Summary
Regulatory T cells (Tregs) can suppress autoimmune diseases such as type 1 diabetes (T1D), but their in vivo activity during suppression remains poorly characterized. In T1D, Treg activity has been demonstrated in the pancreatic lymph node, but little has been studied in the pancreas, the site of autoimmune islet destruction. In this study we induced islet-specific Tregs from the BDC-6.9 TCR-Tg mouse by activation of T cells in the presence of TGF-β. These Tregs can suppress spontaneous diabetes as well as transfer of diabetes into NOD.scid mice by diabetic NOD spleen cells or activated BDC-2.5 TCR-Tg Th1 effector T cells. In the latter transfer model, we observed infiltration of the pancreas by both effector T cells and Tregs, suggesting that Tregs are active in the inflammatory site and are not just restricted to the draining lymph node. Within the pancreas, we demonstrate that Treg transfer causes a reduction in the number of effector Th1 T cells and macrophages, and also inhibits effector T cell cytokine and chemokine production. While we found no role for TGF-β in vitro, transfection of effector T cells with a dominant negative TGF-β receptor demonstrated that in vivo suppression of diabetes by TGF-β-induced Tregs is TGF-β-dependent.
Keywords: Regulatory T cells, Autoimmunity, Tolerance, Diabetes, Rodent
Introduction
While regulatory T cells (Tregs) have been shown to suppress immune responses in a variety of disease models, how Tregs function in sites of inflammation remains poorly understood. The NOD mouse model of type 1 diabetes (T1D), in which T cell immunity against islet cell antigens in the pancreas results in autoimmune destruction of the insulin-producing beta cells, has been a valuable model for examination of in vivo Treg activity. Anti-islet autoimmunity can be inhibited by transfer of “natural” CD4+CD25+ Tregs, [1–4], or by induced Tregs which upregulate the Treg transcription factor Foxp3 after activation of CD4 T cells in the presence of TGF-β [5, 6]. Despite the many examples of Treg inhibition in T1D, the process by which Tregs affect autoimmune inflammation is not well defined.
A variety of mechanisms have been proposed to explain the suppressive effects of Tregs. One early hypothesis was that Tregs prevent proliferation of other lymphocytes by acting as a sink for IL-2 [7], an idea which has been recently revived [8]. Several reports have concluded that Tregs function by cytotoxic killing of effector cells, mediated by perforin [9], granzyme B [10], or FasL [5]. Other labs have reported suppressive roles for CTLA-4 on Tregs [11], and also for various Treg-secreted cytokines such as IL-10 [12, 13], IL-9 [14] and IL-35 [15]. More than any other factor, however, the suppressive cytokine TGF-β has been postulated to be the primary mediator of Treg function.
Although TGF-β has been frequently examined as a key player in Treg-mediated suppression, conclusions regarding the suppressive role of this cytokine have been inconsistent. While Treg production of TGF-β has been reported by some labs [5, 16, 17], others have been unable to reproduce such results [18]. A role for TGF-β during in vitro suppression by Tregs remains inconclusive, as blocking studies have yielded mixed results [16, 18–20]. Further evidence against a role for TGF-β was demonstrated in a study showing effective in vitro suppression by TGF-β-deficient Tregs [18], as well as by a demonstration of Treg-mediated inhibition of T effectors deficient for the TGF-β signaling molecule Smad3 [21]. These in vitro findings have not always been consistent with in vivo studies of TGF-β. Treg-mediated suppression has been blocked in vivo with TGF-β specific antibodies [3, 22], and studies of effector T cells that express a dominant-negative TGF-β receptor have indicated that inhibition by Tregs is TGF-β-dependent [17, 23–25], although one study of Smad3 deficient mice showed that Treg suppression of inflammatory bowel disease was TGF-β-independent [26].
With regard to Tregs in T1D, recent studies focusing on Tregs in the draining pancreatic lymph node have shown that transfer of Tregs results in a decrease in the number of Th1 effector T cells in the lymph node [5], and also that Tregs appear to interfere with the interaction between T effectors and dendritic cells [27]. However, it is notable that in studies of T1D, little has been reported on Treg function and the requirements for TGF-β in the pancreas, the actual site of immune-mediated destruction. We address the in vivo behavior of Tregs in this report through the use of the BDC-6.9 T cell receptor transgenic (TCR-Tg) NOD mouse model, as a source of CD4 T cells induced through culture with TGF-β to produce a Foxp3+ inhibitory Treg population [6]. These TGF-β-induced Tregs can suppress diabetes induced in NOD and NOD.scid mice by adoptive transfer of islet-specific T effectors derived from a second TCR-Tg mouse, the BDC-2.5 TCR-Tg/NOD mouse. We have previously demonstrated ex vivo analysis of cytokine production by pancreas-infiltrating cells after transfer of diabetogenic Th1 T cell clones [28], and in this study, we show how effector function of Th1 T cells and macrophages is affected by the presence of TGF-β-induced Tregs in the pancreas during diabetes induction. We also demonstrate unequivocally that these TGF-β-induced Tregs require the presence of TGF-β in vivo for suppression of diabetes.
Results
Prevention of adoptive transfer of diabetes by TGF-β-induced Tregs
The treatment of CD4 T cells from the BDC-6.9 TCR-Tg mouse with anti-CD3 and TGF-β resulted in TGF-β-induced Tregs with high levels of Foxp3 expression, and little to no production of IFN-γ upon stimulation with antigen/MHC [6]. Upon phenotypic examination by intracellular cytokine staining of TGF-β-induced Tregs, TGF-β was found to be elevated but detection of this cytokine was inconsistent; only very low levels of IL-10 were present [6]. We also investigated chemokine production by TGF-β-induced Tregs and found that in addition to decreased levels of RANTES in TGF-β-induced Tregs as previously reported [5], there were also lower amounts of MIP-1α, MIP-1β, and MIP-1γ. Cytokine/chemokine production by TGF-β-induced Tregs is summarized in Table 1.
Table I.
TGF-β-induced Tregs express increased levels of Foxp3 and produce reduced levels of IFN-γ and chemokines.a
| Foxp3 | IFN-γ | TGF-βb | RANTES | MIP-1α | MIP-1β | MIP-1-γ | |
|---|---|---|---|---|---|---|---|
| Th1 | 3.4 | 75.3 | 2.4 | 6.0 | 50.3 | 25.3 | 11.4 |
| Treg | 94.3 | 11.3 | 15.9 | 1.8 | 20.6 | 13.6 | 3.3 |
Th1 effector cells and TGF-β-induced Tregs were derived from BDC-6.9 TCR-Tg CD4 cells as described in the Materials and Methods. Intracellular Foxp3 protein production was analyzed by flow cytometry and production of cytokines was measured by intracellular staining after stimulation with anti-CD3. Values displayed indicate the percentage of Foxp3 or cytokine-positive cells from representative experiments.
TGF-β could not be detected in all experiments.
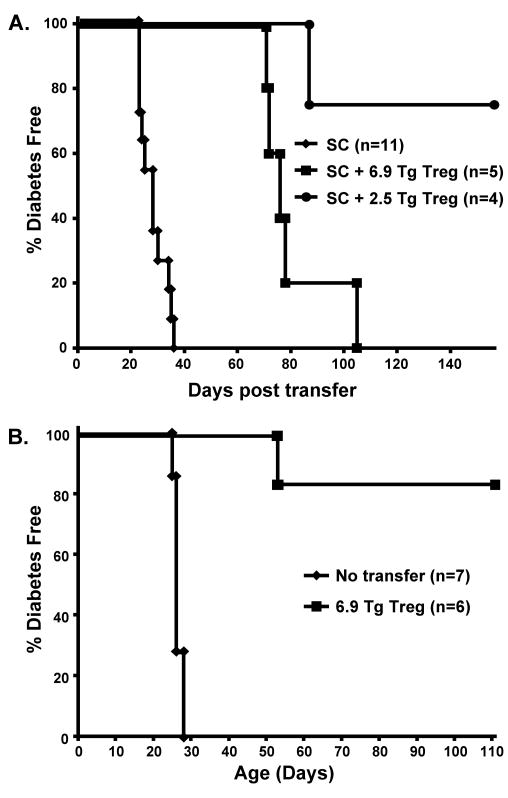
We have previously shown the ability of TGF-β-induced BDC-6.9 TCR-Tg Tregs to suppress adoptive transfer of diabetes by BDC-2.5 TCR-Tg T cells into both NOD and NOD.scid recipient mice [6]. In order to assess whether these TGF-β-induced Tregs can suppress a polyclonal population of diabetogenic CD4 and CD8 T cells, as would be encountered during natural development of T1D, we assessed the capacity of TGF-β-induced TCR-Tg Tregs to inhibit transfer of diabetes by spleen cells harvested from spontaneously diabetic NOD mice. We injected 1 × 107 spleen cells into young NOD.scid recipients (< 2 weeks old), some of which received a cotransfer of an equal number of BDC-2.5 TCR-Tg or BDC-6.9 TCR-Tg TGF-β-induced Tregs. A majority of the mice receiving BDC-2.5 TCR-Tg TGF-β-induced Treg cotransfers were protected from diabetes (p < 0.01). BDC-6.9 TCR-Tg TGF-β-induced Tregs significantly delayed diabetes an average of 52 days (p < 0.002), although these mice did eventually develop diabetes (Fig 1A), suggesting that greater numbers of TGF-β-induced Tregs may be required to completely suppress in this model. Nevertheless, the results demonstrate that both BDC-2.5 and BDC-6.9 TCR-Tg TGF-β-induced Tregs can suppress polyclonal populations of diabetogenic NOD T cells.
Figure 1. 6.9 TCR-Tg TGF-β-induced Tregs suppress diabetes transfer by diabetic NOD spleen cells and also spontaneous diabetes in 2.5 TCR-Tg/NOD.scid recipients.
A. NOD diabetic spleen cells (SC) (1 × 107) were injected into NOD.scid mice ≤ 2 weeks of age either alone or co-injected with TGF-β-induced Tregs (1 × 107) from either 6.9 TCR-Tg/NOD.C6 mice or 2.5 TCR-Tg/NOD mice. B. 2.5 TCR-Tg/NOD.scid mice were injected at ≤ 18 days of age with 6.9 TCR-Tg TGF-β-induced Tregs (1 × 107). Diabetes incidence was measured by daily urine glucose readings, and positive readings were confirmed by blood glucose analysis.
Suppression of spontaneous diabetes is of particular clinical relevance for human diabetes. Other labs have demonstrated that transfer of natural or TGF-β-induced Tregs can reduce spontaneous diabetes in NOD mice [1, 2, 5]. We tested suppression by BDC-6.9 TCR-Tg TGF-β-induced Tregs in a rapid model of spontaneous diabetes, the BDC-2.5 TCR-Tg/NOD.scid mouse. In contrast to the BDC-2.5 TCR-Tg/NOD mouse, the BDC-2.5 TCR-Tg/NOD.scid contains no Tregs, and diabetes spontaneously develops in three to five weeks [29, 30]. We chose this system because it is much more aggressive, rapid, and consistent than is development of T1D in the spontaneous NOD model. Fig 1B demonstrates that transfer of TGF-β-induced Tregs significantly reduces spontaneous diabetes incidence in the BDC-2.5 TCR-Tg/NOD.scid mouse (p < 0.01), completely preventing disease in most cases (Fig 1B). This outcome demonstrates that BDC-6.9 TCR-Tg TGF-β-induced Tregs can suppress spontaneous diabetes, a result that is particularly dramatic in this accelerated model in which all mice are diabetic within five weeks.
TGF-β-induced Tregs reduce numbers of effector T cells and macrophages in the pancreas
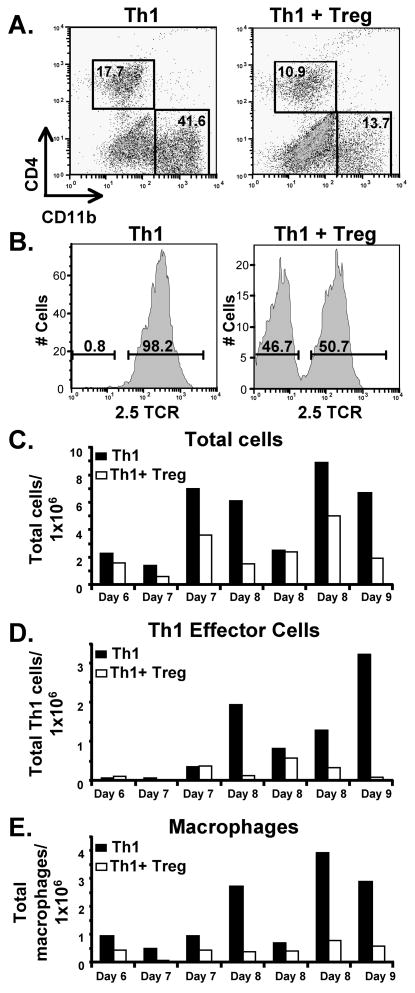
A major objective of this study was to analyze the effects of TGF-β-induced Tregs on pancreas-infiltrating leukocytes during inhibition of diabetes. Because spontaneous diabetes develops slowly and with wide variation from mouse to mouse in NOD mice, it was advantageous to examine an accelerated model of diabetes in which diabetes develops rapidly and consistently, as is the case with transfer of islet-reactive TCR-Tg T cells. We transferred BDC-2.5 TCR-Tg Th1 effector T cells into adult NOD.scid recipients alone or in the presence of BDC-6.9 TCR-Tg TGF-β-induced Tregs. Six to nine days following transfer, pancreata were harvested, digested with collagenase, and homogenized to isolate infiltrating leukocytes. Transferred T cells were identified by staining for CD4, and macrophages were identified as CD11b-positive cells (Fig 2A), which we confirmed were macrophages by positive co-staining for F4/80 (data not shown). Pancreas-infiltrating Th1 cells and TGF-β-induced Tregs were differentiated by staining the Th1 cells with the clonotypic antibody aBDC [31] which is specific for the BDC-2.5 TCR (Fig 2B). In mice injected with Th1 cells alone, the average number of total infiltrating cells in the pancreas increased over time, although there was significant variation from experiment to experiment (Fig 2C). Very few infiltrating cells were observed in the pancreas before day six (not shown), and cell numbers were at their highest on days eight and nine. In mice co-injected with TGF-β-induced Tregs, the total cell infiltrate was significantly reduced by an average of approximately 50% (p = 0.01) (Fig 2C). The number of Th1 cells in the pancreas was decreased in mice only by days eight and nine after transfer (Fig 2D). While the decrease in Th1 cells was not quite statistically significant (p = 0.10), reduced Th1 numbers were consistently observed in all four analyses of pancreas infiltrate on days 8 and 9. Macrophage infiltration in the pancreas was evaluated by CD11b staining (Fig 2A). The presence of TGF-β-induced Tregs strongly reduced macrophage numbers at all time points (Fig 2E) (p = 0.02).
Figure 2. TGF-β-induced Treg-mediated suppression of T cell and macrophage infiltration into the pancreas.
2.5 TCR-Tg Th1 cells (2 × 106) were transferred into NOD.scid mice alone or in a cotransfer with 6.9 TCR-Tg TGF-β-induced Tregs (1 × 107). The pancreas from each was harvested after 6–9 days and pancreata from 2–3 mice in each group were pooled for each experiment. A. After collagenase digestion, cells were stained with fluorescent antibodies for CD4 and CD11b and analyzed by flow cytometry. B. The infiltrating CD4+ cells were separated by flow cytometry into 2.5 TCR+ (Th1) and 2.5 TCR- (Treg) populations. C. Total live cells infiltrating the pancreas were determined by counting leukocytes on a hemacytometer after collagenase digestion of pooled pancreata for each condition. Average cell numbers per pancreas are shown for each of seven separate experiments in which pancreata were harvested on the day indicated on the x axis. D. Th1 cell infiltrate was calculated based on the percent of CD4+ 2.5 TCR+ T cells. E. Macrophage numbers were calculated based on the percent of CD11b+ cells.
Suppression of inflammatory cytokines in the pancreas by TGF-β-induced Tregs
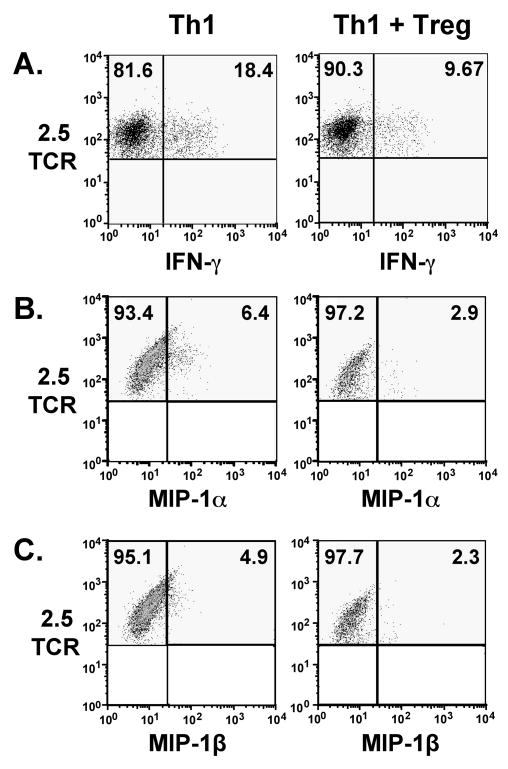
In previous studies of TGF-β-induced Treg-mediated suppression of diabetes induced by BDC-2.5 TCR-Tg Th1 cells, we have demonstrated suppression of effector T cell IFN-γ as illustrated in Fig. 3A from a different experiment), and TNF [6]. In this report, we have extended our ex vivo data to evaluation of chemokine production by pancreas-infiltrating T cells 6–9 days after transfer. T cells were activated with plate-bound anti-CD3 antibody in the presence of brefeldin A, followed by intracellular staining and analysis by flow cytometry. We observed production of low levels of the chemokines MIP-1α and MIP-1β by the Th1 cells, and production of these chemokines was reduced in the presence of TGF-β-induced Tregs (Fig 3B,C). We observed little production of cytokines by TGF-β-induced Tregs, including TGF-β and IL-10 (data not shown).
Figure 3. Cytokine and chemokine production by pancreas-infiltrating Th1 T cells is decreased in the presence of TGF-β-induced Tregs.
6–9 days after T cell transfer as described in Fig. 2, pancreata were harvested and pooled pancreatic cell suspensions from 2–3 mice per group were activated with plate-bound anti-CD3 (5 μg/ml) for 5 h in the presence of GolgiPlug. Cells were then stained for intracellular cytokines and chemokines. Plots are gated on CD11b-, CD4+, BDC-2.5 TCR+ cells. Quadrants were set based on staining with isotype control antibodies. (A.) IFN-γ, (B.) MIP-1α production and (C) MIP-1β production are suppressed in the presence of TGF-β-induced Tregs. Data shown represent consistent results from at least three experiments for each cytokine.
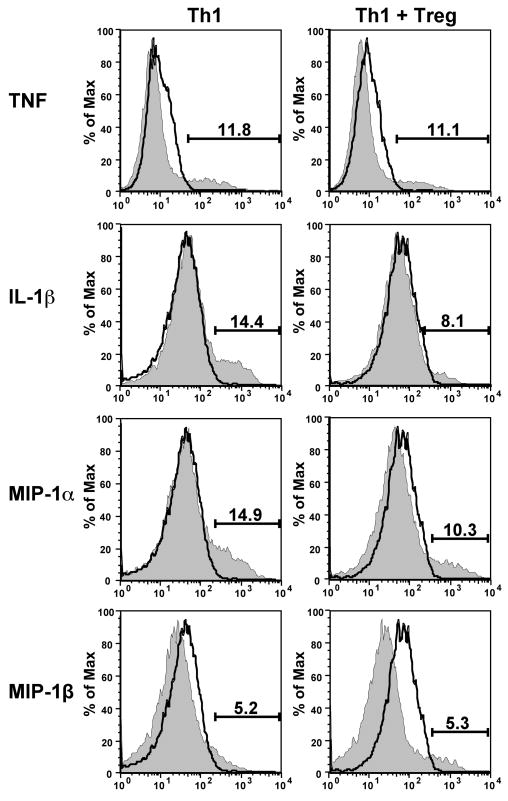
In addition to analysis of T cell cytokines, we investigated production of cytokines by infiltrating macrophages. We detected macrophage production of TNF, IL-1β, MIP-1α and MIP-1β. In the presence of TGF-β-induced Tregs, macrophage production of TNF and MIP-1β was consistently unaffected (Fig 4), while IL-1β and MIP-1α production was reduced in about half of the mice examined. Although we found no clear effect of TGF-β-induced Tregs on cytokine production by macrophages in the pancreas, because macrophages numbers were consistently reduced by TGF-β-induced Tregs, the total cytokine load produced by macrophages was also reduced, a result that might significantly reduce damage to beta-cells.
Figure 4. Effect of TGF-β-induced Tregs on macrophage cytokine production in the pancreas.
6–9 days after T cell transfer as described in Fig. 2, pancreata from 3–4 mice per group were harvested and pooled pancreatic cell suspensions were cultured for 5 h in the presence of brefeldin A before staining for intracellular cytokines. The histograms shown are gated on CD11b+ cells. In the experiment shown, IL-1β and MIP-1α but not TNF or MIP-1β production was suppressed. Histogram outlines indicate isotype control staining and shaded histograms indicate cytokine-specific staining. In four repeats of this experiment, we observed IL-1β and MIP-1α reduction in two cases.
Blocking TGF-β with soluble TGF-βR in vitro does not affect TGF-β-induced Treg suppression
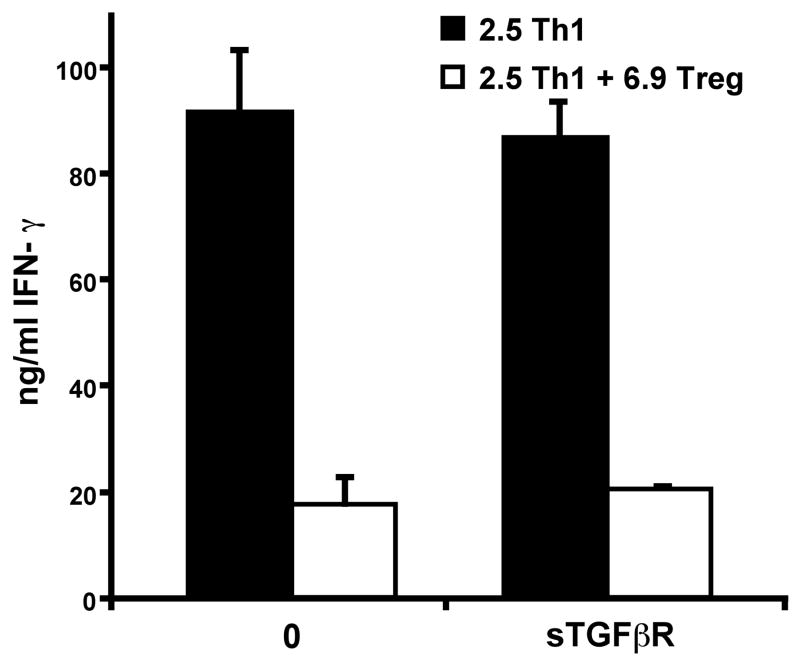
As TGF-β has been frequently shown to play a role in Treg suppression, particularly for TGF-β-induced Tregs [19, 32], and because our 6.9 TCR-Tg TGF-β-induced Tregs can produce TGF-β, we examined whether TGF-β plays a role in the suppressive effects of these TGF-β-induced Tregs by blocking TGF-β in vitro with soluble TGF-β receptor (TGF-βRII). We measured the 2.5 TCR-Tg Th1 effector T cell response to APCs presenting beta-cell antigen in the absence or presence of 6.9 TCR-Tg TGF-β-induced Tregs. Addition of soluble TGF-βRII had no effect on suppression of IFN-γ (Fig 5), but could completely block the expression of Foxp3 induced by 2 ng/ml of recombinant TGF-β (data not shown). Because secreted IL-10 has been shown to be a suppressive factor in some Treg populations [12, 33], we assessed the role of IL-10 in this system by addition of a blocking IL-10 antibody, but observed no effect on suppression (data not shown). These results suggest that neither IL-10 nor TGF-β are necessary for in vitro suppression by TGF-β-induced Tregs.
Figure 5. In vitro suppression by TGF-β-induced Tregs is not blocked by soluble TGF-ƒÀR.
2.5 TCR-Tg Th1 cells were activated in vitro with PECs (5 × 104) presenting β-cell antigen as described in the Materials and Methods. The Th1 cells (1 × 105) were co-cultured alone or with 6.9 TCR-Tg TGF-β-induced Tregs (1 × 105), in the presence/absence of 2 ng/ml soluble TGF-βR to block TGF-β. After 24 h, the supernatants were harvested and soluble IFN-γ measured by ELISA. Error bars indicate standard deviation from the mean of triplicate wells. The experiment was repeated 4 times with consistent results.
TGF-β is required for suppression of Th1 effectors during diabetes induction
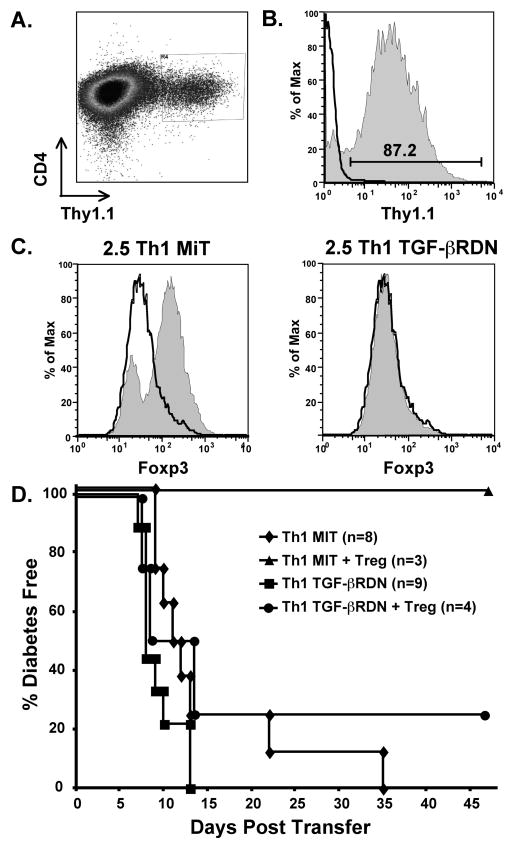
To analyze the role of TGF-β in vivo, we specifically interfered with the interaction of TGF-β on diabetogenic Th1 effectors by transducing those cells with dominant-negative TGF-βRII. We chose this approach because we could be confident that transferred effector T cells would not respond to TGF-β, whereas it is difficult to verify complete blocking of in vivo TGF-β by an antibody. We obtained a retroviral vector (MiT-TGF-βRDN) that contains cDNA for a dominant-negative TGF-βRII that lacks the cytoplasmic tail of the receptor [34]. The vector also contains cDNA for Thy1.1, allowing us to identify and isolate the transduced cells based on Thy1.1 expression on NOD T cells that normally express Thy1.2. To control for the effects of transduction, some T cells were transduced with the empty vector (MiT) that expresses only Thy1.1. For our in vivo assessment of the role of TGF-β during suppression, we transduced 2.5 TCR-Tg Th1 cells with MiT or MiT-TGF-βRDN. Cells in each group were sorted for Thy1.1 (Fig 6A), and Thy1.1+ cells were expanded in vitro with IL-2 to increase yields. After expansion, stable Thy1.1 expression was confirmed by flow cytometry (Fig 6B). To confirm that TGF-βRDN-transduced effectors did not respond to TGF-β we activated both MiT and MiT-TGF-βRDN-transduced cells in the presence of TGF-β using our Treg induction protocol. We found that while T cells transduced with the emptor vector could still be induced by TGF-β to upregulate Foxp3, MiT-TGF-βRDN-transduced cells did not upregulate Foxp3 in response to TGF-β (Fig 6C). This result demonstrates that the TGF-βRDN is dominant over the wildtype TGF-βRII that is still expressed on the cell surface. The transduced effector T cells were then transferred into NOD.scid mice in the absence or presence of 6.9 TCR-Tg TGF-β-induced Tregs. Both Th1 populations transferred diabetes into all mice, although some of the MiT-transduced cells took longer to induce disease (Fig 6D). While TGF-β-induced Tregs effectively suppressed diabetes in mice injected with MiT-transduced Th1 cells (p=0.02), the TGF-β-induced Tregs failed to suppress transfer by MiT-TGF-βRDN-transduced Th1 cells (p=0.69). This result demonstrates that suppression of diabetes mediated by TGF-β induced Tregs is TGF-β dependent.
Figure 6. Th1 effectors expressing a non-functional TGF-βRII are not suppressed in vivo by TGF-β-induced Tregs.
2.5 TCR-Tg Th1 T cells were transduced with the empty MiT vector or MiT-TGF-βRDN. A. Transduced cells were isolated by sorting for expression of Thy1.1, shown here on Th1 cells after MiT-TGF-βRDN transduction. B. After sorting, cells were expanded for three days with IL-2 before adoptive transfer. Thy1.1 expression, shown here on MiT-TGF-βRDN-transduced cells, remained high after expansion. The histogram outline represents isotype control staining and the shaded histogram represents Thy1.1 specific staining. C. MiT and MiT-TGF-βRDN-transduced cells were activated in the presence of TGF-β under Foxp3-inducing conditions. The histogram outlines and the shaded histograms represent Foxp3 expression on cells that were activated in the absence or presence of TGF-β, respectively. Foxp3 expression increased on MiT-transduced cells but not MiT-TGF-βRDN-transduced cells. D. 2.5 TCR-Tg Th1 cells (1 × 106) transduced with empty MiT vector or MiT-TGF-βRDN were transferred into adult NOD.scid mice in the absence or presence of 6.9 TCR-Tg TGF-β-induced Tregs (2 × 106). TGF-β-induced Tregs effectively suppressed diabetes induction by Th1 cells containing the MiT vector (p = 0.02), but did not suppress diabetes induction by Th1 cells containing the MiT-TGF-βRDN vector (p = 0.69). Data are combined from four experiments.
Discussion
This study reveals that during suppression of autoimmune inflammation in the pancreas, TGF-β-induced Tregs have three major effects on pancreas-infiltrating cells: Th1 effector cell numbers are decreased, Th1 effector cytokines are reduced, and effector macrophage numbers are decreased. Although the percentage of macrophages producing cytokines was not strongly affected by TGF-β-induced Tregs, the reduction in total macrophage numbers resulted in a reduced macrophage cytokine load. We also observed large numbers of TGF-β-induced Tregs in the pancreas, suggesting that these effects may be the result of TGF-β-induced Treg activity not just in the draining lymph node [5, 27], but in the inflammatory tissue as well. Based on our data, it is not clear which of the observed suppressive effects is the primary or dominant effect. Our data show that T cells (both TGF-β-induced Tregs and Th1 cells) begin to accumulate in the pancreas on day 6 after injection into NOD.scid recipients, at which point there is no detectable effect of TGF-β-induced Tregs on Th1 cell numbers. It was not until day 8 that we observed TGF-β-induced Treg-dependent reduction in numbers of Th1 effectors in the pancreas. These Th1 cells are presumably recruited to the pancreas after encounter with antigen presented in the pancreatic lymph node. We observed that production of Th1 cytokines is decreased at early time points, demonstrating that TGF-β-induced Tregs affect the activity of Th1 effectors in the pancreas before a reduction in cell numbers is seen. We hypothesize that inhibition of cytokine and chemokine production by effector T cells is an immediate consequence of TGF-β-induced Treg activity in the pancreas, and because macrophage recruitment into the pancreas is largely dependent on recruitment by chemokine-producing T cells, the numbers of macrophages in the pancreas are reduced. As macrophages are key mediators of islet destruction [35, 36], and because the most dramatic impact of TGF-β-induced Tregs in this system is the reduction of macrophage numbers in the pancreas, we feel that this effect is critical to protection from development of T1D. It follows that Th1 cell numbers decrease at later time points as a result of reduced interaction with macrophages in the pancreas, and also due to reduced chemokine-mediated recruitment. Other possible mechanisms of suppression, such as Treg-mediated cytotoxicity, will require further investigation. Finally, it should be noted that while we have previously found a strong correlation between Th1 cytokine production in the pancreas and diabetes pathogenesis in NOD mice [28], the roles of cytokines such as IFN-γ and TNF in T1D have been somewhat controversial as some reports have indicated that there may not be an absolute requirement for these cytokines under all conditions [37, 38]. The observed reduction in levels of IFN-γ and TNF by TGF-β-induced Tregs during suppression of diabetes transfer supports the conclusion that these cytokines play an important role in diabetes pathogenesis.
Another contribution of this study is the demonstration that TGF-β is a critical mediator of the suppressive activity of TGF-β-induced 6.9 TCR-Tg Tregs in vivo, despite the fact that we did not observe a role for this cytokine during in vitro suppression. Although the mechanism by which TGF-β-induced Tregs can suppress other CD4 T cells in vitro is unclear, our experiments with Th1 effectors expressing MiT-TGF-βRDN demonstrate that in vivo suppression is dependent on the interaction of TGF-β with those T effectors. This outcome is consistent with the findings of others [24], and we find it likely that while the mechanism of in vitro inhibition is TGF-β-independent, there are additional requirements for in vivo inhibition of autoimmunity, at least one of which is dependent on the action of TGF-β on effector T cells. The requirement for suppression of T cells by TGF-β also supports the conclusion that suppression of autoimmunity is not mediated solely through inhibition of macrophages or dendritic cells. It may be that TGF-β is also required for other aspects of in vivo suppression, such as inhibition of APC function or maintenance of a Treg population, but these possibilities were not addressed in this study. It is of interest to note that in a recent report, TGF-β was found to suppress naive islet-antigen specific CD8 T cells, but not memory CD8 T cells [39]. We have made similar observations with CD4 T cell clones which represent fully differentiated memory T cell effectors. In experiments in which diabetogenic CD4 T cell clones were used to transfer disease, TGF-β-induced Tregs were ineffective (Tonkin and Haskins, unpublished). In the model we have presented, the BDC-2.5 TCR-Tg Th1 cells come from a mouse where some T cells have interacted with their cognate antigen, but a majority of the cells may still be naive, and therefore more easily suppressed in their relatively immature state. In sum, our work confirms and extends the findings of other in vivo studies in which Tregs failed to suppress T effectors that expressed a dominant-negative TGF-βRII [17, 23–25] or lacked TGF-βRII [40].
It is not clear whether natural Tregs and TGF-β-induced Tregs are distinct populations with unique mechanisms of suppression, but the results we report here are the first to show that TGF-β-induced Tregs require TGF-β for in vivo T cell suppression. Although we did not observe TGF-β production by TGF-β-induced Tregs in vivo, it is possible that TGF-β was made at different time points or locations not evaluated or that the TGF-β-induced Tregs may have induced production of TGF-β by other cells. Such a possibility is consistent with findings by Fahlen et al [24], who showed that TGF-β-deficient Tregs could suppress in a TGF-β-dependent manner. It has been postulated that suppression by Tregs involves the ability of Tregs to educate other T cells to adopt a regulatory phenotype and that suppression by these educated Tregs is TGF-β-dependent [12, 41, 42]. Such a mechanism could explain the requirement for TGF-β in our in vivo studies of diabetes suppression. It also follows that the TGF-β-induced Treg-mediated reduction of cytokines and chemokines produced by Th1 effector cells may be a result of the action of TGF-β on those T effectors. Our findings thus provide some new clues as to the in vivo role of TGF-β during Treg-mediated suppression.
Materials and Methods
Mice
NOD and NOD.scid breeding mice were initially acquired from The Jackson Laboratory or the Barbara Davis Center for Childhood Diabetes (Denver, CO), and were housed in specific pathogen-free conditions at the
University of Colorado Denver (UCD) Center for Laboratory Animal Care (CLAC). Experimental animals were monitored for development of disease by urine glucose (Diastix, Bayer) and hyperglycemia confirmed by One Touch Ultra glucometer (Life Scan, Milpitas, CA). Mice were considered diabetic when blood glucose levels were >15 mM (270 mg/dl). The BDC-2.5 TCR-Tg/NOD and BDC-6.9 TCR-Tg/NOD mice were produced using TCR genes from diabetogenic T cell clones BDC-2.5 and BDC-6.9 respectively [43, 44]. NOD congenic mice lacking the BDC-6.9 antigen (NOD.C6) were produced as previously described [44] and were crossed with BDC-6.9 TCR-Tg/NOD mice to produce BDC-6.9 TCR-Tg/NOD.C6 mice.
The BDC-2.5 TCR-Tg/NOD mouse (hereafter referred to as BDC-2.5 TCR-Tg) was used as a source of Th1 effector T cells. Because BDC-6.9 TCR-Tg/NOD.C6 (hereafter, BDC-6.9 TCR-Tg) mice do not become diabetic and are therefore easier to maintain, the BDC-6.9 TCR-Tg on the NOD.C6 background was used routinely as a source of CD4 T cells for TGF-β-induced Tregs instead of BDC-6.9 TCR-Tg/NOD mice; moreover, TGF-β-induced Tregs derived from both strains were indistinguishable from one another. All procedures were in accordance with Institutional Animal Care and Use Committee guidelines and approved by the UCHSC Animal Care and Use Committee.
Induction of Th1 and Treg cells
Th1 effector cells and TGF-β-induced Tregs were generated by purification of CD4 T cells from 2–3 month-old BDC-2.5 TCR-Tg or BDC-6.9 TCR-Tg mice by harvesting spleen and lymph nodes, followed by positive selection with magnetic anti-CD4 Microbeads (Miltenyi). CD4 T cells were resuspended at 1 × 106 cells/ml in complete medium (CM). CM is DMEM supplemented with 44 mM sodium bicarbonate, 0.55 mM L-arginine, 0.27 mM L-asparagine, 1.5 mM L-glutamine, 1 mM sodium pyruvate, 50 mM 2-ME, 10 mM HEPES, and 10% FCS. T cells were activated by placing 5 × 106 cells per well in 6-well tissue culture plates coated with 1 μg/ml anti-CD3 (BD Bioscience). For Th1 cells, 100 U/ml of recombinant human IL-2 (National Cancer Institute) was added to the media. For induction of Tregs, medium was supplemented with 100 U/ml IL-2, 3 ng/ml human TGF-β1 (Peprotech), and 2.5% anti-IFN-γ supernatant from the XMG 1.2 hybridoma. Cells were incubated at 37° for 3 days and then harvested. Foxp3 protein expression was evaluated with the anti-mouse Foxp3 Staining Set (eBioscience).
Adoptive transfer of T cells
Th1 and TGF-β-induced Treg cells were injected into recipients immediately upon harvest after the 3-day induction period. For most experiments, Th1 effector T cells were obtained from the 2.5 TCR-Tg mouse and TGF-β-induced Tregs from the BDC-6.9 TCR-Tg mouse so that Th1 T cells could be easily distinguished from TGF-β-induced Tregs via the BDC-2.5 TCR antibody [31]. In NOD.scid recipients, age 6–12 weeks, BDC-2.5 TCR-Tg Th1 cells (2 × 106) were injected i.v., with or without BDC-6.9 TCR-Tg TGF-β-induced Tregs (1 × 107). In some experiments we adoptively transferred diabetes into NOD.scid recipients (< 2 weeks old) by transfer of spleen cells (1 × 107) harvested from spontaneously diabetic NOD mice. Some of these mice received cotransfers of 6.9 TCR-Tg or 2.5 TCR-Tg TGF-β-induced Tregs (1 × 107). We also evaluated the ability of BDC-6.9 TCR-Tg TGF-β-induced Tregs to prevent spontaneous diabetes in BDC-2.5 TCR-Tg/NOD.scid recipients by injection of the TGF-β-induced Tregs (1 × 107) before the mice were 18 days old.
Intracellular cytokine staining
For intracellular cytokine staining, cytokine secretion was blocked with brefeldin A, “GolgiPlug” (BD Biosciences) for 4–5 h at 37°. Cells were then harvested, washed, and resuspended in staining buffer (0.5% BSA/PBS) containing GolgiPlug. For identification of T cells and macrophages, cells were incubated for 30 min at 4°C in staining buffer containing GolgiPlug, FITC-conjugated anti-mouse CD4 (GK 1.5) and PerCPCy5.5-conjugated CD11b (M1/70). 2.5 TCR-Tg Th1 cells were identified with the clonotypic anti-2.5 TCR antibody [31] purified from aBDC B cell hybridoma supernatant on a protein G column, conjugated to biotin and detected with streptavidin-PE. Cells were fixed in 2% paraformaldehyde for 10 min, then washed and resuspended in staining buffer containing 0.05% saponin to permeabilize the cells. Antibody binding for intracellular cytokines was carried out for 30 min at 4°C with APC-conjugated anti-mouse IFN-γ (XMG1.2, Biolegend), anti-mouse TNF (MP6-XT22, BD-Biosciences), or an appropriate isotype control antibody and cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences). The B cell hybridoma for the anti TGF-β monoclonal antibody (1D11) was a gift from Linda Bradley (Sidney Kimmel Cancer Center, San Diego, CA), and antibody was purified from hybridoma supernatant on a protein G column and conjugated to FITC with the Fluorotag FITC Conjugation Kit (Sigma). Antibodies specific for IL-1β and the chemokines RANTES, MIP-1α, MIP-1β, and MIP1γ were polyclonal goat IgG antibodies (R&D systems) that were detected with a Cy5-anti-goat IgG secondary antibody (Vector).
Ex vivo analysis of pancreas-infiltrating T cells
For ex vivo analysis, pancreata were harvested from 2–3 mice per group 6–9 days after T cell transfer and were pooled for mice in each experimental group. Pancreata were digested with 5 mg/ml collagenase (Sigma) at 37°C for ~30 min with periodic vortexing, followed by homogenization in CM. Cells from each group were divided in half and cultured in the presence of GolgiPlug for 4–5 h at 37° C with no stimulation or in wells containing anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) to amplify T cell cytokine production. (Macrophage cytokine production did not require any additional stimulation ex vivo.) Cells were then harvested and stained for surface markers and intracellular cytokines.
Analysis of in vitro suppression of cytokine production
For analysis of TGF-β-induced Treg-mediated suppression of IFN-γ produced by Th1 cells, IL-2-expanded Th1 or TGF-β-induced Treg cells (4 × 105/ml) were cultured with 2 × 105/ml thioglycolate-elicited peritoneal macrophages (PEC) as antigen presenting cells (APC). Islet cells (4 × 104/ml) from NOD mice were used as antigen. After 24 h, the supernatant was harvested and assayed for IFN-γ by ELISA using paired antibodies (BD Biosciences). In some assays we blocked IL-10 with 8 μg/ml purified anti-IL-10 antibody (JES6-1A12, BD Biosciences) or blocked TGF-β with 2 ng/ml soluble TGF-βRII/Fc chimera (R&D Systems). Soluble cytokines were measured after 24 h by specific ELISA using paired capture and biotinylated-detection antibodies (BD Biosciences), followed by streptavidin-HRP (Sigma) and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma) as the substrate. Assays were read on a Titertek Multiskan Plus platereader at 405 nm and cytokine concentrations determined by comparison to recombinant cytokine standards.
Transduction of Th1 effectors with MiT-TGF-βRDN
An empty retroviral vector, MSCV-IRES-Thy1.1 (MiT) [45], and MiT containing cDNA for a dominant-negative TGF-β receptor (MiT-TGF-βRDN) [34] were gifts from Laurent Gapin (National Jewish Health). The dominant-negative TGF-βRII is truncated so that it lacks the cytoplasmic tail, and the cDNA was cloned into MiT between the BglII and NotI sites in the lab of Bill Schiemann (University of Colorado Health Sciences Center). MiT and MiT-TGF-βRDN were transfected into phoenix packaging cells with fugene reagent (Roche). Retrovirus-containing supernatants were harvested after 48 h and 72 h of culture. 2.5 TCR-Tg CD4 T cells were activated to become Th1 effectors by activation with 1 μg/ml plate-bound anti-CD3 in 6-well tissue culture plates at 1 × 106 cells/ml CM. After 48 h of activation, the CM was replaced with retroviral supernatant containing 8 μg/ml polybrene and the plate centrifuged at 1300 g for 120 min. After centrifugation T cells were incubated at 37°C for 1–2 h, after which retroviral supernatant was replaced with CM. After 24 h T cells were harvested and stained with anti-Thy1.1 antibody (HIS51, Biolegend) and Thy1.1-expressing cells isolated on a MoFlo cell sorter (Dako). To evaluate responsiveness to TGF-β, transduced T cells were immediately activated in the presence of TGF-β with our Treg-inducing protocol and Foxp3 expression tested after three days. Transduced Th1 cells were otherwise expanded with 100 U/ml IL-2 for 3–4 days and injected into NOD.scid recipients alone (1 × 106) or in a cotransfer with 6.9 TCR-Tg TGF-β-induced Tregs (2 × 106). Recipient mice were then monitored for diabetes incidence.
Statistical analysis
Statistical significance of the effect of Tregs on diabetes transfer was determined by a Wilcoxon test of survival calculated by JMP software (SAS Institute). Significances of differences in numbers of pancreas-infiltrating T cells and macrophages were determined by paired Student’s t test. A p value of less than 0.05 was considered significant.
Acknowledgments
This work was supported by research grants from the Juvenile Diabetes Research Foundation (JDRF 1-2004-49) and NIH (RO1 DK50561).
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002:12287–12292. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You S, Belghith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S, Garcia C, Waldmann H, Bach JF, Chatenoud L. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 4.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, Ziegler SF, Bradley LM. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- 6.Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J Immunol. 2008;181:4516–4522. doi: 10.4049/jimmunol.181.7.4516. [DOI] [PubMed] [Google Scholar]
- 7.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 8.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 9.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 11.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 12.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Zhu J, Yang Y. Protection against autoimmunity in nonlymphopenic hosts by CD4+ CD25+ regulatory T cells is antigen-specific and requires IL-10 and TGF-beta. J Immunol. 2005;175:4283–4291. doi: 10.4049/jimmunol.175.7.4283. [DOI] [PubMed] [Google Scholar]
- 14.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 15.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Kitani A, Strober Wi. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 20.You S, Thieblemont N, Alyanakian MA, Bach JF, Chatenoud L. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol Rev. 2006;212:185–202. doi: 10.1111/j.0105-2896.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 21.Schramm C, Huber S, Protschka M, Czochra P, Burg J, Schmitt E, Lohse AW, Galle PR, Blessing M. TGFbeta regulates the CD4+CD25+ T-cell pool and the expression of Foxp3 in vivo. Int Immunol. 2004;16:1241–1249. doi: 10.1093/intimm/dxh126. [DOI] [PubMed] [Google Scholar]
- 22.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 23.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du W, Wong FS, Li MO, Peng J, Qi H, Flavell RA, Sherwin R, Wen L. TGF-beta signaling is required for the function of insulin-reactive T regulatory cells. J Clin Invest. 2006;116:1360–1370. doi: 10.1172/JCI27030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kullberg MC, Hay V, Cheever AW, Mamura M, Sher A, Letterio JJ, Shevach EM, Piccirillo CA. TGF-beta1 production by CD4+ CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol. 2005;35:2886–2895. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 27.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantor J, Haskins K. Effector function of diabetogenic CD4 Th1 T cell clones: a central role for TNF-alpha. J Immunol. 2005;175:7738–7745. doi: 10.4049/jimmunol.175.11.7738. [DOI] [PubMed] [Google Scholar]
- 29.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobbs C, Haskins K. Comparison of a T cell clone and of T cells from a TCR transgenic mouse: TCR transgenic T cells specific for self-antigen are atypical. J Immunol. 2001;166:2495–2504. doi: 10.4049/jimmunol.166.4.2495. [DOI] [PubMed] [Google Scholar]
- 31.Kanagawa O, Militech A, Vaupel BA. Regulation of diabetes development by regulatory T cells in pancreatic islet antigen-specific TCR transgenic nonobese diabetic mice. J Immunol. 2002;168:6159–6164. doi: 10.4049/jimmunol.168.12.6159. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mekala DJ, Alli RS, Geiger TL. IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:11817–11822. doi: 10.1073/pnas.0505445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 35.Yoon JW, Jun HS, Santamaria P. Cellular and molecular mechanisms for the initiation and progression of beta cell destruction resulting from the collaboration between macrophages and T cells. Autoimmunity. 1998;27:109–122. doi: 10.3109/08916939809008041. [DOI] [PubMed] [Google Scholar]
- 36.Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol. 2007;179:5760–5767. doi: 10.4049/jimmunol.179.9.5760. [DOI] [PubMed] [Google Scholar]
- 37.Yang XD, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, McDevitt HO. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol. 2001;166:1352–1359. doi: 10.4049/jimmunol.166.2.1352. [DOI] [PubMed] [Google Scholar]
- 39.Filippi CM, Juedes AE, Oldham JE, Ling E, Togher L, Peng Y, Flavell RA, von Herrath MG. Transforming growth factor-beta suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes. 2008;57:2684–2692. doi: 10.2337/db08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 44.Pauza ME, Dobbs CM, He J, Patterson T, Wagner S, Anobile BS, Bradley BJ, Lo D, Haskins K. T-cell receptor transgenic response to an endogenous polymorphic autoantigen determines susceptibility to diabetes. Diabetes. 2004;53:978–988. doi: 10.2337/diabetes.53.4.978. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, White J, Zhu Y, Kappler J, Marrack P. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]