. [DOI] [PubMed] [Google Scholar]
- 76.Nakashima N, et al. The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J Biol Chem. 2000;275:12889–12895. doi: 10.1074/jbc.275.17.12889. [DOI] [PubMed] [Google Scholar]