Abstract
Purpose
To evaluate the effect of tumor hypoxia on the expected cell killing by clinically used regimens of stereotactic ablative radiotherapy (SABR), and to determine the extent to which the negative effect of hypoxia could be prevented using a clinically available hypoxic cell radiosensitizer.
Materials and Methods
We have calculated the expected level of tumor cell kill from clinically used regimens of SABR both with and without the assumption of 20% of the tumor cells being hypoxic using the standard LQ model and the universal survival curve (USC) modification. We compare the results obtained with our own clinical data on lung tumors of different sizes and with the published data of others. We also have calculated the expected effect on cell survival of adding the hypoxic cell sensitizer etanidazole at clinically achievable drug concentrations.
Results
Modeling tumor cell killing with any of the currently used regimens of SABR produces results that are inconsistent with the majority of the clinical findings if tumor hypoxia is not considered. However, with the assumption of tumor hypoxia the expected level of cell killing is consistent with the clinical data. For only some of the smallest tumors are the clinical data consistent with no tumor hypoxia, but there could be other reasons for the sensitivity of these tumors. The addition of etanidazole at clinically achievable tumor concentrations produces a large increase in the expected tumor cell kill from the large radiation doses used in SABR.
Conclusions
The presence of tumor hypoxia is a major negative factor in limiting the curability of tumors by SABR at radiation doses that are tolerable to surrounding normal tissues. However, this negative effect of hypoxia could be overcome by the addition of clinically tolerable doses of the hypoxic cell radiosensitizer etanidazole.
Keywords: Hypoxic cell radiosensitizer, Etanidazole, Stereotactic ablative radiotherapy (SABR), Stereotactic body radiation therapy (SBRT), Stereotactic radiosurgery (SRS)
A century of clinical experience as well as numerous preclinical studies have demonstrated the superiority of multiple small doses of irradiation over large single doses or a few large fractions in achieving local tumor control for acceptable normal tissue toxicity. A major reason for this is that hypoxic cells are universally resistant to killing by radiation and so their prevalence in tumors results in low levels of tumor cell killing for single doses of radiation. The decreased oxygenation of tumor cells is a result of structural and functional disturbances of the tumor vasculature that inhibit the normal delivery of oxygen (1, 2). Fractionation of radiation however greatly mitigates the protection afforded by tumor hypoxia because of the phenomenon of reoxygenation (3, 4), the process by which the hypoxic cells surviving a given radiation dose become oxygenated prior to the next radiation dose most likely as a result of fluctuating tumor blood flow (5). While hypoxia has been shown to be associated with increased metastasis (6, 7), treatment failure even with conventionally fractionated radiotherapy in head and neck and cervix tumors with high levels of hypoxia can be attributed primarily to the decreased sensitivity of hypoxic tumor cells to ionizing radiation (8, 9).
So, with the overwhelming evidence for the superiority of conventionally fractionated irradiation over large single doses why is there growing interest in the use of stereotactic ablative radiotherapy (SABR), also known as stereotactic body radiation therapy (SBRT) or in the brain stereotactic radiosurgery (SRS)? The answer lies in technological advances that have created the ability to conform and intensify the radiation dose to relatively small tumors while minimizing the volume of normal tissue irradiated, originally in the brain and subsequently in other anatomic sites. Besides the obvious convenience to the patient of one or a few doses vs. 6 weeks or more of daily treatments, emerging clinical results of SABR appear highly promising compared to more conventional radiotherapy techniques. Do these results contradict the radiobiological principles learned in the preceding decades? As we will argue, quite the opposite is true.
A few simple calculations illustrate the discrepancy between clinical results and radiobiological modeling when hypoxia is not considered, as well as the magnitude of the potential impact of hypoxic cells on SABR. If we assume standard linear-quadratic (LQ) modeling parameters for the sensitivity of tumor cells to radiation (10), i.e., α = 0.35 and α/β = 10, the predicted tumor cell kill for several currently used SABR regimens (11–14) is shown in Table 1. Note that the prediction of 27.4 logs of cells kill for 60 Gy/3 fractions would be sufficient to control a spherical tumor of 4 km diameter with 99% probability assuming complete packing with 109 clonogenic cells/mL! It has been argued that LQ modeling overestimates the killing from large doses per fraction, and indeed modeling with a “universal survival curve” (USC) that becomes log-linear with large fraction size predicts a substantial decrement in biologically effective dose (BED) from 180 Gy to 132 Gy for the same regimen (15). This corresponds to 19 logs of kill, still sufficient to control a tumor of 6 meter diameter with 99% probability!
Table 1.
Predicted tumor cell killing for different SABR regimens with or without 20% hypoxic fraction
| Fractionation regimens currently in use | Predicted Logs of Cell Kill | |||
|---|---|---|---|---|
| Without Hypoxia | With Hypoxia | |||
| LQ | USC | LQ | USC | |
| 25 Gy/1fraction (11, 12) | 13.3 | 8.1 | 3.3 | 3.2 |
| 50 Gy/4 fractions (13) | 17.1 | 14.9 | 6.7 | 6.7 |
| 60 Gy/3 fractions (14, 15) | 27.4 | 19.0 | 7.7 | 7.7 |
LQ: linear quadratic model; USC: universal survival curve model; Model parameters: α=0.35, β =0.035, D0=1.25 Gy, Dq=1.8 Gy, DT=6.4 Gy, oxygen enhancement ratio OER=2.8, hypoxic fraction HF=0.2. With hypoxia, the difference between the LQ and USC models is trivial because scaling of the dose-response curve by the OER for hypoxic cells results in the transition dose that differentiates them (DT) being comparable to or larger than the dose per fraction.
This is clearly not the clinical experience, as 60 Gy/3 fractions does not control 99% of even small, peripheral lung lesions. While single fraction SRS of brain metastases is commonly perceived to result in very high tumor control rates, this was not the case in the recently reported EORTC 22952–26001 trial, in which treatment of brain metastases with a median diameter of 2 cm (maximum 4 cm) with SRS alone to a dose of 20 Gy to the 80% isodose line resulted in an in-field progression rate of 31% (16), a result quite consistent with radiosurgery series of relatively larger brain metastases (in the size range often treated elsewhere in the body) with adequate follow up by MR imaging. In fact, several analyses of local control of lung tumors after SABR demonstrate a marked dose-response relationship, with poor local control when the BED is less than 100 Gy (17, 18), a dose predicted by modeling to be easily tumor sterilizing. In particular the Indiana University and University of Colorado experiences demonstrated that doses of 54–66 Gy/3 fractions were needed to reach 88–90% 3-year local control of stage I lung cancer and limited pulmonary and hepatic metastases (14, 19, 20). Thus, far higher doses than predicted by standard modeling based on in vitro data for fully oxygenated cells are needed to achieve the promising results reported clinically, indicating the presence of a universal resistance mechanism in vivo.
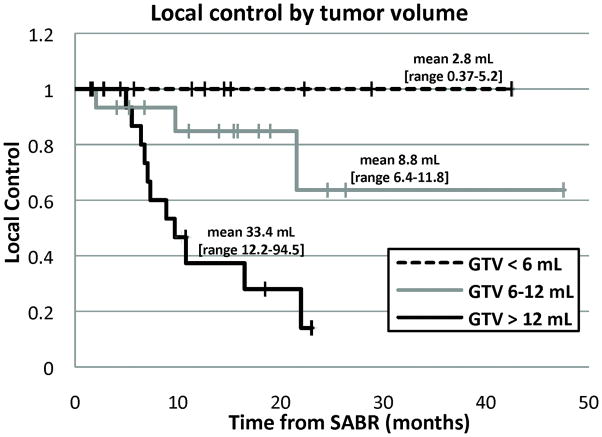
In addition, there appears to be an important influence of tumor size. The Stanford dose escalation experience using single fraction SABR of 15–30 Gy for treatment of limited pulmonary tumors demonstrates a critical dependence of local control on gross tumor volume (GTV), with a Kaplan-Meier estimate of local control at 11 months of 100% for tumors < 6 mL, 93% for tumors 6–12 mL, and 47% for tumors > 12 mL (Figure 1), with the majority of patients receiving 25 or 30 Gy (11, 12). This is very similar to the findings from the University of Heidelberg (21). It seems unlikely that the large difference seen in Figure 1 in tumor control with size could be the result of different numbers of tumor cells, as the difference in average volume between the largest and smallest groups of tumors in the series was only 1 log, a small difference compared to the predicted killing by these doses based on standard modeling. Thus, assuming these differences hold up with longer follow up, there must be a resistance mechanism present in larger tumors that is not apparent in smaller tumors.
Figure 1.
Local control of lung tumors treated with single fraction SABR (mostly 25–30 Gy) as a function of tumor size.
Is hypoxia a plausible explanation for these findings? We know that approximately 90% of all solid tumors have median oxygen concentrations less than those typically found in normal tissues (22, 23). A study of resectable lung cancer in particular found that 10 of 20 tumors had radiobiologically significant hypoxia with a median pO2 of less than 15 mmHg (7 with a median < 5 mmHg) by intraoperative oxygen histography, and 10 of 16 had immunohistochemical evidence of hypoxic gene induction by CA-IX staining (24). Interestingly, an analysis of 518 brain metastases treated with SRS found one year local control rates of 90%, 76%, and 57% for lesions with homogeneous, heterogeneous, and ring-like patterns of contrast enhancement, respectively, suggesting an effect of differing levels of necrosis and hypoxia (25). Table 1 also shows the result of modeling considering hypoxia, using standard parameters of a hypoxic fraction of 20%, an oxygen enhancement radio (OER) of 2.8, and reoxygenation between fractions (10). Accounting for hypoxia, even the intensive 60 Gy/3 fractions regimen is predicted to be barely sufficient to control a small tumor, indicating that hypoxia is more than sufficient to bridge the gap between simple radiobiological modeling and the clinical results.
It should also be mentioned that the relatively high rates of local control of some tumors after even single fraction SABR of 20–25 Gy (at least with short-term follow-up), for example in series of lung (Figure 1), pancreas, and spine tumors (12, 21, 26, 27), fall between what would be expected by modeling with and without hypoxia. This suggests the possible contribution of other mitigating factors such as: 1. No hypoxia in some tumors (e.g., the smallest ones); 2. Only a small proportion (one in 102–104) of tumor cells are clonogenic stem cells (28 and M. Diehn unpublished observations); 3. An active immune response is sufficient to eradicate microscopic residual tumor; 4. High single doses of radiation cause acute damage to the endothelial cells of the tumor vasculature (29). All of these would be predicted to increase tumor control by SABR. Thus, taking into consideration all of the clinical data the last three potentially improve the fit to the observed results, but only after first accounting for the dominant effect of hypoxia.
Nevertheless, clinical investigation has led to SABR regimens of relatively high efficacy. So do we still need to worry about hypoxia? The extreme ablative doses required to overcome the radioresistance of hypoxic cells clinically come at a significant cost. Even with highly conformal therapy, normal tissue toxicity is still a problem with SABR as demonstrated in a phase II trial of SABR for lung tumors in which there was excessive toxicity comparing patients with central (perihilar and central mediastinal) lesions with those with more peripheral lesions that do not have adjacent critical normal tissues (30). Radiation pneumonitis (14), brachial plexopathy (31), rib fracture and chest wall pain (32, 33), and dermatitis (34) have all been reported in series of SABR for lung tumors, as have gastric and duodenal ulcers in SABR of pancreatic cancer (26). SABR is contraindicated if significant volumes of normal tissues, particularly those with “serial” organization, are in or adjacent to the target volume. Clearly, there is room to improve the therapeutic ratio of SABR.
Some 30 years ago Fowler and colleagues showed that the inferiority of large single doses of radiation in achieving cure of transplanted mouse mammary tumors for a given level of skin reaction could be entirely overcome if the resistance of the hypoxic cells in the tumors was eliminated by pretreatment of the mice with a large dose of the hypoxic cell radiosensitizer misonidazole (35). In other words, the lower efficacy of single doses in effecting tumor cure was not the result of lower repair on the tumor cells relative to the normal skin cells but by the presence of tumor hypoxia. This raises the important question of whether a rationally designed second generation hypoxic cell radiosensitizer such as etanidazole could improve the control of hypoxic tumors by SABR while decreasing normal tissue toxicity by reducing the radiation dose. Using the same radiobiological parameters as before, the benefit of such a sensitizer can be estimated as shown in Table 2. Note that even with the modest dose of 20 Gy in a single fraction with a sensitizer giving an enhancement ratio (SER) of 2.5, the expected cell kill (6–8.2 logs) is comparable to that for the very large dose of 60 Gy/3 fractions (7.7 logs, Table 1) in the presence of hypoxia without a sensitizer. In other words, with a sufficient dose of sensitizer, the same tumor control could potentially be achieved with just one fraction of the three fraction regimen. It should be noted that even with reduced dose SABR, we would still expect ablation of normal tissues encompassed within the target volume, but the volume of normal tissues receiving ablative doses in the regions surrounding the target volume would be much lower. This would for example allow treatment of lesions closer to the proximal bronchial tree than the relatively large 2 cm exclusion zone currently recommended (30).
Table 2.
Predicted tumor cell killing with different SERs for the hypoxic cells
| Fractionation Regimen | Predicted logs of cell kill | ||||
|---|---|---|---|---|---|
| Sensitizer Enhancement Ratio (SER) | |||||
| 1.0* | 1.7 | 2.0 | 2.5 | ||
| 25 Gy/1fr | LQ | 3.3 | 6.5 | 8.3 | 11.7 |
| USC | 3.2 | 5.3 | 6.3 | 7.7 | |
| 20 Gy/1fr | LQ | 2.6 | 4.8 | 6.0 | 8.2 |
| USC | 2.6 | 4.3 | 5.0 | 6.0 | |
SER of 1 corresponds to no sensitizer.
How large an SER could we expect with etanidazole? Clinical studies with the well-tolerated single dose of 12 g/m2 of etanidazole found peak tumor bed concentrations of the drug at 45 minutes after an i.v. injection, with a mean of 988 μg/ml and a median of 430 μg/ml, which would translate into an SER for the hypoxic cells of 2.0 to 2.5 (36, 37). On the other hand, it has been shown that the SER of nitroimidazoles rapidly approaches 1 with rising oxygen concentrations, such that their effect is negligible in the presence of oxygen even well below physiologic levels (38). Thus no radiosensitization of normal tissues is to be expected, as demonstrated in early preclinical studies (39) and in numerous clinical trials (40).
While many clinical trials of etanidazole and fractionated radiation have been conducted, only two have tested single doses of radiation with single doses of etanidazole: RTOG 89-06 was a phase I dose escalation study of etanidazole up to 12 g/m2 with single dose intraoperative radiation therapy (IORT) (37), and RTOG 95-02 was a phase I-B study of etanidazole 12 g/m2 with single dose SRS of brain tumors (41). Both had primary endpoints of toxicity, and efficacy was not reported. Both demonstrated that a single dose of etanidazole at 12 g/m2 is well tolerated with a total of 72 patients receiving that dose. Neither study demonstrated enhanced normal tissue sensitivity to radiation. Based on the promising tissue concentrations of etanidazole demonstrated in RTOG 89-06 as described above, a multi-institutional phase III trial of IORT for locally recurrent rectal cancer with or without etanidazole has been proposed and is now actively in the planning phase. Also of particular interest is a small but tantalizing randomized phase III trial of another 2-nitroimidazole drug, doranidazole, that was tested with 25 Gy single fraction radiotherapy for unresectable pancreas cancer, demonstrating a significant benefit of 23% vs. 0% overall survival at 3 years, with or without doranidazole, respectively (42). Intraoperative radiotherapy was used as a means of delivering definitive conformal radiation to the intact tumor without attempting resection, providing the closest analogy to SABR with a hypoxic cell radiosensitizer yet studied. It is also of relevance that early studies of Urtasun and colleagues with the first generation hypoxic radiosensitizer, metronidazole, demonstrated improved survival giving relatively large fractions (and substandard total radiation doses) to glioblastomas combined with the sensitizer (43) but the combination was not superior to that of standard fractionated radiotherapy without a sensitizer (44).
In summary, a clinical trial of the addition of a high single dose of etanidazole to a single dose of SABR is justified because: 1. There is ample clinical evidence that hypoxia is present in human solid tumors; 2. Based on modeling considerations this hypoxia is a highly plausible explanation for the tumor control rates seen in clinical trials of SABR; 3. Clinical trials have demonstrated the safety of administering single doses of etanidazole sufficient to produce tissue concentrations that pre-clinical experiments have shown should overcome most of the marked radiation resistance conferred by tumor hypoxia; 4. There is a need to increase the therapeutic ratio of SABR. Single dose sensitizer-enhanced SABR, if proven safe and effective for a wide range of tumor sizes and anatomic locations, would be a substantial advance over the current state of the art of using massive SABR doses to overcome hypoxic resistance, and would open the possibility of additional sites for treatment, for treatments for second primary tumors and new metastases, and salvage of local recurrences after prior radiation.
Footnotes
Conflict of Interest Notification: BL has received speaking honoraria from Accuray, Inc., and Varian Medical Systems. JMB has equity holdings in Proacta, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown JM. Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol Med Today. 2000;6:157–162. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- 2.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 3.Kallman RF. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology. 1972;105:135–142. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- 4.Kallman RF, Dorie MJ. Tumor oxygenation and reoxygenation during radiation therapy: their importance in predicting tumor response. Int J Radiat Oncol Biol Phys. 1986;12:681–685. doi: 10.1016/0360-3016(86)90080-5. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 6.Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 7.Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 8.Brizel DM, Dodge RK, Clough RW, et al. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 9.Rofstad EK, Sundfor K, Lyng H, et al. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer. 2000;83:354–359. doi: 10.1054/bjoc.2000.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat Res. 1997;147:541–550. [PubMed] [Google Scholar]
- 11.Le Q-T, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1:802–809. [PubMed] [Google Scholar]
- 12.Loo BW, Shen J, Quinlan-Davidson S, et al. Tumor size is a critical determinant of local control in single fraction stereotactic radiotherapy of pulmonary tumors. Int J Radiat Oncol Biol Phys. 2008;72:S467–S468. [Google Scholar]
- 13.Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 16.Kocher M, Mueller RP, Abacioglu MU, et al. Adjuvant Whole Brain Radiotherapy vs. Observation after Radiosurgery or Surgical Resection of 1–3 Cerebral Metastases – Results of the EORTC 22952–26001 Study. Int J Radiat Oncol Biol Phys. 2009;75:S5. [Google Scholar]
- 17.Wulf J, Baier K, Mueller G, et al. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol. 2005;77:83–87. doi: 10.1016/j.radonc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 19.Timmerman RD, Park C, Kavanagh BD. The North American experience with stereotactic body radiation therapy in non-small cell lung cancer. J Thorac Oncol. 2007;2:S101–112. doi: 10.1097/JTO.0b013e318074e4fa. [DOI] [PubMed] [Google Scholar]
- 20.McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112–118. doi: 10.1016/j.ijrobp.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 21.Hof H, Muenter M, Oetzel D, et al. Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (NSCLC) Cancer. 2007;110:148–155. doi: 10.1002/cncr.22763. [DOI] [PubMed] [Google Scholar]
- 22.Vaupel PW, Hockel M. Oxygenation status of human tumors: A reappraisal using computerized pO2 histography. In: Vaupel PW, Kelleher DK, Gunderoth M, editors. Tumor Oxygenation. Stuttgart: Gustav Fischer Verlag; 1995. pp. 219–232. [Google Scholar]
- 23.Brown JM. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 24.Le Q-T, Chen E, Salim A, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–1514. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 25.Goodman KA, Sneed PK, McDermott MW, et al. Relationship between pattern of enhancement and local control of brain metastases after radiosurgery. Int J Radiat Oncol Biol Phys. 2001;50:139–146. doi: 10.1016/s0360-3016(00)01584-4. [DOI] [PubMed] [Google Scholar]
- 26.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 28.Bertolini G, Roz L, Perego P, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 31.Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol. 2009;93:408–413. doi: 10.1016/j.radonc.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Pettersson N, Nyman J, Johansson KA. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: a dose- and volume-response analysis. Radiother Oncol. 2009;91:360–368. doi: 10.1016/j.radonc.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Dunlap NE, Cai J, Biedermann GB, et al. Chest Wall Volume Receiving >30 Gy Predicts Risk of Severe Pain and/or Rib Fracture After Lung Stereotactic Body Radiotherapy. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who’s at risk? Int J Radiat Oncol Biol Phys. 2008;72:1283–1286. doi: 10.1016/j.ijrobp.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Fowler JF, Sheldon PW, Denekamp J, et al. Optimum fractionation of the C3H mouse mammary carcinoma using x-rays, the hypoxic-cell radiosensitizer Ro-07-0582, or fast neutrons. Int J Radiat Oncol Biol Phys. 1976;1:579–592. doi: 10.1016/0360-3016(76)90139-5. [DOI] [PubMed] [Google Scholar]
- 36.Brown JM. Clinical trials of radiosensitizers: what should we expect? Int J Radiat Oncol Biol Phys. 1984;10:425–429. doi: 10.1016/0360-3016(84)90063-4. [DOI] [PubMed] [Google Scholar]
- 37.Halberg FE, Cosmatis D, Gunderson LL, et al. RTOG #89-06: a phase I study to evaluate intraoperative radiation therapy and the hypoxic cell sensitizer etanidazole in locally advanced malignancies. Int J Radiat Oncol Biol Phys. 1994;28:201–206. doi: 10.1016/0360-3016(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 38.Ling CC, Michaels HB, Epp ER, et al. Interaction of misonidazole and oxygen in the radiosensitization of mammalian cells. Int J Radiat Oncol Biol Phys. 1980;6:583–589. doi: 10.1016/0360-3016(80)90386-7. [DOI] [PubMed] [Google Scholar]
- 39.Stone HB. Misonidazole in fractionated radiotherapy of a murine mammary carcinoma: comparison of tumor and normal tissue response. Int J Radiat Oncol Biol Phys. 1988;14:957–962. doi: 10.1016/0360-3016(88)90018-1. [DOI] [PubMed] [Google Scholar]
- 40.Overgaard J, Horsman MR. Modification of Hypoxia-Induced Radioresistance in Tumors by the Use of Oxygen and Sensitizers. Semin Radiat Oncol. 1996;6:10–21. doi: 10.1053/SRAO0060010. [DOI] [PubMed] [Google Scholar]
- 41.Drzymala RE, Wasserman TH, Won M, et al. A phase I-B trial of the radiosensitizer: etanidazole (SR-2508) with radiosurgery for the treatment of recurrent previously irradiated primary brain tumors or brain metastases (RTOG Study 95-02) Radiother Oncol. 2008;87:89–92. doi: 10.1016/j.radonc.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Karasawa K, Sunamura M, Okamoto A, et al. Efficacy of novel hypoxic cell sensitiser doranidazole in the treatment of locally advanced pancreatic cancer: long-term results of a placebo-controlled randomised study. Radiother Oncol. 2008;87:326–330. doi: 10.1016/j.radonc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Urtasun R, Band P, Chapman JD, et al. Radiation and high-dose metronidazole in supratentorial glioblastomas. N Engl J Med. 1976;294:1364–1367. doi: 10.1056/NEJM197606172942503. [DOI] [PubMed] [Google Scholar]
- 44.Urtasun R, Feldstein ML, Partington J, et al. Radiation and nitroimidazoles in supratentorial high grade gliomas: a second clinical trial. British Journal of Cancer. 1982;46:101–108. doi: 10.1038/bjc.1982.171. [DOI] [PMC free article] [PubMed] [Google Scholar]