Summary
Using a human papillomavirus (HPV) cervicovaginal murine challenge model, we microscopically examined the in vivo mechanisms of L1 virus-like particle (VLP) and L2 vaccine-induced inhibition of infection. In vivo HPV infection requires an initial association with the acellular basement membrane (BM) to induce conformational changes in the virion that permit its association with the keratinocyte cell surface. By passive transfer of immune serum, we determined that anti-L1 antibodies can interfere with infection at two stages. Similarly to active VLP immunization, transfer of high L1 antibody concentrations prevented BM binding. In the presence of low concentrations of anti-L1 antibodies, virions associated with the BM, however binding to the epithelial cell surface was not detected. Regardless of the concentration, L2 vaccine-induced antibodies allow BM association, but prevent association with the cell surface. This is the first study to examine the mechanisms of vaccine-induced inhibition of virus infection in vivo.
Introduction
Papillomaviruses (PVs) are a family of small, non-enveloped viruses which encapsidate an 8 kb double stranded circular DNA genome. More than 100 HPV genotypes (types) have been described, with each type being classified based primarily on differences in the amino acid sequence of the major capsid protein, L1 (Bernard et al., 2010; de Villiers et al., 2004). The virus capsid also contains the minor protein, L2, whose N-terminal domain is highly conserved amongst the PV family (Gambhira et al., 2007; Pereira et al., 2009). Human papillomaviruses (HPVs) are the primary etiological agents involved in the development of cervical neoplasia. More than 10 HPV types can cause cervical cancer, with HPV16 and HPV18 accounting for approximately 70% of cases (Muñoz et al., 2004; Schiffman et al., 2007). HPVs have also been implicated as a causative agent of other ano-genital and oropharyngeal cancers, as well as benign genital and cutaneous warts (Giuliano et al., 2008).
L1 can self-assemble into virus-like particles (VLPs) comprised of 72 pentameric capsomers. L1 VLPs contain immunodominant epitopes that elicit strong type-specific immune responses capable of inhibiting PV infection in animal model systems (Breitburd et al., 1995; Christensen et al., 1996; Kirnbauer et al., 1992; Suzich et al., 1995). Virion-binding antibodies are thought to act as the primary mechanism for inhibition, as passively-administered sera from animals vaccinated with VLPs from the cottontail rabbit papillomavirus (CRPV) and canine oral papillomavirus (COPV) confer protection against type-specific challenge (Breitburd et al., 1995; Suzich et al., 1995). The strong immunogenicity of L1 has led to the development of two commercial L1 VLP-based vaccines: Cervarix, a bivalent vaccine targeting HPV16/18 (Paavonen et al., 2009), and Gardasil, a quadrivalent formulation consisting of VLPs of HPV6/11/16/18 (Muñoz et al., 2010) (HPV6 and HPV11 cause most cases of genital warts). Both vaccines are highly effective at preventing infection and neoplastic lesions caused by the targeted HPV types. The immunity generated by L1 VLP vaccination is PV type-restricted due to sequence divergence in the surface loops of the L1 capsid proteins among PV types (Carter et al., 2006). In contrast, the minor capsid protein, L2, has recently been recognized as an attractive alternative vaccine target, due to the evidence that, when removed from its normal context in the virion, the highly conserved N-terminal region of L2 contains epitope(s) capable of generating broadly cross-type neutralizing antibodies (Gambhira et al., 2007; Jagu et al., 2009; Roden et al., 2000).
Elucidation of the mechanisms that underlie vaccine-induced protection can provide insight into the effectiveness of the currently licensed vaccines and assist in the assessment and design of future vaccines, including those based on L2. The mechanisms whereby vaccine-induced antibodies prevent infection are reasonably well understood for a number of viruses in cultured cell systems. By contrast, knowledge of how anti-virion antibodies prevent infection in vivo is limited because few animal models of virus binding, entry, and infection have been extended to a microscopic examination of the relevant tissue (Miller et al., 2005; Ong et al., 2008). Mechanisms of in vivo inhibition of virus infection could be particularly informative for HPV vaccines that target L1 or L2, as we have recently identified substantial differences between HPV infection in cultured cells and that observed in vivo utilizing a murine cervicovaginal challenge (CVC) model (Kines et al., 2009; Roberts et al., 2007). The CVC model and analysis of the infectious steps were made possible by development of high titer HPV pseudovirions (PsV), in which the authentic L1 and L2 capsid proteins encapsidate a reporter plasmid (e.g., luciferase). Expression of the reporter gene can be used as a surrogate for virus infection (Buck and Thompson, 2007). The pseudovirions have been well characterized and are believed to behave similarly to authentic virus during the establishment phase of the infectious process (Day et al., 2004; Florin et al., 2004; Gambhira et al., 2007). These properties make them well suited for examination of the early events in PV infection.
While the initial steps in HPV infection can occur on the surface of immortalized cultured cell lines, the in vivo CVC model has shown that, remarkably, the earliest steps in HPV infection occur strictly on the acellular basement membrane (BM) prior to transfer of the virion to its target epithelial cell, the keratinocyte (Kines et al., 2009; Roberts et al., 2007). The initial step in in vivo infection is binding of the virion to BM heparan sulfate proteoglycans (HSPG) at sites exposed due to epithelial damage. This step, which is L1-dependent but L2-independent, is followed by a conformational change in the capsid that renders the L2 N-terminus susceptible to proprotein convertase (PC) cleavage (furin and/or PC5/6). PC cleavage leads to exposure of a major N-terminal cross-neutralization epitope on L2 (a.a. 17–36) as well as exposure of a previously occluded region of L1, resulting in virion transfer and stable association with an undetermined receptor on the epithelial cell surface. By comparison, in cultured cells PsV bind avidly to cell surface HSPG followed by PC cleavage of L2 and exposure of the L2 cross-neutralization epitope on the cell surface (Day et al., 2007). The pseudovirions subsequently bind to a second, undetermined receptor, also located on the cell surface (Day et al., 2008). PsV can also bind to the extracellular matrix (ECM) produced by cultured cells. However, ECM binding is not equivalent to in vivo BM binding, in that ECM binding is largely independent of HSPG interaction and does not result in efficient cleavage of L2. Therefore, in cultured cells, the key early steps in infection take place on the cell surface, while in the murine CVC model, as discussed above, the steps are spatially separate, with initial HSPG binding and PC cleavage occurring on the BM, prior to transfer to the epithelial cell surface.
The above differences make it difficult to predict the in vivo mechanisms by which the antibodies induced by L1 VLP- and L2-based vaccines act to prevent infection. Therefore, we have undertaken an analysis of the protection induced by both of these vaccines in the CVC model and correlated protection with the infectious steps abrogated by vaccination or passive transfer of vaccine-induced antibodies.
Results
Vaccination with HPV16 L1 VLPs induces type-restricted protection in the mouse CVC model
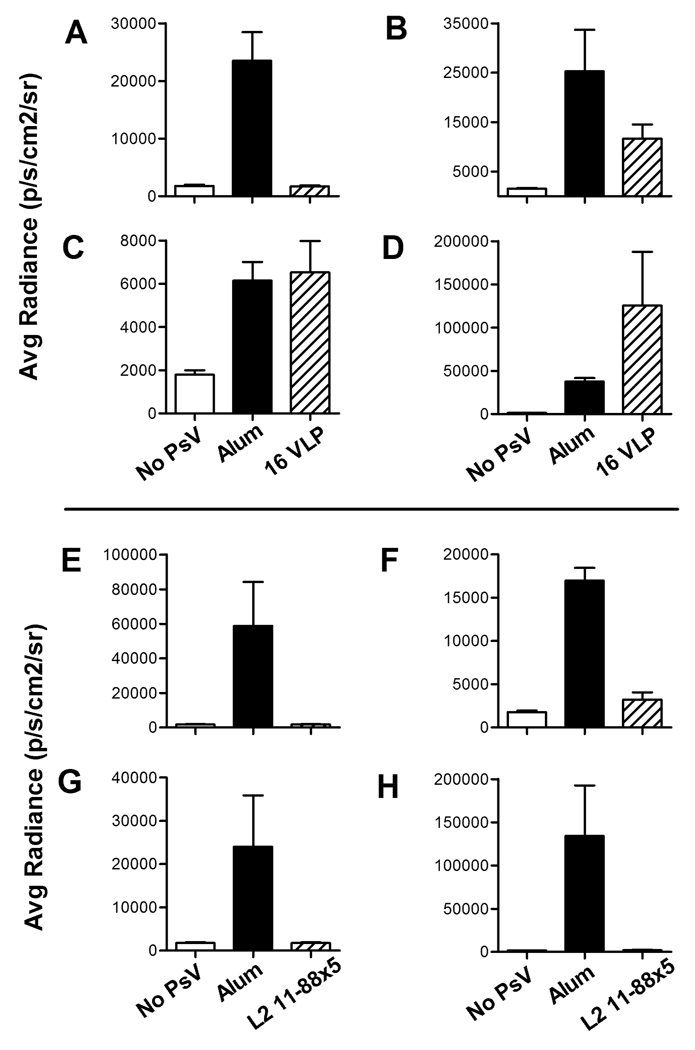
To examine the type specificity of L1 VLP-induced protection from cervicovaginal challenge, mice were vaccinated with either alum alone or HPV16 L1 VLPs (precipitated onto alum) three times at two week intervals, and then vaginally challenged, two weeks after the final immunization, with 10 micrograms of HPV pseudovirions of types 16, 31, 45, or 58 (Figure 1, A–D). Antibodies generated after VLP vaccination are type-restricted in their ability to neutralize virus in vitro (Christensen and Kreider, 1990; Giroglou et al., 2001; Pastrana et al., 2004), and the HPV16 L1 VLPs induced strong protection against HPV16 vaginal challenge (100%, p=0.0012, Figure 1A). HPV16, 31, and 58 are phylogenetically grouped in the α9 species (Bernard et al., 2010), with 16 being more closely related to 31 than to 58, and this correlated with the partial protection observed against HPV31 (57.3%, p=0.0826, Figure 1B), and lack of protection against HPV58 (NS, Figure 1D). In the mice challenged with HPV58, the notable difference in the radiance between the HPV16 VLP-vaccinated and alum-vaccinated control was driven by outliers in the VLP group and was not significant. HPV45 is even more divergent from HPV16, as it is a member of the α7 species, which also includes HPV18. This divergence correlates with the lack of protection from HPV45 infection after 16L1 VLP vaccination (NS, Figure 1C). Importantly, the results in the CVC model are qualitatively similar to the protection against the α9 species seen in women in the clinical efficacy trials of the commercial vaccines for protection against 6 months persistent infection. It was found that women who were given either of the commercial vaccines were strongly protected against HPV16, partially protected against HPV31, and not protected against HPV58 (the HPV45 results in women cannot be readily compared, as the commercial vaccines also contain VLPs from HPV18, which as noted above is an α7 species virus) (Brown et al., 2009; Paavonen et al., 2009). Thus, the murine model is capable of recapitulating the protection observed in vaccinated women.
Figure 1. Protection from infection after HPV16 L1 VLP or L2 11–88x5 vaccination.
Mice were vaccinated with Alum only (solid bars), 5µg HPV16 L1 VLPs or 25µg L2 11–88x5 (hatched bars) and challenged with (A, E) HPV16, (B, F) HPV31, (C, G) HPV45 or (D, H) HPV58. Unvaccinated mice were challenged with vehicle only (CMC; open bars) to establish background luminescence. Values on y-axis are average radiance (p/s/cm2/sr). Data represent the mean +/− SEM of five mice/group from a representative experiment.
HPV virion localization in vivo after vaccination with HPV16 L1 VLPs
To identify at which step HPV16 L1 VLP vaccination interferes with viral infection, vaccinated mice were vaginally challenged with either HPV16 or HPV45 PsV, which were labeled with Alexa Fluor 488 (AF488). AF488-coupled PsV have been utilized in previous in vivo studies and have binding (Kines et al., 2009) and infectivity (Roberts et al., 2007) profiles similar to unmodified PsV, which were used in the experiment in Figure 1. We opted to utilize AF488-coupled PsV for the binding analysis to facilitate virion detection in the presence of vaccine-induced anti-L1 antibodies.
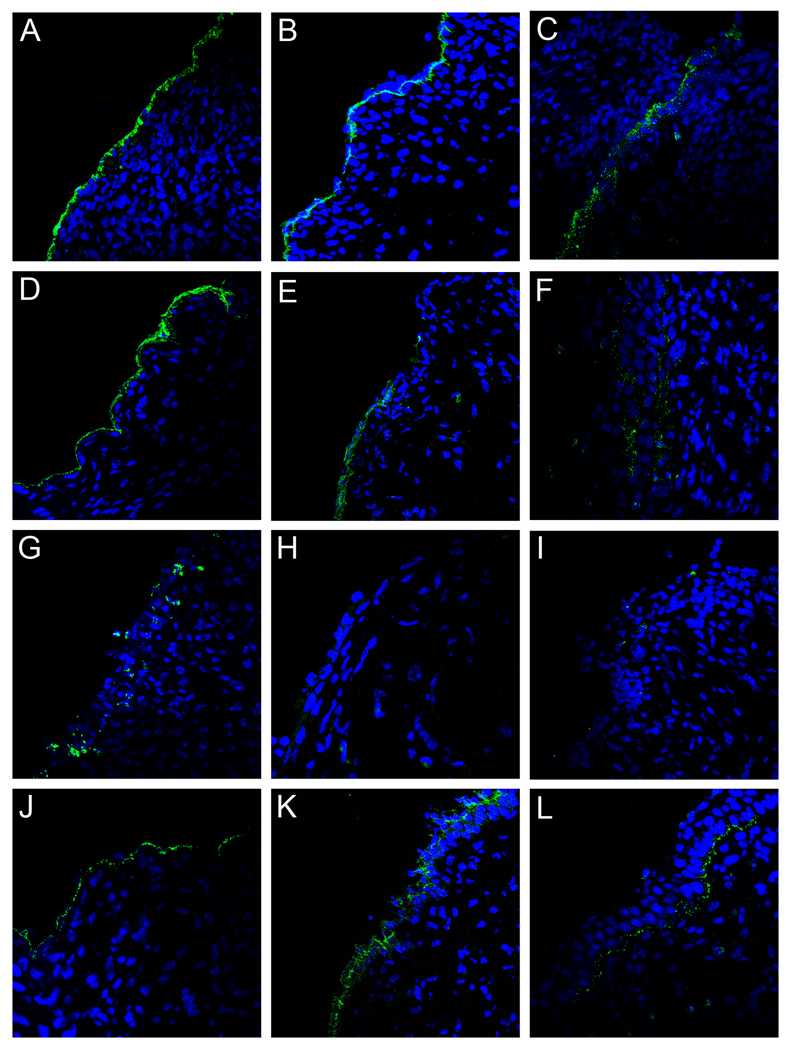
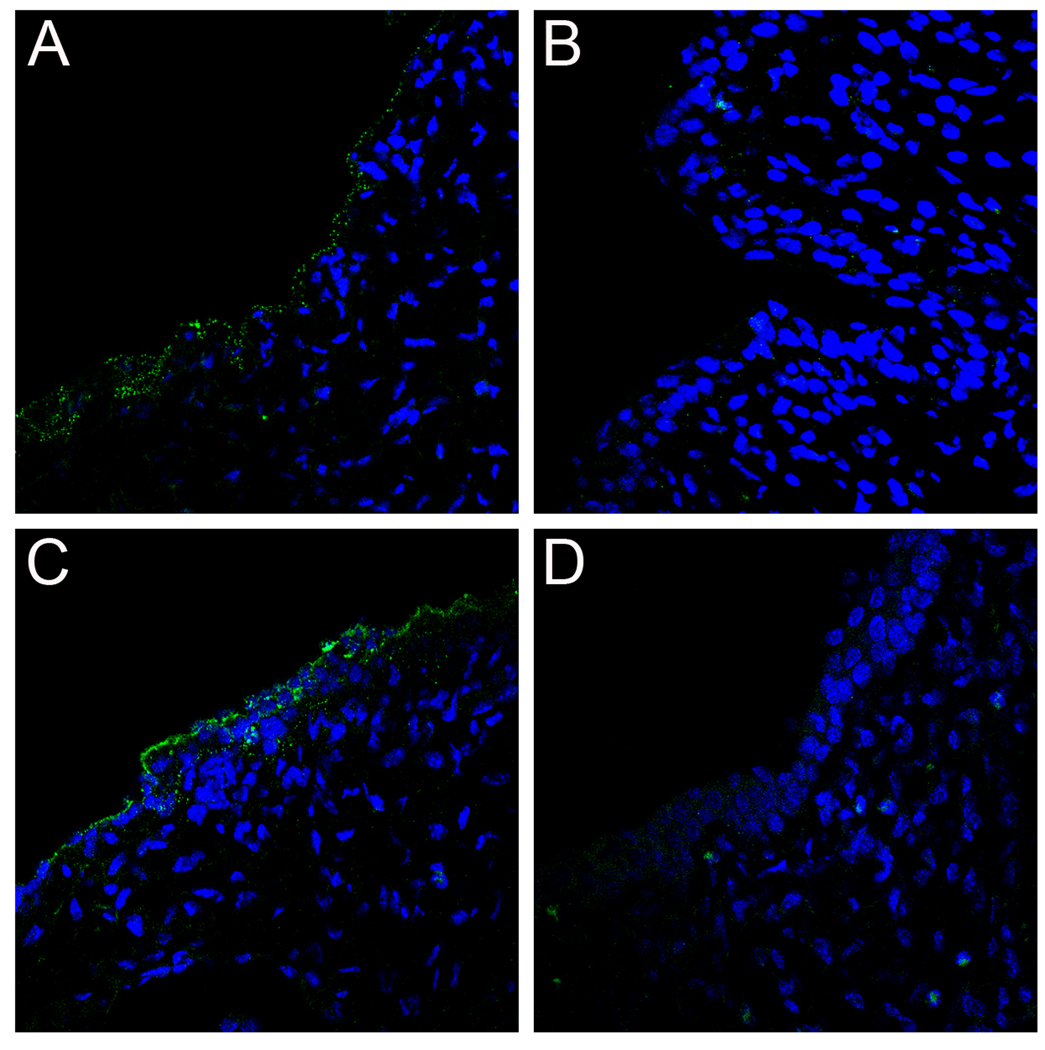
Tissues were harvested at either 4 or 18 hours following PsV challenge, and the localization of the capsids was determined. In alum-treated mice, as predicted from previous work in naïve mice (Kines et al., 2009), both the HPV16 and HPV45 capsids initially localized to the exposed BM (Figures 2A and 2D), the L2 17–36 epitope was readily detected at the 4 hour time point after virus challenge (Figures 2B and 2E), and by 18 hours, some virions were associated with the surface of the re-epithelializing cellular layer, although some residual virions on the BM were clearly visible (Figures 2C, 2F and 2L) as previously reported (Kines, et al. 2009). By contrast, in mice vaccinated with HPV16 L1 VLPs and challenged with HPV16 PsV, the viral capsids never associated with the BM. Instead, 4 hours after HPV16 challenge, the capsids were found associated with large cellular aggregates within the lumen of the cervicovaginal tract (Figure 2G). Further investigation determined that the cellular aggregates were primarily comprised of neutrophils, and the antibody/virus complex was found predominantly on these cells (Figure 3B). The capsids also bound neutrophils in alum-immunized mice (Figure 3A), although to a lesser extent, indicating that this interaction can occur in the absence of virion-specific antibodies. The L2 17–36 epitope was undetectable (Figure 2H) at 4 hours, and by 18 hours, the amount of detectable virus was greatly decreased to near background levels (Figure 2I), possibly due to degradation by the neutrophils. The above changes were specific for HPV16 L1 VLP-induced protection, as the HPV16-vaccinated mice challenged with HPV45 displayed virion localization and L2 epitope exposure similar to that of alum-treated mice, which correlated with the lack of protection against HPV45 (Figures 2J–2L).
Figure 2. PsV capsid localization and L2 exposure following VLP immunization.
Pseudovirion capsids were evaluated for their ability to interact with the BM and epithelium and expose the L2 cross-neutralization epitope following generation of an antibody response via immunization with either adjuvant only or HPV16 VLPs and adjuvant. HPV16 pseudovirions in tissue from alum-immunized animals are shown in panels A–C. Panels A and C show the localization of AF488-coupled capsids (green) at 4 hours and 18 hours, respectively. Panel B shows the exposure of the L2 17–36 epitope at 4 hours following delivery of uncoupled pseudovirions, detected with a rabbit polyclonal antiserum against L2 a.a. 17/36 (green). Detection of HPV45 capsids is shown in panels D–F. AF488-coupled capsids are shown in panels D and F at 4 hours and 18 hours, respectively. The L2 epitope exposure at 4 hours is shown in panel E. The remaining panels show the analogous experiments performed in the VLP-immunized animals. HPV16 pseudovirion detection is shown in panels G–I. Capsid binding is shown for the two time points in panels G and I. L2 epitope exposure at 4 hours is shown in panel H. HPV45 pseudovirus binding is shown in panels J–L. Capsid association is in panels J and L at 4 hours and 18 hours. L2 exposure is in panel K. Images are representative of five animals tested for each condition examined.
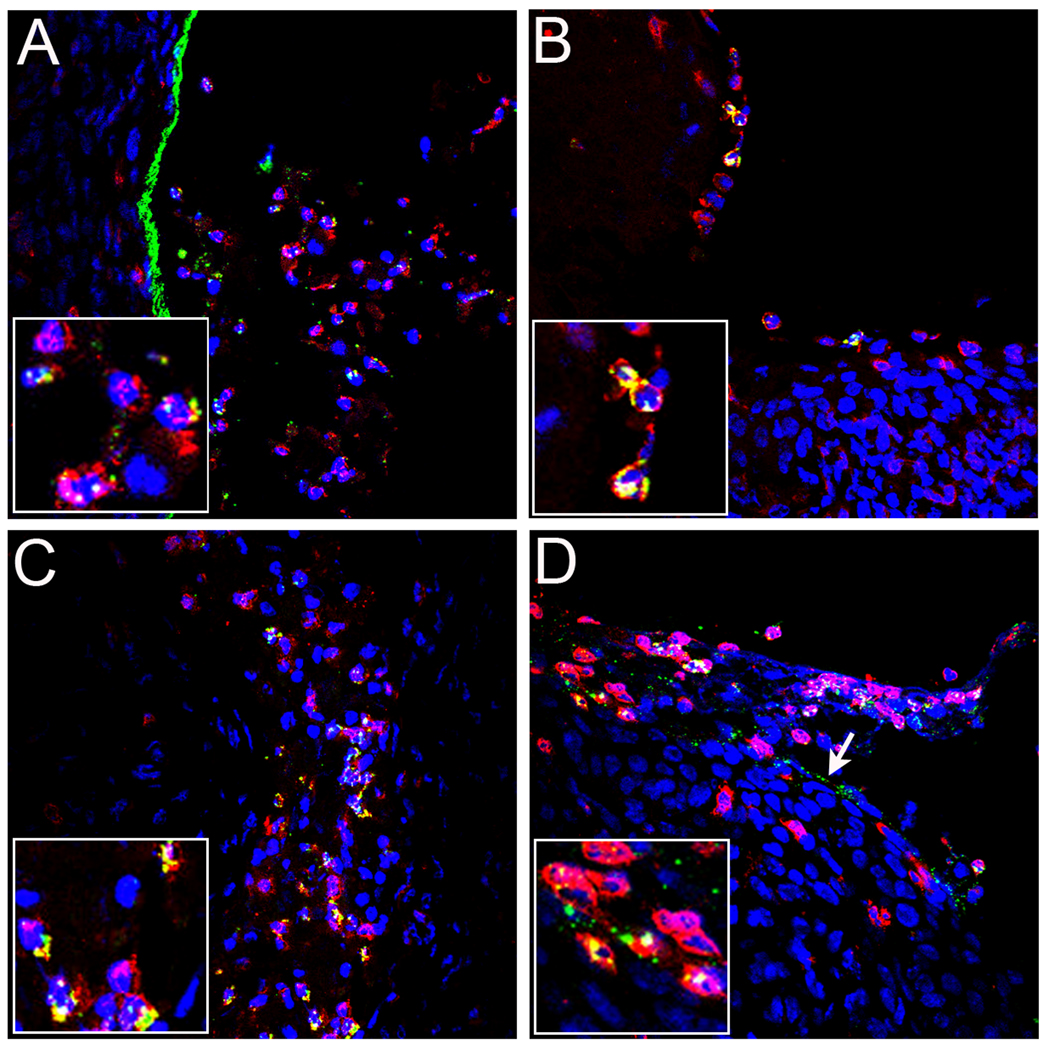
Figure 3. Colocalization of pseudovirus with neutrophils.
HPV16 pseudovirus was instilled into the vaginal tract of an alum-vaccinated mouse (panel A), an HPV16 VLP-vaccinated mouse (panel B), or a mice that received passively-transferred rabbit anti-L1 immune serum (panels C, high volume and D, low volume). Alexa Fluor 488-coupled pseudovirus (green) was used in the animals shown in panels A and B. In panels C and D, the antibody-bound pseudovirus was detected with Alexa Fluor 488-coupled donkey anti-rabbit secondary antibody (green). BM-associated virions evident in panel D are indicated with the arrow. Neutrophils were detected in all panels with a rat anti-neutrophil antibody and Alexa Fluor 594-coupled donkey anti-rat secondary antibody (red). All tissues were harvested at 4 hours post-instillation. Images are representative of five animals tested for each condition examined.
Prevention of BM Binding by Passive Transfer of anti-HPV16 L1 Immune Serum is Antibody Concentration-Dependent
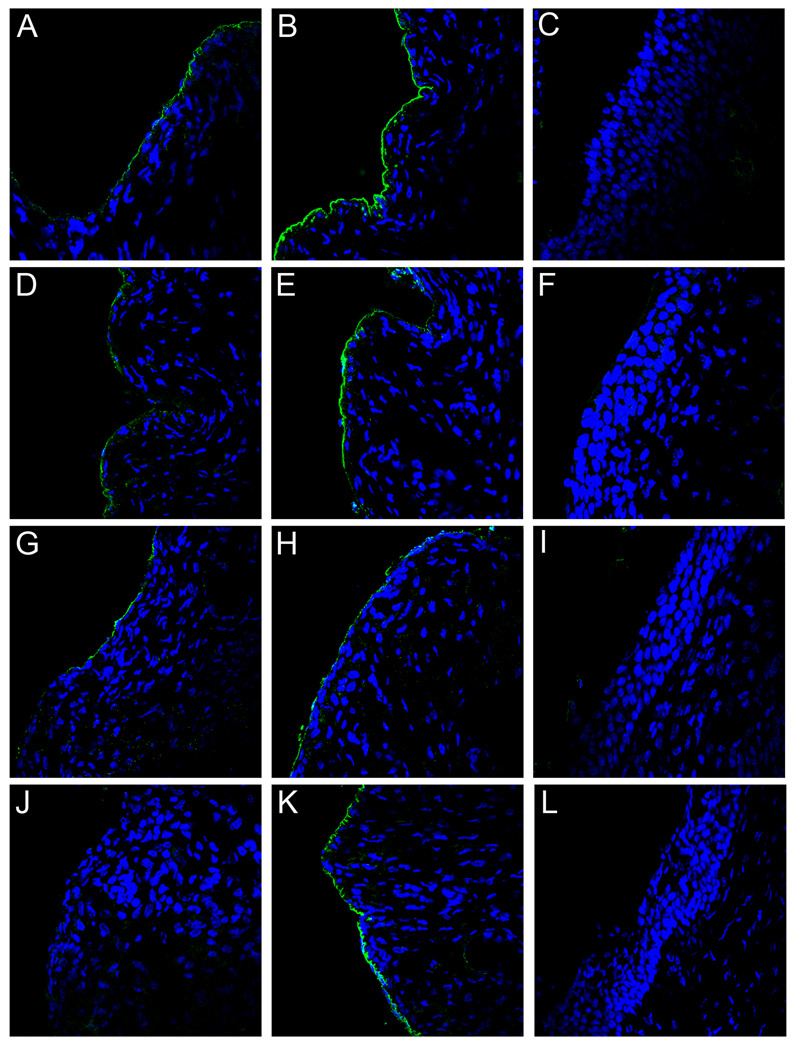
As noted earlier, transfer of immune serum from L1 VLP vaccinees to naïve recipients as a means of passive immunization can confer protection in animal PV models. We decided to verify this with our vaccination protocol and to confirm that the observed effects on PsV binding utilizing the CVC model following VLP-vaccination can be attributed solely to the induced antibodies. Therefore, we passively transferred high titer serum from a rabbit immunized with HPV16 L1 VLPs into naïve mice. As expected, the passive transfer of a high serum volume (20µl) resulted in strong protection (> 99.9%; p=0.0026) against HPV16 PsV challenge and no protection from infection with HPV45 (Figures S1A and S1B). Microscopic analysis of tissue from mice challenged with HPV16 revealed a pattern of rabbit antibody-bound PsV identical to the PsV pattern seen following vaccination. This included the lack of BM binding at 4 hours (Figure 4C), association of the antibody-PsV complexes with lumenal neutrophils (Figure 3C), and greatly diminished amounts of detectable virus at 18 hours (Figure 4D). Passive transfer of pre-immune rabbit serum gave results that were similar to the alum-immunized controls (Figures 4A and 4B). Because there is no formation of antibody-capsid complexes in the presence of pre-immune serum (Figure 4A), we demonstrate the presence of PsV with anti-HPV16 L1 staining (Figure 4B). Additionally, the BM deposition of HPV45 virus in the presence of the high volume anti-16 L1 serum was similar to pre-immune control animals, providing further evidence for type-specific immunity (data not shown). These data verify that serum antibodies can account for all of the microscopic findings observed in the mice protected by HPV16 VLP vaccination.
Figure 4. Localization of capsids following passive transfer of anti-VLP sera.
Either pre-immune or rabbit anti-HPV16 VLP immune sera was passively transferred into mice and the localization of capsids analyzed at either 4 or 18 hours following delivery. The capsid-antibody complexes were detected with Alexa Fluor 488-conjugated donkey anti-rabbit (green). Detection of binding of pre-immune sera at 4 hours is shown in panel A. Detection of capsids in the same tissue with an anti-HPV16 L1 serum is shown in panel B. Detection of capsid-antibody complexes following the transfer of a high volume of immune serum is shown in panels C (4 hrs.) and D (18 hrs.). Detection of capsid-antibody complexes following the transfer of a low volume of immune serum is shown in panels E (4 hrs.) and F (18 hrs.). Detection of exposure of L2 following low volume passive transfer is shown in panel G (4 hrs.). This detection required blocking the passively transferred rabbit serum with unlabeled donkey anti-rabbit serum prior to binding of the rabbit anti-L2 serum which was detected with an Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (green). Complete blocking of the low volume passive transfer was achieved. The omission of the anti-L2 serum, but inclusion of the Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody, resulted in no detectable signal (panel H). The experiment could not be performed with the high volume passive transfer due to the inability to achieve complete blocking (data not shown). Images are representative of five animals tested for each condition examined. See also Figures S1, S2, S3.
It is known that vaccine-induced antibodies wane over time in vaccine subjects (Olsson et al., 2007; Romanowski et al., 2009). Therefore, we wanted to examine protection from infection and mechanisms of inhibition in the presence of low amounts of circulating anti-HPV16 L1 VLP antibodies. We performed a microscopic examination of tissue from mice that were subjected to an HPV16 PsV challenge following passive transfer of the lowest volume of the immune rabbit serum (0.2µl) that was still highly protective from type-specific challenge (>97%; p=0.0029) (Figure S1A). (The transfer of one-half as much volume (0.1µl) resulted in only 90% protection [unpublished data]). When the 0.2µl volume was transferred, the microscopic appearance following HPV16 PsV challenge differed substantially from what had been observed above with the higher volume (20µl). The most striking difference was that at the 4 hour time point, the input HPV16 PsV could be found associated with the BM (Figure 4E). This pattern of deposition was qualitatively similar to that found in control tissues of mice that received pre-immune serum, albeit present at substantially reduced levels. To confirm that this staining truly represented association with the BM, we examined the co-localization of the capsids with nidogen, a marker of the BM (Figures S2A and S2B). Additionally, we determined that the L2 17–36 epitope was exposed on these BM-bound capsids at 4 hours (Figure 4G; Figure 4H shows complete blocking of passively transferred antibody prior to immune detection of L2). Substantial binding of virions by neutrophils was also evident at 4 hrs (Figure 3D). However, when we examined tissues harvested at the 18 hour time point, we were unable to detect bound PsV, indicating loss of particles from the BM and lack of stable association with the epithelial cell surface (Figure 4F). Thus, the lower volume of immune serum did not completely prevent BM binding or L2 epitope exposure, although it did completely abrogate infection. This result indicates an additional mechanism of neutralization is evident at lower antibody concentrations. As observed with the high volume anti-16L1 sera transfer, HPV45 binding was unaffected by low volume transfer, and was similar to controls (data not shown).
The differences in the steps inhibited in vivo by the higher and lower volumes of immune serum prompted us to ask whether similar observations might be made in cultured cells. We found that at both the high concentration and the low concentration of serum that inhibited infection of cultured cells (data not shown), the PsV was prevented from binding to the ECM, but bound robustly to the cell surface, unlike what was seen in vivo (Figures S3E and S3F). We suspect that PsV/antibody complexes bind to HSPG determinants on the cells since the complexes did not bind pgsa-745 cells, which lack all GAGs due to a mutation in a processing enzyme, or to sodium chlorate treated HaCaT cells, which express under-sulfated HSPG (unpublished observations). However, exposure of the L2 epitope did correlate with the in vivo observations, in that it was not exposed at high serum concentrations but became exposed at low concentrations (compare Figures S3C and S3D with Figure 4G).
In vivo protection from infection after vaccination with HPVL2 11–88x5
We also examined the protection elicited by an L2-based vaccine and studied its mechanism of protection. We used a candidate L2 vaccine consisting of a single polypeptide, designated L2 11–88x5, which is composed of the conserved N-terminal sequence of amino acids 11–88 from the L2 of the distantly related HPV types 1, 5, 6, 16, and 18. L2 11–88x5 can induce cross-neutralizing antibodies both in mice and rabbits, as determined by a tissue culture-based neutralization assay, and can protect mice against cutaneous challenge with HPV16 PsV (Jagu et al., 2009). To determine if broad cross-protection occurs in the CVC in vivo model, we vaccinated mice with alum alone or L2 11–88x5 (precipitated onto alum) three times at two week intervals, and subsequently vaginally challenged them with HPV types 16, 31, 45, and 58 two weeks following the final immunization (Figure 1E–1H). We observed 99.9% (p=0.0278), 90.4% (p<0.0001), 100% (p=0.0993), and 99.7% (p=0.0272) inhibition of infection, respectively, indicating that effective cross-protection against cervicovaginal infection was generated. It is noteworthy that the L2 11–88x5 vaccine induced good protection against HPV31, HPV45, and HPV58, although none of these HPV types is present in the immunogen.
HPV capsid localization in vivo after vaccination with L2 11–88x5
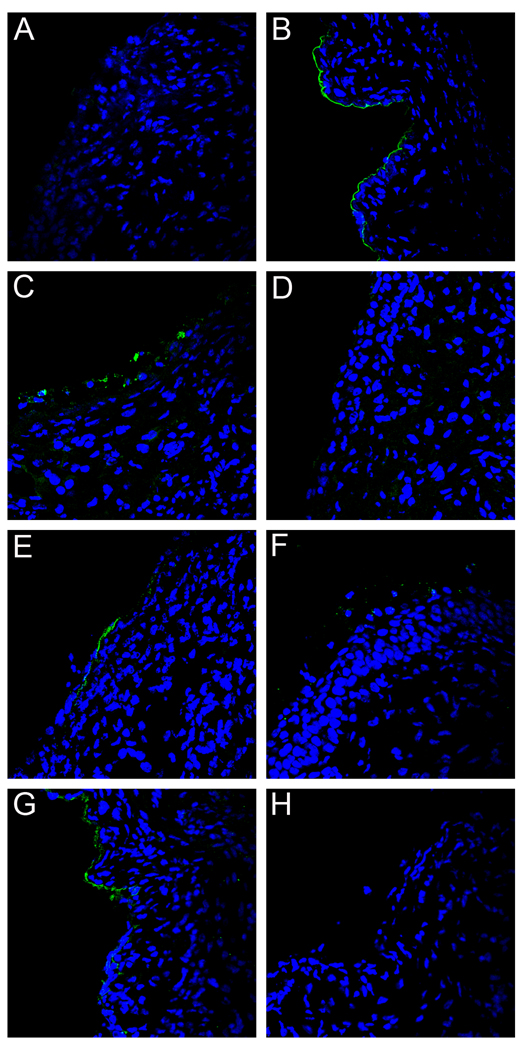
The substantial in vivo cross-protection induced by L2 11–88x5 vaccination prompted us to examine at which step antibodies raised to this immunogen interfere with the infectious process. Given that the L2 cross-neutralization epitope is not exposed until after the virus has bound to the BM, it seemed unlikely that these antibodies would prevent BM binding. Indeed, following vaccination with L2 11–88x5 and vaginal challenge with HPV16 or HPV45, capsids of both HPV types localized on the BM at 4 hours, as determined by staining with an anti-L1 antibody (Figures 5A and 5C). Unexpectedly, by 18 hours, both HPV16 and HPV45 capsids were undetectable on the BM or elsewhere in the tissue (Figures 5B and 5D). Unfortunately, interference from vaccine-induced antibodies prevented us from directly monitoring the L2 epitope exposure in these mice. However, as L2 17–36 exposure is detectable by 4 hours, it seems likely that exposure occurs as in control animals, which would enable the vaccine-induced L2 antibodies to bind to the epitope(s) that becomes exposed on the BM. This antibody engagement appears to lead to the premature separation of the virus from the BM and its failure to stably bind the epithelial cells.
Figure 5. Capsid localization and L2 exposure following L2 immunization.
Capsid binding and localization was examined in mice that had been immunized with the L2 11–88x5. HPV16 capsid binding was examined at 4 hours (panel A) and 18 hours (panel B) post-instillation (green). Likewise, HPV45 capsid binding was examined at 4 hours (panel C) and 18 hours (panel D). Rabbit anti-L1 serum against the appropriate type was used for staining in order to show capsid localization. Images are representative of five animals tested for each condition examined.
L2 antibodies induced by L2 11–88x5 vaccination prevent the stable engagement of BM bound virus with epithelial cells
Most of the above speculation could be tested by passive transfer of immune sera from L2 11–88x5 vaccinated animals. Therefore, we transferred a high volume (100µl) and a low volume (20µl) of immune rabbit serum into naïve mice to assess protection from infection by HPV16 and HPV45 PsV. Additionally, we microscopically evaluated viral localization events associated with this challenge (Figure 6). Passive transfer of 100µl immune serum protected the mice from both HPV16 and HPV45 PsV challenge by 100% (p=0.0035 and p<0.0001, respectively) and transfer of 20ul immune serum completely protected them from HPV16 (99.6%, p=0.0036) and to a lesser degree, HPV45 (90%, p=0.0003) (Figures S1C and S1D). Following challenge with either HPV type, the microscopic observations, as determined by detection of rabbit antibody-bound-PsV complexes, were indistinguishable, irrespective of the volume of serum transferred, from what had been observed in the mice immunized with L2 11–88x5. This includes BM binding at 4 hours post-challenge (HPV16 Figures 6A, 6B, 6D, 6E; HPV45 Figures 6G, 6H, 6K) and loss of both HPV16 and HPV45 by 18 hours (Figures 6C, 6F and 6I, 6L respectively). The presence of anti-L2 antibody bound to HPV45 at 4 hours was below the level of detection in the presence of low serum passive transfer (Figure 6J); however, we could visualize virions on the BM with an anti-L1 antibody (Figure 6K). Thus, the protection mediated by L2 11–88x5 vaccination and the associated microscopic changes can all be attributed to the vaccine-induced antibodies.
Figure 6. Localization of capsids following passive transfer of anti-L2 sera.
Rabbit anti-L2 immune serum was passively transferred into mice and the localization of capsids analyzed at either 4 hours (panels A, B, D, E, G, H, and K) or 18 hours (panels C, F, I, and L) following delivery. Panels A–F show localization of HPV16. Panels G–L show localization of HPV45. The capsid-antibody complexes at 4 hours were detected with Alexa Fluor 488-conjugated donkey anti-rabbit (green) in panels A, D, G and J, showing weak staining of the antibody-capsid complexes following either transfer of high volume (panels A and G) or low volume (panel D) of immune serum. Visualization of anti-L2 bound to HPV45 capsids in the low volume transfer was below the threshold of detection (panel J). Detection of capsids (instead of antibody-bound capsids) with anti-L1 serum is shown for the same sections in the adjacent panels (B, E, H and K). Detection of capsids with the anti-L1 serum at 18 hours is shown in panel C for high volume, HPV16, panel F for low volume, HPV16, panel I for high volume, HPV45, and panel L for low volume, HPV45. Images are representative of five animals tested for each condition examined. See also Figure S1.
Discussion
The in vivo mechanisms by which active immunization actually protects from subsequent challenge have not been previously analyzed microscopically for any infectious agent. Cell culture-based assays have provided a proxy for this understanding, but it is unclear how closely these systems mimic what is occurring in the host animal (Ochsenbauer and Kappes, 2009; Se-Thoe et al., 2000; Stanley et al., 2006). Utilizing the murine CVC model to examine the process of in vivo HPV infection, we recently reported that, in contrast to what is described for cultured cell infection, the initial steps for in vivo HPV infection take place on the BM. These extracellular events, which involve the transition of the mature capsid to a PC-cleaved form with exposed L2 neutralization epitope(s), are necessary to effect stable association with the epithelial cell surface. Here we have used this model to address how these characteristics of HPV infection might contribute to the mechanisms of protection induced by the current HPV L1 VLP vaccine and a candidate L2 vaccine, thus providing the first mechanistic examination of antibody-dependent inhibition of virus infection in vivo.
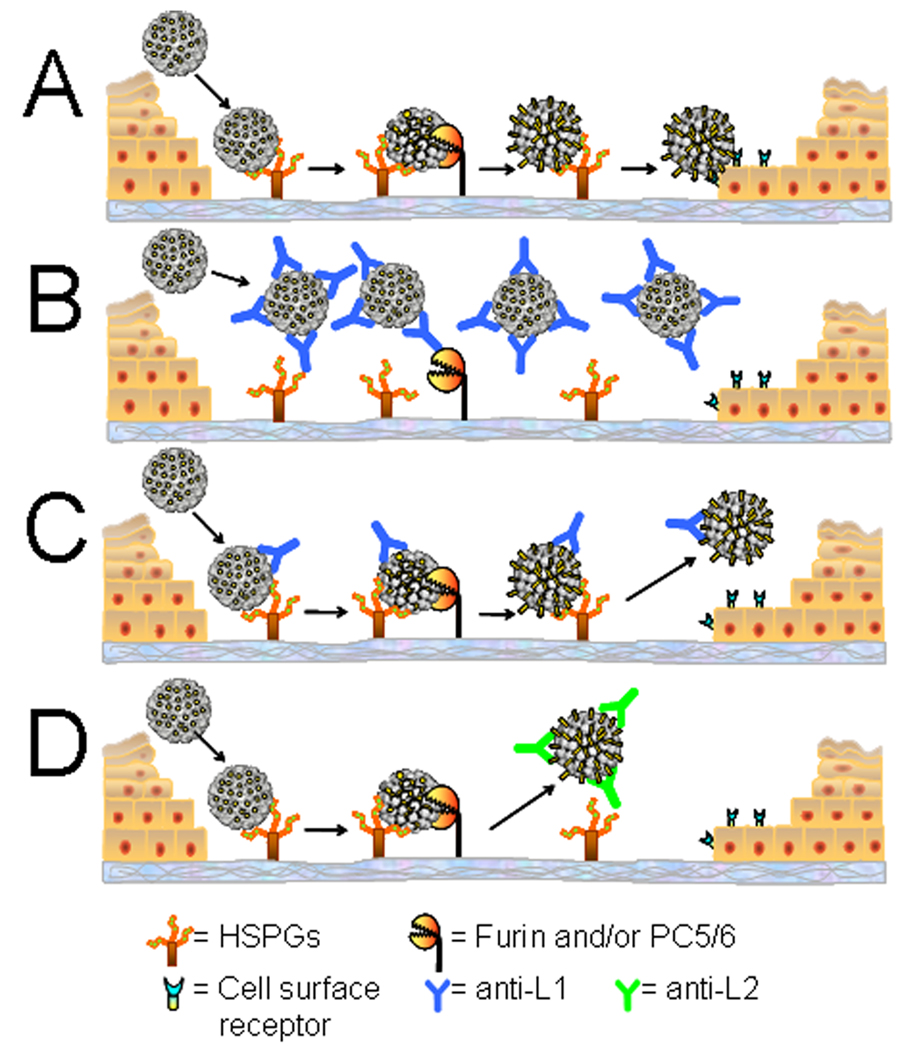
To examine vaccine-induced protection from PV infection in vivo, we compared capsid binding, localization and L2 epitope exposure in control animals with those either immunized with VLPs, or receiving passively transferred L1-immune serum. The latter approach allows us to analyze the inhibition of infection at various quantities of virion-specific antibodies. Through these analyses, we have identified two distinct mechanisms of inhibition mediated by polyclonal antibodies against L1, with the predominant mechanism depending upon antibody level. As discussed below, some aspects of these mechanisms were not predicted from cell culture models. A model summarizing these mechanisms is presented in Figure 7. The events observed in unimmunized animals are shown in Figure 7A. In contrast, prevention of infection mediated by VLP-vaccination or transfer of the higher amount of polyclonal serum is depicted in Figure 7B. We postulate that a high antibody to virus particle ratio results in an immunoglobulin-coated capsid, which effectively precludes the interaction of the capsid proteins with HSPG on the BM, the required first step in infection. The soluble virion/antibody complexes are subsequently scavenged by neutrophils possibly through opsonization. However, we cannot rule out the possibility that some virion/antibody complexes transiently associate with the BM prior to rapid removal by infiltrating neutrophils.
Figure 7. Model of HPV binding and interference by vaccine-induced antibodies.
Panel A depicts normal in vivo binding, cleavage, and transfer of the virion to the epithelial cell surface. BM binding is blocked in the presence of high concentrations of anti-L1 antibodies (B), however at low concentrations (C), anti-L1 antibodies prevent stable engagement of the cell surface receptor. Anti-L2 antibodies bind only after the initial conformation change on the BM and cleavage of L2 by furin or PC5/6 (D) and also prevent stable binding to the cell surface.
By contrast, low concentrations of serum that are still strongly protective against infection only partially prevent BM binding (Figure 7C). However, engagement of the secondary L1 receptor on the epithelial cell surface by the capsid is prevented and the virus is lost from the tissue. Based on the results with neutralizing monoclonal antibodies on cultured cells, we had previously hypothesized that low amounts of L1 antibodies might neutralize by cross-linking the capsomers, thereby preventing a concerted conformational change in the capsid required for exposure of the L2 N-terminus to furin cleavage (Day et al., 2007). However, our current results clearly demonstrate that the L2 17–36 epitope is exposed on BM in vivo in the presence of low concentrations of neutralizing antibodies. Therefore, it seems likely that low concentrations of antibodies function by direct inhibition of capsid interaction with the secondary keratinocyte-specific receptor. Inhibition of this interaction might require fewer capsid-bound antibodies than are necessary to block binding to HSPG on the BM. It is also possible that, under conditions of low antibody occupancy, an additional undefined conformational change is prevented that is essential for epithelial cell transfer.
Both of the mechanisms of L1-based neutralization observed in this study are likely to be relevant to protection of VLP-vaccinated women, since the initially high peak titers observed after the standard three dose regimen decline rapidly in the first one to two years and then plateau at a 20 to 100-fold lower level (Olsson et al., 2007; Romanowski et al., 2009). The striking similarity of peak titers and of the type-restriction of protection seen in our model after L1 VLP vaccination and the degree seen in clinical trials of HPV VLP vaccines makes it likely that the results obtained herein are relevant to cervicovaginal HPV infections in women. Moreover, they suggest that the long duration of protection seen with vaccination may be more attributable to the inhibition mechanism seen at lower antibody levels.
Following L2 vaccination, we observed near normal levels of BM association of both homologous and heterologous types. This result was not unexpected, as we have previously demonstrated that the major L2 neutralization epitope(s) are poorly exposed in mature capsids and become exposed gradually on the BM (Kines et al., 2009). Therefore, the anti-L2 antibodies would bind inefficiently to the input capsids. However, following deposition on the BM and PC cleavage, anti-L2 antibodies would be able to bind to their newly exposed target epitope(s). We speculate that antibody-bound capsids fail to stably engage the epithelial cell surface because L2 antibody binding sterically hinders engagement of the keratinocyte specific receptor. This receptor would normally be bound via a surface of L1 that is exposed after PC cleavage of L2 (compare Figures 7A and 7D). The loss of the virions from the BM mediated by anti-L2 antibodies might indicate that the conformational change leading to exposure of the L2 cross-neutralization epitope(s) also results in a reduced affinity for HPSG. Interestingly, the protective antibodies induced by the L2 vaccine lead to changes that are microscopically similar to those seen at low concentrations of the anti-L1 VLP serum, although the epitopes bound by the respective antibodies are clearly distinct. It is encouraging to L2 vaccine prospects that protection may be induced by the same mechanism possibly responsible for long term protection following VLP vaccination.
Important aspects of these results were not predicted from the analysis of neutralization mechanisms in cultured cells, further underscoring the utility of the in vivo analysis. These differences arise in part because in immortalized cultured cells, HPV can directly bind either to HSPG on the cell surface or to non-HSPG ECM determinants, while in vivo there appears to be a strict initial binding to BM-associated HSPG. This understanding, in fact, minimizes the apparent discrepancies between the in vivo and in vitro observations for L2 neutralization. In cultured keratinocytes, we found that following HSPG-mediated cell surface association, the L2 cross-neutralization epitope was exposed, and that antibody engagement prevented transfer to the secondary cell surface receptor. This caused a loss of capsids from the cell surface and sequestration on the ECM. The existence of this additional capsid binding site in vitro leads to retention of the neutralized capsids in the cell culture system. More substantive differences are obvious when comparing the anti-L1 neutralization mechanisms. For example, the same rabbit polyclonal serum that at high concentrations prevents virus from binding to HSPG on the BM in vivo, blocks ECM binding, but does not affect association of the virus with cell surface HSPG on cultured cells. Instead this serum prevents infection by interfering with the stable engagement of the virus with the secondary cell surface receptor and subsequent endocytosis. This in vitro result is unchanged whether neutralizing with high concentrations or low concentrations of serum. In the in vitro assay, the main difference observed between the two concentrations, is that the L2 17–36 epitope is exposed at the low concentration, but not at the high concentration of serum. The binding of high occupancy virion/antibody complexes to cultured cells but not the BM could have several explanations. Modifications of HSPG on cell surfaces and ECM have been shown to differ even within the same cell type (Stow and Farquhar, 1987). The HSPG composition of the in vivo BM is likely to be even more complex as it arises from multiple cell types. Therefore, the relative affinity of the antibody and different forms of HSPG for a common or overlapping binding site on the capsid could account for the difference. In addition, it has been hypothesized that that there may be more than one HSPG binding site on the capsids (Selinka et al., 2007). Antibodies that block the capsid determinant that binds BM-like HSPG on cells may not block the site that binds to a second class of cell surface HSPG.
A noteworthy feature of the candidate L2 vaccine, L2 11–88x5, is that it targets cryptic cross-neutralization epitopes. Our challenge studies demonstrate broad cross-protection against all HPV types tested, including those not present in this L2 immunogen. This result provides important reassurance that the remarkably broad cross-neutralizing activity previously observed with in vitro neutralization assays is not an artifact that might result from the substantial differences in the initial events of infection that occur in vivo versus in vitro. Other viruses, such as HIV, expose cryptic functional domains transiently after cell surface attachment, thereby limiting the opportunity to induce neutralizing antibodies directed against them (Zwick and Burton, 2007). Thus, the theoretical advantage that targeting cryptic epitopes could result in the induction of broadly protective antibodies is usually outweighed by the joint challenges of generating a strong immune response to such epitopes and the brief exposure of the epitopes during infection (Haynes and Montefiori, 2006; Kelker et al., 2010). L2 may represent an exceptional opportunity for success because the cryptic L2 epitopes are exposed for several hours while the virus resides on the BM.
The demonstration that passive systemic transfer of anti-L1 VLP or anti-L2 immune sera can protect mice from cervicovaginal challenge confirms two concepts related to the mechanism of action of prophylactic HPV vaccines, and provides an approach to test the protective activity of any sera. First, because with the results observed after passively administering sera from immunized animals recapitulate both the protection and the microscopic appearance following direct vaccination, the results provide formal proof that antibodies mediate the predominant protective activity of both vaccines. Second, following vaccination, the possibility that some of the virus-specific antibodies in the genital tract are locally produced by mucosal antibody-secreting B cells, cannot be formally eliminated. However, the passive serum transfer results directly confirm that systemic circulating vaccine-induced antibodies are capable of preventing HPV infection of the female genital tract, either by exudation at the site of trauma or transudation into the cervicovaginal mucus. Finally, the reproducible effectiveness of passively transferred sera in the prevention of genital tract HPV infection raises the possibility that this assay could be used to derive a surrogate immune correlate of protection for the sera collected from clinical vaccination trials. We plan to evaluate this possibility.
Experimental Procedures
Pseudovirions
HPV16 L1 VLPs used for immunization were prepared as described in (Harro et al., 2001). All pseudovirions were produced as previously described (Buck and Thompson, 2007). SDS-PAGE analysis was performed to determine the concentration of the HPV16 L1 content of the pseudovirus preparation. For those experiments requiring fluorescently labeled pseudovirus, after Optiprep purification, particles were conjugated to Alexa Fluor 488 according to manufacturer’s protocol (Invitrogen), and then placed over a second Optiprep gradient to remove unconjugated fluorophore.
Animal studies
Six- to eight-week-old female BALB/cAnNCr mice were obtained from the National Cancer Institute and housed and handled in accordance with the NCI approved guidelines. Experimental protocols were approved by the National Cancer Institute’s Animal Care and Use Committee. Animals were immunized subcutaneously with 5µg of 16L1 VLPs or 25ug of 6His-tagged L2 11–88x5 peptide (Jagu et al., 2009) precipitated onto Imject Alum (Thermo) to a final volume of 100µl. Animals were immunized three times, with each injection spaced two weeks apart. Anti-L1 rabbit serum (in vitro EC50=1:10,950,000) used in the passive transfer studies was obtained from a 16L1 VLP-immunized rabbit and has been previously described (Roden et al., 1996). Anti-L2 polyclonal rabbit serum (in vitro EC50=1:20,605) was similarly generated against the L2 11–88x5 peptide.
In vivo pseudovirus delivery and infection
The mice were exposed to male mouse bedding for 7 days and received 3mg of Depo-provera (Pfizer) 5 days prior to infection (Johnson et al., 2009). Animals were pre-treated with 4% nonoxynol-9 and subsequently infected with pseudovirus as previously described (Cuburu et al., 2009; Johnson et al., 2009; Roberts et al., 2007). A PsV inoculum of 10 µg, based on L1 content, was used regardless of type in order to equalize the potential number of target epitopes for the neutralizing antibodies. The particle to infectivity ratio varies among types, thus explaining the difference in luminescent signal observed for the positive controls. For those mice receiving the passively transferred rabbit serum, serum was delivered neat or diluted in 1×PBS to a final volume of 100µl and was administered intraperitoneally 24 hours prior to infection. Infection was measured 48 hours after pseudovirus delivery as previously described (Cuburu et al., 2009; Johnson et al., 2009). Raw data was computed using Living Image software (Caliper Life Sciences). An identical region of interest (ROI) was drawn around the luciferase signal emitted from each mouse, and the average radiance within the ROI was determined. For binding analyses, mice were sacrificed at 4 or 18 hours following pseudovirus instillation and tissues were treated as described in Kines et al., 2009.
Antibodies and immunofluorescent staining
The rabbit polyclonal antiserum against HPV16 L1, the rat anti-HPV16 L1 polyclonal serum,and the rabbit antiserum, 17/36, that recognizes aa17–36 of L2 was previously described (Kines et al., 2009). The rat anti-nidogen monoclonal antibody was obtained from US Biological. Neutrophils were detected with a rat anti-neutrophil antibody (Santa Cruz Biotechnology). Tissues were stained as previously described (Kines et al., 2009). For L2 17–36 detection following passive transfer of the low volume of rabbit immune VLP serum, it was necessary to first prevent detection of the input serum by blocking with unlabeled donkey anti-rabbit serum. Complete blocking was confirmed by detection with Alexa Fluor 488-coupled donkey anti-rabbit serum. The 17/36 antiserum was then used for specific detection of the L2 epitope without interference from the delivered serum. Following staining, all sections were mounted with Prolong Gold mounting solution (Invitrogen). Microscopy was performed on a Zeiss LSM 510 confocal system interfaced with a Zeiss Axiovert 100M microscope. Images were collated with Adobe Photoshop software.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism Software, in which a one-tailed unpaired t-test was used to determine p-values.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and by National Cancer Institute, SPORE in Cervical Cancer, P50 CA098252 and CA118790 to R.B.S. Roden, and a Prevent Cancer Foundation, Alexandria, VA fellowship to S. Jagu. D. R. Lowy and J. T. Schiller are inventors of intellectual property owned by the US government for the L1 vaccine. D. R. Lowy, J. T. Schiller, S. Jagu and R.B.S. Roden are inventors on intellectual property owned by the US government and Johns Hopkins University for the L2 vaccine. R. B. S. Roden has been a paid consultant of Merck & Co, Inc., and both S. Jagu and R. B. S. Roden have received unrestricted educational grant funding from GlaxoSmithKline. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernard H-U, Burk RD, Chen Z, van Doorslaer K, Hausen Hz, de Villiers E-M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitburd F, Kirnbauer R, Hubbert N, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J, Lowy D. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, et al. The Impact of Quadrivalent Human Papillomavirus (HPV; Types 6, 11, 16, and 18) L1 Virus-Like Particle Vaccine on Infection and Disease Due to Oncogenic Nonvaccine HPV Types in Generally HPV-Naive Women Aged 16–26 Years. The Journal of Infectious Diseases. 2009;199:926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- Buck CB, Thompson CD. Production of papillomavirus-based gene transfer vectors. Curr Protoc Cell Biol. 2007;Chapter 26(Unit 26 21) doi: 10.1002/0471143030.cb2601s37. [DOI] [PubMed] [Google Scholar]
- Carter JJ, Wipf GC, Madeleine MM, Schwartz SM, Koutsky LA, Galloway DA. Identification of Human Papillomavirus Type 16 L1 Surface Loops Required for Neutralization by Human Sera. J. Virol. 2006;80:4664–4672. doi: 10.1128/JVI.80.10.4664-4672.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N, Reed C, Cladel N, Han R, Kreider J. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ND, Kreider JW. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J. Virol. 1990;64:3151–3156. doi: 10.1128/jvi.64.7.3151-3156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuburu N, Kweon M-N, Hervouet C, Cha H-R, Pang Y-YS, Holmgren J, Stadler K, Schiller JT, Anjuere F, Czerkinsky C. Sublingual Immunization with Nonreplicating Antigens Induces Antibody-Forming Cells and Cytotoxic T Cells in the Female Genital Tract Mucosa and Protects against Genital Papillomavirus Infection. J Immunol. 2009;183:7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Baker CC, Lowy DR, Schiller JT. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14252–14257. doi: 10.1073/pnas.0404229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Lowy DR, Schiller JT. Heparan Sulfate-Independent Cell Binding and Infection with Furin-Precleaved Papillomavirus Capsids. J. Virol. 2008;82:12565–12568. doi: 10.1128/JVI.01631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Thompson CD, Buck CB, Pang Y-YS, Lowy DR, Schiller JT. Neutralization of Human Papillomavirus with Monoclonal Antibodies Reveals Different Mechanisms of Inhibition. J. Virol. 2007;81:8784–8792. doi: 10.1128/JVI.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E-M, Fauquet C, Broker TR, Bernard H-U, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Florin L, Becker KA, Sapp C, Lambert C, Sirma H, Muller M, Streeck RE, Sapp M. Nuclear Translocation of Papillomavirus Minor Capsid Protein L2 Requires Hsc70. J. Virol. 2004;78:5546–5553. doi: 10.1128/JVI.78.11.5546-5553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007;81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroglou T, Sapp M, Lane C, Fligge C, Christensen ND, Streeck RE, Rose RC. Immunological analyses of human papillomavirus capsids. Vaccine. 2001;19:1783–1793. doi: 10.1016/s0264-410x(00)00370-4. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, Muñoz N, Schiffman M, Bosch FX. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26 Suppl 10:K17–K28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro CD, Pang Y-YS, Roden RBS, Hildesheim A, Wang Z, Reynolds MJ, Mast TC, Robinson R, Murphy BR, Karron RA, et al. Safety and Immunogenicity Trial in Adult Volunteers of a Human Papillomavirus 16 L1 Virus-Like Particle Vaccine. J. Natl. Cancer Inst. 2001;93:284–292. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Montefiori DC. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Review of Vaccines. 2006;5:347–363. doi: 10.1586/14760584.5.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RBS. Concatenated Multitype L2 Fusion Proteins as Candidate Prophylactic Pan-Human Papillomavirus Vaccines. J. Natl. Cancer Inst. 2009;101:782–792. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009;83:2067–2074.. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker HC, Itri VR, Valentine FT. A Strategy for Eliciting Antibodies against Cryptic, Conserved, Conformationally Dependent Epitopes of HIV Envelope Glycoprotein. PLoS ONE. 2010;5:e8555. doi: 10.1371/journal.pone.0008555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proceedings of the National Academy of Sciences. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim E-Y, Ma Z-M, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, et al. Propagation and Dissemination of Infection after Vaginal Transmission of Simian Immunodeficiency Virus. J. Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz N, Bosch FX, Castellsagué X, Díaz M, Sanjose Sd, Hammouda D, Shah KV, Meijer CJLM. Against which human papillomavirus types shall we vaccinate and screen? the international perspective. International Journal of Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Muñoz N, Kjaer SK, Sigurdsson K, Iversen O-E, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Impact of Human Papillomavirus (HPV)-6/11/16/18 Vaccine on All HPV-Associated Genital Diseases in Young Women. J. Natl. Cancer Inst. 2010;102:325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- Ochsenbauer C, Kappes JC. New virologic reagents for neutralizing antibody assays. Current Opinion in HIV & AIDS. 2009;4:418–425. doi: 10.1097/COH.0b013e32832f011e. [DOI] [PubMed] [Google Scholar]
- Olsson S-E, Villa LL, Costa RLR, Petta CA, Andrade RP, Malm C, Iversen O-E, Høye J, Steinwall M, Riis-Johannessen G, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Ong KCB, Badmanathan MM, Devi SP, Leong KLD, Cardosa MJP, Wong KTMMF. Pathologic Characterization of a Murine Model of Human Enterovirus 71 Encephalomyelitis. Journal of Neuropathology & Experimental Neurology. 2008;67:532–542. doi: 10.1097/NEN.0b013e31817713e7. [DOI] [PubMed] [Google Scholar]
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. The Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Buck CB, Pang Y-YS, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Pereira R, Hitzeroth II, Rybicki EP. Insights into the role and function of L2, the minor capsid protein of papillomaviruses. Archives of Virology. 2009;154:187–197. doi: 10.1007/s00705-009-0310-3. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Roden R, Hubbert N, Kirnbauer R, Christensen N, Lowy D, Schiller J. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 1996;70:3298–3301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden RBS, Yutzy WH, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor Capsid Protein of Human Genital Papillomaviruses Contains Subdominant, Cross-Neutralizing Epitopes. Virology. 2000;270:254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, Somani R, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–1985. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. The Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- Se-Thoe SY, Ling AE, Ng MML. Alteration of virus entry mode: A neutralisation mechanism for dengue-2 virus. Journal of Medical Virology. 2000;62:364–376. doi: 10.1002/1096-9071(200011)62:3<364::aid-jmv9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Selinka H-C, Florin L, Patel HD, Freitag K, Schmidtke M, Makarov VA, Sapp M. Inhibition of Transfer to Secondary Receptors by Heparan Sulfate-Binding Drug or Antibody Induces Noninfectious Uptake of Human Papillomavirus. J. Virol. 2007;81:10970–10980. doi: 10.1128/JVI.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. Vaccine. 2006;24:S106–S113. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- Stow JL, Farquhar MG. Distinctive populations of basement membrane and cell membrane heparan sulfate proteoglycans are produced by cultured cell lines. The Journal of Cell Biology. 1987;105:529–539. doi: 10.1083/jcb.105.1.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick MB, Burton DR. HIV-1 Neutralization: Mechanisms and Relevance to Vaccine Design. Current HIV Research. 2007;5:608–624. doi: 10.2174/157016207782418443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.