Abstract
Background
The Chinese mitten crab Eriocheir sinensis belongs to the Class Crustacea, Decapoda, Brachyura. The spermatozoon of this species is of aflagellated type, it has a spherical acrosome surrounded by the cup-shaped nucleus, which are unique to brachyurans. For the past several decades, studies on the spermatogenesis of the mitten crab mainly focus on the morphology. Compared with the extensive study of molecular mechanism of spermatogenesis in mammals, relatively less information is available in crustacean species. Myosin Va, a member of Class V myosin, has been implicated in acrosome biogenesis and vesicle transport during spermatogenesis in mammals. In the present study we demonstrate the expression and cellular localization of myosin Va during spermatogenesis in E. sinensis.
Methodology/Principal Findings
Western blot demonstrated that myosin Va is expressed during spermatogenesis. Immunocytochemical and ultrastructural analyses showed that myosin Va mainly localizes in the cytoplasm in spermatocytes. At the early stage of spermiogenesis, myosin Va binds to the endoplasmic reticulum vesicle (EV) and proacrosomal granule (PG). Subsequently, myosin Va localizes within the proacrosomal vesicle (PV) formed by PG and EV fusion and locates in the membrane complex (MC) at the mid spermatid stage. At the late spermatid stage, myosin Va is associated with the shaping nucleus and mitochondria. In mature spermatozoon, myosin Va predominates in acrosomal tubule (AT) and nucleus.
Conclusions/Significance
Our study demonstrates that myosin Va may be involved in acrosome biogenesis and nuclear morphogenesis during spermatogenesis in E. sinensis. Considering the distribution and molecular characteristics of myosin Va, we also propose a hypothesis of AT formation in this species. It is the first time to uncover the role of myosin Va in crustacean spermatogenesis.
Introduction
Spermatogenesis refers to the process which diploid spermatogonia develop into haploid mature spermatozoa. It can be divided into three phases: the mitotic phase, meiotic phase, and spermiogenesis phase. At the mitotic phase, the spermatogonia develop into primary spermatocytes through mitosis. During the meiotic phase, primary spermatocytes change into secondary spermatocytes via meiosis. At the spermiogenesis phase, spermatids differentiate into well shaped spermatozoa through organelle reorganization and new structure formation [1], [2]. Three major events occur during spermiogenesis in mammals, i.e., nuclear condensation, acrosomal shaping, and mid-piece formation.
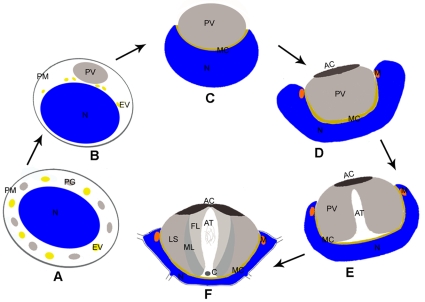
However, not all the sperm share the same features during spermiogenesis. In most crustaceans, the sperm has a characteristic acrosome complex and has no flagellum [3]–[6]. Chinese mitten crab Eriocheir sinensis (Crustacea, Decapoda) is a commercial aquatic species widely bred in China. It has been a gastronomic favorite of consumers for centuries. In recent two decades, this species has been used as a model for studying the crustacean spermatogenesis. Studies on the mechanism of spermatogenesis of this species are helpful to the culture and management of this crab. Spermiogenesis in the Chinese mitten crab E. sinensis has been divided into three stages, the early stage, mid stage and late stage (Figure 1) [3]. At the early stage, the endoplasmic reticulum vesicle (EV) generates dense proacrosomal granule (PG), which distribute in the spermatid cytoplasm (Figure 1A). At the mid stage, the proacrosomal granule (PG) and part of the endoplasmic reticulum vesicle (EV) aggregate into one vesicle designated as proacrosomal vesicle (PV) adjacent to the nucleus (Figure 1B). Later, the nucleus initiates to wrap up PV and a thin layer of membrane complex (MC) is sandwiched between the PV and the nucleus, and then the spermatid discards most of the cytoplasm (Figure 1C). During the late stage, the PV invaginates to form the acrosomal tubule (AT) and some mitochondria are surrounded by the nuclear membrane at subapical region of the spermatid (Figure 1D, E); finally, the nucleus becomes cup-shaped with several radial arms (RA) surrounding the acrosome (Figure 1F). In mature spermatozoon, a nuclear cup surrounds the acrosome, the latter is composed of an apical cap (AC), three layers (fibrous layer FL, middle layer ML and lamellar structures LS) and the acrosomal tubule (AT) (Figure 1F) [7]–[9].
Figure 1. A model of spermiogenesis in Chinese mitten crab E. sinensis.
(A) At early stage proacrosomal granule (PG) and the endoplasmic reticulum vesicle (EV) distribute in the spermatid cytoplasm around the nucleus (N). PM indicates the plasma membrane. (B–C) At mid stage, proacrosomal granule (PG) and the endoplasmic reticulum vesicle (EV) aggregate into proacrosomal vesicle (PV) (B), subsequently, the nucleus (N) initiates to wrap up proacrosomal vesicle (PV) and the membrane complex (MC) emerges between proacrosomal vesicle (PV) and the nucleus (N) (C). The spermatid discards most of the cytoplasm (C). (D–F) At late stage, the proacrosomal vesicle (PV) invaginates to form the acrosomal tubule (AT) (D, E). The mature spermatozoon consists of acrosome with apical cap (AC), acrosomal tubule (AT) and three layers (fibrous layer FL, middle layer ML and lamellar structures LS), centriole (C) at the base of acrosomal tubule (AT), the nuclear cup with several radial arms (RA) and mitochondria (M) (F).
Comparing to the mammalian spermatozoon with the acrosome covered most of the elongated nucleus, the Chinese mitten crab E. sinensis spermatozoon has a characteristic organization with a cup-shaped nucleus surrounded the acrosomal complex. Motor proteins play important roles during acrosome biogenesis and nuclear morphogenesis [9]–[13]. KIFC1, a microtubule-dependent molecular motor, has been shown in acrosomal formation during spermiogenesis in the Chinese mitten crab E. sinensis in our lab [9]. Class V myosin (myosin V), actin-based molecular motor, has a head domain with actin-binding site, a neck domain, and a tail domain responsible for cargo binding and is involved in a diverse range of cellular functions [14], [15]. Myosin V transports multiple cargos along actin filaments, including membrane-bound organelles and vesicles, such as ER vesicles [16], [17], mitochondria [18], [19], melanosomes [20], secretory granules in neurons [21], [22], endosomes and AMPA receptors [23], [24], GLUT4 vesicles [25], neurofilaments [26], mRNA and mRNP complex [27]–[32]. It is also associated with microtubules and kinesins [33]–[38]. Myosin V has been demonstrated to be involved in spermatogenesis [11], [12], [39]. It has a role in shaping and stabilizing the actin-based investment cones during Drosophila spermiogenesis [12]. Myosin Va, a member of myosin V, has been shown to transport the Golgi originated proacrosomal vesicles to the acroplaxome, then facilitate these vesicles coalescing into the acrosome [40]. It has also been suggested that myosin Va is involved in anchoring the acrosome to the acroplaxome and mobilizing vesicles along F-actin during the intramanchette transport [10]. Given the special pattern of spermatogenesis in E. sinensis and molecular characteristic of myosin Va, we determine to study the function of myosin Va during spermatogenesis in Chinese mitten crab E. sinensis.
In the present study, we report the expression of myosin Va during spermatogenesis in E. sinensis. We demonstrate that myosin Va is associated with EV and PG at an early spermatid stage and is localized in PV and MC at the mid spermatid stage. At the late spermatid stage, myosin Va bounds to mitochondria and distributes within the differentiating nucleus and AT. It suggests a role of myosin Va in acrosome biogenesis and nuclear shaping. We also suppose a model about the AT formation. Our findings reveal the first time that myosin Va may function in acrosome biogenesis and nuclear morphogenesis during crustacean spermatogenesis.
Materials and Methods
Animals
Seventy adult males of E. sinensis (Crustacea, Decapoda, Brachyura) were obtained from a local fishery market in Hangzhou, China. The specimens were maintained in aerated tanks for two weeks. Six adult male ICR mice were purchased from the Zhejiang University Laboratory Animal Center, and animal usage was also approved by the center. The permit number is SCXK 2007-0029. All procedures were carried out in accordance with the laws and polices by this animal center. For use of Chinese mitten crab, no approval is needed in China.
Antibodies
Anti-Myosin Va (LF-18) was purchased from Sigma (St. Louis, Mo., USA). Rabbit Anti-β-actin was purchased from BIOS (China). FITC Conjugated Monoclonal Anti-actin was purchased from Sigma (St. Louis, Mo., USA). HRP Conjugated Goat anti-Rabbit IgG was purchased from Immunology Consultants Laboratory, Inc. Texas. Red-conjugated Affinipure Goat Anti-Rabbit IgG was purchased from Protein Tech Group, Inc.
Western Blot Analysis
Testis from adult E. sinensis and brain from ICR mice were homogenized in RAPI Lysis Buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS) containing protease inhibitors and centrifuged at 14,000 rpm. The supernatant were ultrafiltrated by Centricon Centrifugal Filter Unit YM-30 (Millipore). After that the protein concentration was determined by the Coomassie brilliant blue method. Samples containing equal amounts of protein were separated on 8% gels (SDS-PAGE) and electrophoretically transferred to the PVDF membrane (Bio-Rad). Anti-Myosin Va antibody was used at a dilution of 1∶200. The membrane were incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody diluted 1∶1000. Immunoblots were developed using Pierce ECL Western Blotting Substrate (Thermo).
Immunofluorescence
Testes were obtained from adult male E. sinensis and were fixed overnight in 4% paraformaldehyde (PFA) in phosphate buffered saline (0.1 M PBS, PH 7.4). The tissues were rinsed three times in PBS and were incubated overnight in 0.5 M sucrose in PBS, then were embedded in Tissue-Tek® O.C.T. Compound, and cut into 5-µm frozen sections. The sections were washed with PBS for 15 min at room temperature, then blocked in 6% BSA in TBST (20 mM Tris, pH 7.5, 154 mM NaCl, 2 mM EGTA, 2 mM MgCl2, 0.1% Triton X-100) for 1 hour. Testis sections were incubated with anti-myosin Va antibodies (1∶100 dilution) in blocking buffer (2% BSA, 0.1% azide in TBST) for 1 h, then rinsed 3 times in TBST. The primary antibody was detected with Goat anti-rabbit IgG conjugated to Texas Red (1∶200 dilution, Jackson Immuno Research Laboratories, West Grove, PA., USA). β-Actin was detected by monoclonal anti-β-actin conjugated to FITC (1∶100 dilution, Sigma, St. Louis, MO., USA) and nuclei were stained with DAPI contained in the mounting medium (Vectashield, Vector Laboratories). The intracellular localization of these proteins was observed with a LSM 510 fluorescence microscope fit with appropriate filters and images captured with an Orca II CCD camera (Hamamatsu, Bridgewater, NJ). For controls, the primary antibody was omitted (data not shown).
Colloidal Gold Labeling and Electron Microscopy
Testes were removed from E. sinensis to a separate dish, fixed in 4% paraformaldehyde and 0.1% glutaraldehyde, rinsed 3 times in 0.1 M sodium phosphate buffer, pH 7.4, and one time in distilled water. Samples were then dehydrated with increasing ethanol concentrations and embedded in LR White (Ted Pella, Inc., Redding, CA) overnight, changed to fresh media, and transferred to gelatin capsules and polymerized at 50°C for 24 h. Samples were sectioned on a Reichert ultramicrotome and collected on nickel grids. Grids were incubated with PBS with 50 mM Glycine, pH 7.4 for 20 minutes to inactivate aldehyde. Grids were then blocked in 5% egg albumin for 2 h and then incubated overnight with anti-myosin Va antibodies (diluted 1∶50 in 0.05 M Tris, pH 7.4, 5% egg albumin) at 4°C. The grids were then incubated with goat anti-rabbit IgG conjugated to 25 nm gold (Amersham Pharmacia Biotech, Piscataway, N.J., USA) diluted 1∶10 in 0.02 M Tris, pH 8.2, 5% egg albumin at room temperature. The grids were then washed twice with sodium phosphate buffer, rinsed twice in distilled water, air dried for at least 30 min, and counterstained with uranyl acetate and lead citrate. The primary antibody was omitted in control sections.
Results
Identification of Myosin Va in the testis of E. sinensis
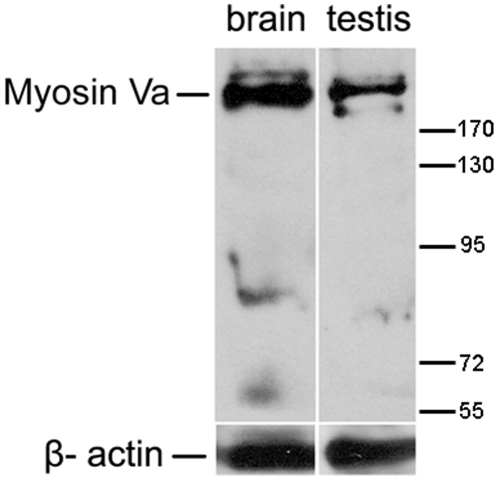
We performed western blot to determine whether myosin Va protein was expressed in the testis. The whole protein extraction of testis in E. sinensis and the brain extraction of ICR male mice known to contain neural vesicles rich in myosin Va were ultrafiltrated. Then, the concentrated solution was probed with an anti-myosin Va polyclonal antibody. In the positive control, the antibody recognized a band about 215 kDa expected for myosin Va in the brain lysate of mice, a similar immunoreactive band was indentified in testicular lysate of E. sinensis (Figure 2). The observed weak band maybe attributed to the products of alternatively spliced variants of myosin Va or the antibody cross-reacted with other members of class V myosins. Western blot analysis demonstrated that myosin Va is expressed in the testis of E. sinensis.
Figure 2. Myosin Va is expressed in the testis of E. sinensis.
Immunoblots of myosin Va in E. sinensis testis and brain extracts of ICR male mice probed with the anti-myosin Va polyclonal antibody. Brain extracts of mice known to contain myosin Va serve as positive control. A similar band was observed in extracts of E. sinensis testis. β-actin was used as a loading control. The molecular weight marker is shown at right.
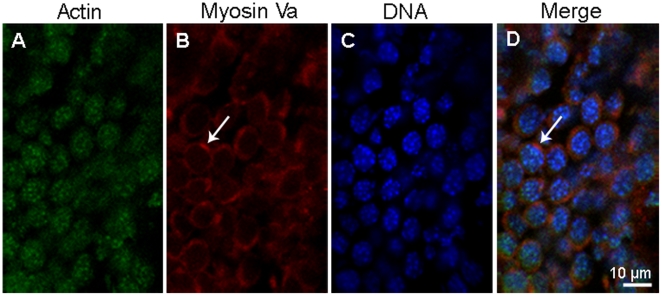
Myosin Va localizes in the cytoplasm at spermatocytes
Immunofluorescent localization results demonstrated that myosin Va distributes in the cytoplasm (Figure 3B, D). Actin shows strong punctuated staining within nucleus and weak diffuse staining in the cytoplasm (Figure 3A, C).
Figure 3. Immunofluorescent localization of myosin Va in spermatocytes of E. sinensis.
Actin (green) is visualized with FITC conjugated monoclonal anti-actin, myosin Va (red), and DNA with DAPI (blue). Myosin Va mainly localizes in the cytoplasm (arrows in B, D) in spermatocytes; actin mainly localizes in the nucleus, while the cytoplasm presents weak diffuse staining. The chromatin granules dispersed in the nucleus can be seen (C).
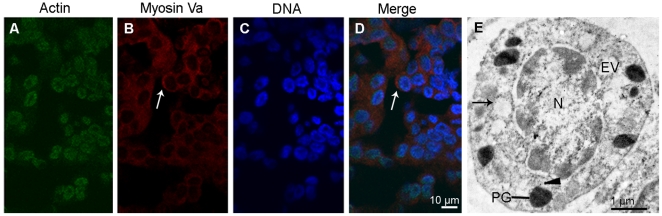
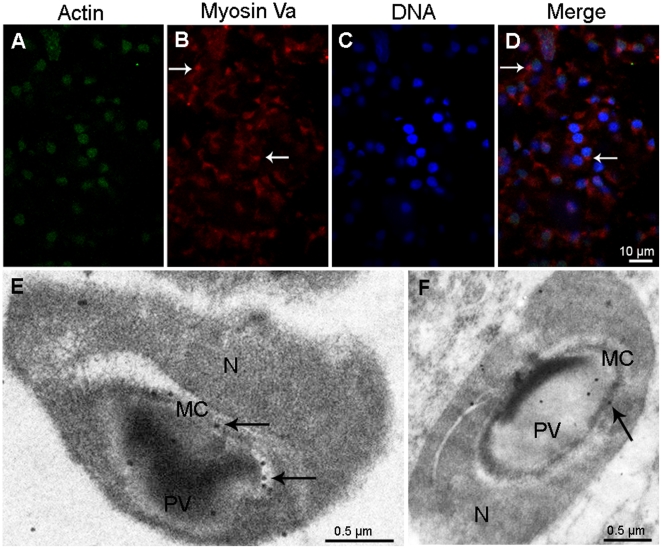
Myosin Va is associated with the membrane of PG and EV at the early spermatid stage
A pronounced feature of early spermatid is the formation of dense granule called PG, which originates from EV. Myosin Va staining is present in the cytoplasm, while the actin staining is prominent in the nucleus (Figure 4A–D). Myosin Va-decorated PG and EV can be observed in the cytoplasm (Figure 4E), which may suggest a role of myosin Va in PG formation and transportation.
Figure 4. The localization of myosin Va at the early stage during E. sinensis spermiogenesis.
(A–D) Immunofluorescent localization of myosin Va in early spermatids. The cytoplasm presents myosin Va staining (arrows in B, D), the chromatin lumps attach to the nuclear membrane (C). (e) Ultrastructural localization of myosin Va. Myosin Va binds to the endoplasmic reticulum vesicle (EV) membrane (arrow) and proacrosomal granule (PG) (arrowhead). The proacrosomal granule (PG) is characterized by high density while the endoplasmic reticulum vesicle (EV) has low density.
Myosin Va is principally localized in PV and MC at the mid spermatid stage
At the early mid spermatid stage, PG and EV accumulate and then fuse to form a large PV close to the nucleus, myosin Va immunoreactive sites can be seen at the PV in the spermatid (Figure 5A–D). Immunogold labeling of myosin Va are visualized along the PV membrane and MC, with a small quantity within the PV at the late mid spermatid stage (Figure 5E, F).
Figure 5. Localization of myosin Va at the mid stage during E. sinensis spermiogenesis.
(A–D) Immunofluorescent localization of myosin Va in early mid stage. Myosin Va staining can be seen at the proacrosomal vesicle (PV) (arrows in B, D). Scale bar = 10 µm. (E–F) Immunogold labeling of myosin Va is present in the membrane complex (MC) and the proacrosomal vesicle (PV) membrane at late mid stage (arrows in E, F). E shows longitudinal section and F shows cross section of mid stage spermatid.
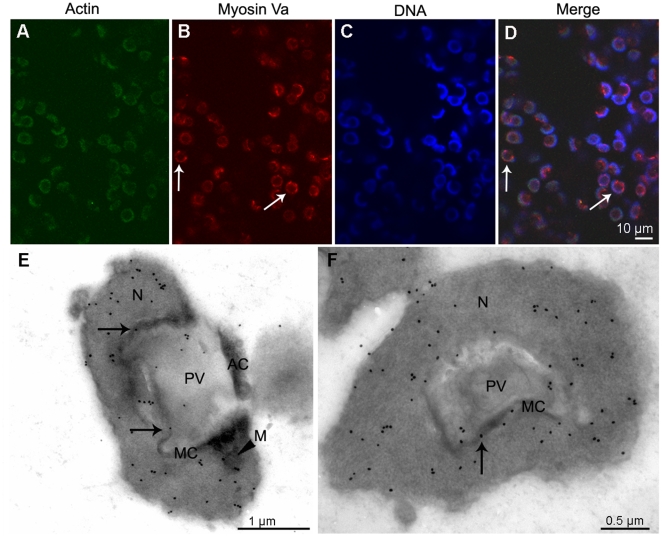
Myosin Va localizes in the nucleus, mitochondria, MC and AT at the late spermatid stage
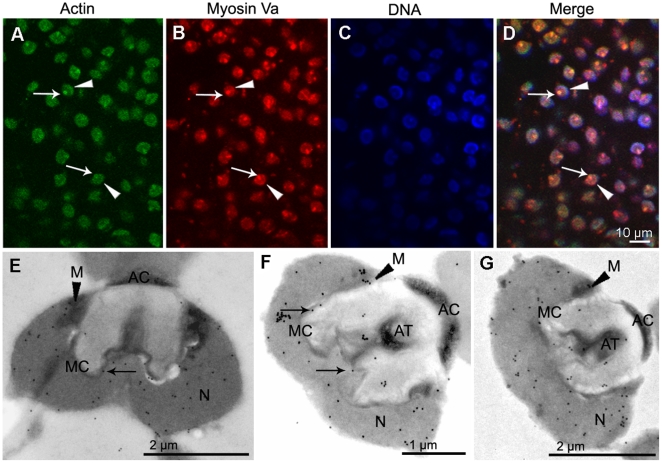
Immunofluorescent results displayed a distinct myosin Va distribution pattern at the late spermatid stage (Figure 6, 7). We discovered that at the beginning of the late spermatid stage, when the nucleus initiates to protrude at the bottom of the nuclear cup, accompanied by acrosome invagination, myosin Va allocates within the evaginated nucleus part and MC (Figure 6E, F). The mitochondria also display myosin Va immunoreactivity (Figure 6E). As spermiogenesis is in progress, myosin Va becomes associated with the evaginated nuclear membrane while the nucleus is protruding (Figure 7E). This is documented by the location of its immunofluorescence signal (Figure 7B, D). After the AT formation, a part of myosin Va is retained in the AT (Figure 7F). Immunofluorescent results demonstrated that AT displays a prominent actin staining which is colocalized with myosin Va (Figure 7D). In mature spermatozoon with well developed acrosome and nucleus, abundant myosin Va is distributed in the nucleus and AT (Figure 7G). The mitochondria are decorated with myosin Va at the late spermatid stage (Figure 7E–G).
Figure 6. Localization of myosin Va at the beginning of late stage during E. sinensis spermiogenesis.
(A–D) Immunofluorescent localization of myosin Va. Myosin Va concentrates in the nucleus (arrows in B and D). (E–F) Immunogold labeling of myosin Va. Prominent myosin Va labeling is present in the nucleus (N), and some associates with the nuclear membrane and the membrane complex (MC) (arrows in E, F), as well as mitochondria (M) (arrowhead in E). E. longitudinal section and F. cross section of the initiation of late stage.
Figure 7. Localization of myosin Va at the late stage during E. sinensis spermiogenesis.
(A–D) Immunofluorescent localization of myosin Va. Myosin Va distributes in the nucleus (N) (arrowhead in B,D) and acrosomal tubule (AT) (arrow in B,D), actin staining is present in the nucleus (N) (arrowhead in A,D) and acrosomal tubule (AT) (arrow in A,D), myosin Va colocalizes with actin (D). (E–G) Immunoelectron microscopy analyses of myosin Va. (E) When the acrosomal tubule (AT) begin to form, myosin Va associates with the nuclear membrane and the membrane complex (MC) (arrow in E). (F) After the acrosomal tubule (AT) formation, myosin Va bounds to the nuclear membrane and localizes in the membrane complex (MC) (arrows in F) and acrosomal tubule (AT). (G) Myosin Va localization in mature spermatozoon. Myosin Va distributes in the nucleus (N) and acrosomal tubule (AT). Mitochondria (M) are decorated with myosin Va labeling (arrowheads in E–G).
Discussion
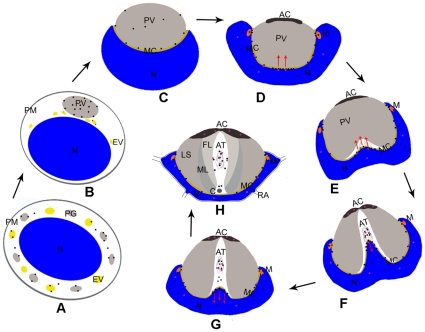
Previous studies have proposed that myosin Va facilitates the transport of proacrosomal vesicles derived from the Golgi apparatus to the acrosome and anchors the developing acrosome to the acroplaxome plate during the nuclear elongation of spermatids in mammals [10], [40]. In addition, myosin Va is localized in the manchette and associated with manchette vesicles, indicating that it participates in the intramanchette transport [10], [41]. Myosin V was shown to be associated with the nucleus and to be involved in the investment cone formation and acrosome biogenesis during the Drosophila spermiogenesis [12]. During spermiogenesis in E. sinensis, PG and EV aggregate and then coalesce into PV adjacent to the nucleus. Later, the nucleus gradually invaginates to a cup-shape with about 20 radiating arms and surrounds the acrosome. Here we showed that myosin Va is associated with PG and EV at the early spermatid stage, and then decorates the PV membrane and MC situated between PV and the nucleus at the mid spermatid stage. Myosin Va is mainly distributed within the nucleus, part of which is associated with the evaginated nuclear membrane during AT formation. Myosin Va bounded mitochondria can be seen during the late spermatid stage. In mature spermatozoon, myosin Va is distributed in the nucleus and AT. A schematic diagram for myosin Va localization and AT formation during E. sinensis spermiogenesis is shown in Figure 8. Given the dynamic distribution and molecular characteristics of myosin Va during E. sinensis spermatogenesis, we propose that myosin Va plays an important role in spermatid differentiation.
Figure 8. A model of myosin Va localization and function during E. sinensis spermiogenesis.
The black point indicates myosin Va and the purple point indicates KIFC1. (A) In early stage, myosin Va is associate with the proacrosomal granule (PG) and the endoplasmic reticulum vesicle (EV) (black point myosin Va). (B, C) In mid stage, myosin Va localizes in the proacrosomal vesicle (PV) (B), as the nucleus (N) begin to wrap proacrosomal vesicle (PV), some myosin Va localizes to the membrane complex (MC) (C). (D–H) A hypothesis of the acrosomal tubule (AT) formation at late stage. At the initiation of late stage, myosin Va and KIFC1 exert a clutching force on the nuclear membrane at the bottom of the nuclear cup to push the membrane complex (MC) forward (red arrows in D, E); meanwhile, myosin Va at the other part of the membrane complex (MC) anchors proacrosomal vesicle (PV) to the nucleus and it also associates with the mitochondria (M) (D, E). When the acrosomal tubule (AT) reaches to the apical cap (AC), the evaginated nucleus (N) initiates to contract by pulling of myosin Va and KIFC1 (red arrows in F, G), some myosin Va interacts with actin in acrosomal tubule (AT), part of KIFC1 translocate to the apical cap (AC) (F, G). In mature spermatozoon, myosin Va localizes in the nucleus (N), membrane complex (MC) and acrosomal tubule (AT), while KIFC1 localizes in the apical cap (AC), nucleus (N) and acrosomal tubule (AT) (H).
Myosin Va may be involved in PV biogenesis
In this study, myosin Va was found to be associated with the membrane of PG and EV in the early spermatids (Figure 4E, 8A). In the mid spermatids, PG and part of EV fuse to form PV, myosin Va signals were found on PV (Figure 5D, 8B). Subsequently the nucleus initiates to invaginate to wrap PV, and myosin Va is associated with the PV membrane and MC (Figure 5E, F, 8C). MC is situated between PV and the nucleus and contains F-actin and materials derived from degenerated EV and mitochondria, which may anchor PV to the nucleus. Myosin Va was demonstrated to associate with the ER in mouse melanoma cells and transport ER vesicles in squid axons [16], [42]. Myosin Va transports the proacrosomal vesicles generated by Golgi apparatus to the acrosome, and then connects acrosomal vesicle to the acroplaxome which is a F-actin containing plate [10], [40]. It is reasonable to assume that myosin Va has a role in transporting PG and EV to the acrosomal pole of the nucleus and facilitating them coalescing into PV, after which it also serves as a candidate protein to connect PV to the nucleus during nuclear invagination.
Myosin Va may function in nuclear shaping and AT formation
The acroplaxome-myosin Va interaction is essential for acrosomal docking and nuclear shaping [10], [11], [40]. It has also been found to be associated with the nucleus during the spermatid individualization in Drosophila [12]. Acroframosome (AFS) consisting of F-actin and microtubule may serve as an anchor to tether the acrosome to the nucleus during spermiogenesis in caridean shrimp Macrobrachium nipponense [43]. MC contains F-actin and locates between the acrosome complex and the nucleus, which may have a similar role in anchoring the developing acrosome to the nucleus during spermiogenesis in Chinese mitten crab.
Du et al. (1988) have found that AT contains MC materials, however, the mechanism of AT biogenesis remains unknown [3]. KIFC1, microtubule-dependent motor protein, has been demonstrated to localize in the AC, AT and nucleus and may have a role in formation and maintaining of AC and AT structure during E. sinensis [9]. Nevertheless, no microtubule is existed in the AT [9], while AT is rich in actin (Figure 7A). Kinesin and myosin V have been demonstrated to be binding partners or act as tethers to function [33], [34], [36], [38]. Based on our result and the previous study, we propose a model of AT shaping (Figure 8D–H). Initially, the basal part of the cup-shaped nucleus protrudes to push the MC forward (Figure 8D, E). Myosin Va has a major distribution in the nucleus and resides in the nuclear membrane and MC (Figure 7E, 8E), therefore, it is possible that myosin Va and KIFC1 pulls the nucleus forward at the bottom of the nuclear cup; meanwhile, myosin Va in other parts of MC attaches the developing acrosome to the nucleus (Figure 8E). After AT reaches to AC at the tip of the acrosome, the nuclear convex contracts to the nuclear basal body via the drawing of myosin Va and KIFC1, while part of KIFC1 translocate to the AC which may function in the acrosome reaction (Figure 8F, G). A small amount of myosin Va and KIFC1 stay in AT interacting with F-actin (Figure 8H). It suggests that myosin Va and KIFC1 may work in concert to facilitate AT formation and fertilization.
Nuclear actin has been implicated in transcription and chromatin remodeling [44]–[46]. Myosin Va has also been proposed to function in nuclear compartmentalization and transcription [47]. What is even more interesting, in contrast to other types of spermatozoon, chromatin in the crab Cancer pagurus sperm is not compacted, which enable the nucleus to penetrate the oocyte more flexibly [48]. Combined with our result, it suggests that myosin Va and actin may participate in the nuclear organization of spermatids during spermiogenesis and fertilization.
In addition, we observed that mitochondria and MC which contained degenerated mitochondria display myosin Va labeling (Figure 5E, F, Figure 6E, 7 E–G, 8 C–H). Myo2, a Class V member, mediates mitochondrial motility directly in yeasts [18]. Therefore, myosin Va may be implicated in mitochondria localization and morphogenesis.
In summary, this work supports the notion that myosin Va is involved in the acrosome biogenesis. In addition, our result suggests a possible mechanism of AT formation. In this process, myosin Va and KIFC1 pull the nucleus to protrude, which is then pushing the MC forward to form the AT. After the AT reaches to the tip of acrosome, the evaginated nucleus retracts to the basal part of the nuclear cup by drawing of myosin Va and KIFC1. Furthermore, our work suggests a novel role of myosin Va in the nuclear morphogenesis during spermiogenesis of E. sinensis.
Acknowledgments
We wish to thank the Sperm Laboratory members for many helpful discussions and Dr. Hans-Uwe Dahms (Sangmyung University, Korea) for constructive comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported in part by the following projects: National Natural Science Foundation of China (http://www.nsfc.gov.cn)(No. 40776079 and 31072198) and National Basic Research Program of China (973 Program) (Grant No 2007CB948104). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 2.Pudney J. Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech. 1995;32:459–497. doi: 10.1002/jemt.1070320602. [DOI] [PubMed] [Google Scholar]
- 3.Du NS, Xue LZ, Lai W. Studies on the sperm of Chinese mitten-handed crab, Eriocheir sinensis (Crustacea, Decapoda). II. Spermatogenesis. Oceanol Limnol Sin. 1988;19:71–75. [Google Scholar]
- 4.Yang WX, Du NS, Lai W. Changes of Golgi apparatus during spermatogenesis of Macrobrachium nipponense. Acta Zoologica Sinica. 1998;44:377–383. [Google Scholar]
- 5.Yang WX, Du NS, Lai W. Junctional relationship between spermatogenic cell and Sertoli cells of freshwater shrimp, Macrobrachium nipponense. Acta Zoologica Sinica. 1999;45:178–186. [Google Scholar]
- 6.Simeó CG, Kurtz K, Chiva M, Ribes E, Rotllant G. Spermatogenesis of the spider crab Maja brachydactyla (Decapoda: Brachyura). J Morphol. 2010;271:394–406. doi: 10.1002/jmor.10805. [DOI] [PubMed] [Google Scholar]
- 7.Du NS, Lai W, Xue LZ. Studies on the sperm of Chinese mitten-handed crab, Eriocheir sinensis (Crustacea, Decapoda). I. The morphology and ultrastructure of mature sperm. Oceanol Limnol Sin. 1987;18:119–125. [Google Scholar]
- 8.Du NS. Fertilization of Chinese mitten crab. Fisheries Science & Technology Information. 1998;25:9–13. [Google Scholar]
- 9.Yu KM, Hou L, Zhu JQ, Ying XP, Yang WX. KIFC1 participates in acrosomal biogenesis, with discussion of its importance for the perforatorium in the Chinese mitten crab Eriocheir sinensis. Cell Tissue Res. 2009;337:113–123. doi: 10.1007/s00441-009-0800-3. [DOI] [PubMed] [Google Scholar]
- 10.Kierszenbaum AL, Rivkin E, Tres LL. The actin-based motor myosin Va is a component of the acroplaxome, an acrosome-nuclear envelope junctional plate, and of manchette-associated vesicles. Cytogenet Genome Res. 2003;103:337–344. doi: 10.1159/000076822. [DOI] [PubMed] [Google Scholar]
- 11.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67:271–284. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 12.Mermall V, Bonafé N, Jones L, Sellers JR, Cooley L, et al. Drosophila myosin V is required for larval development and spermatid individualization. Dev Biol. 2005;286:238–255. doi: 10.1016/j.ydbio.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Yang WX, Sperry AO. C-terminal kinesin motor KIFC1 participates in acrosome biogenesis and vesicle transport. Biol Reprod. 2003;69:1719–1729. doi: 10.1095/biolreprod.102.014878. [DOI] [PubMed] [Google Scholar]
- 14.Reck-Peterson SL, Provance DW, Jr, Mooseker MS, Mercer JA. Class V myosins. Biochim Biophys Acta. 2000;1496:36–51. doi: 10.1016/s0167-4889(00)00007-0. [DOI] [PubMed] [Google Scholar]
- 15.Wu XF, Jung G, Hammer JA., Jr Functions of unconventional myosins. Current Opinion in Cell Biology. 2000;12:42–51. doi: 10.1016/s0955-0674(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 16.Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J Cell Sci. 1998;111:3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- 17.Wollert T, Weiss DG, Gerdes HH, Kuznetsov SA. Activation of myosin V-based motility and F-actin-dependent network formation of endoplasmic reticulum during mitosis. J Cell Biol. 2002;159:571–577. doi: 10.1083/jcb.200204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altmann K, Frank M, Neumann D, Jakobs S, Westermann B. The class V myosin motor protein, Myo2, plays a major role in mitochondrial motility in Saccharomyces cerevisiae. J Cell Biol. 2008;181:119–130. doi: 10.1083/jcb.200709099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh T, Watabe A, Toh-E A, Matsui Y. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22:7744–7757. doi: 10.1128/MCB.22.22.7744-7757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hume AN, Ushakov DS, Tarafder AK, Ferenczi MA, Seabra MC. Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J Cell Sci. 2007;120:3111–3122. doi: 10.1242/jcs.010207. [DOI] [PubMed] [Google Scholar]
- 21.Bittins CM, Eichler TW, Gerdes HH. Expression of the dominant-negative tail of myosin Va enhances exocytosis of large dense core vesicles in neurons. Cell Mol Neurobiol. 2009;29:597–608. doi: 10.1007/s10571-009-9352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desnos C, Huet S, Fanget I, Chapuis C, Böttiger C, et al. Myosin va mediates docking of secretory granules at the plasma membrane. J Neurosci. 2007;27:10636–10645. doi: 10.1523/JNEUROSCI.1228-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlson E, Holzbaur EL. Myosin learns to recruit AMPA receptors. Cell. 2008;135:414–415. doi: 10.1016/j.cell.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, et al. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol Cell Biol. 2007;27:5172–5183. doi: 10.1128/MCB.02298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alami NH, Jung P, Brown A. Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. Neurosci. 2009;29:6625–6634. doi: 10.1523/JNEUROSCI.3829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn BD, Sakamoto T, Hong MS, Sellers JR, Takizawa PA. Myo4p is a monomeric myosin with motility uniquely adapted to transport mRNA. J Cell Biol. 2007;178:1193–1206. doi: 10.1083/jcb.200707080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krauss J, López de Quinto S, Nüsslein-Volhard C, Ephrussi A. Myosin-V regulates oskar mRNA localization in the Drosophila oocyte. Curr Biol. 2009;19:1058–1063. doi: 10.1016/j.cub.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 29.Ohashi S, Koike K, Omori A, Ichinose S, Ohara S, et al. Identification of mRNA/protein (mRNP) complexes containing Puralpha, mStaufen, fragile X protein, and myosin Va and their association with rough endoplasmic reticulum equipped with a kinesin motor. J Biol Chem. 2002;277:37804–37810. doi: 10.1074/jbc.M203608200. [DOI] [PubMed] [Google Scholar]
- 30.Salerno VP, Calliari A, Provance DW, Jr, Sotelo-Silveira JR, Sotelo JR, et al. Myosin-Va mediates RNA distribution in primary fibroblasts from multiple organs. Cell Motil Cytoskeleton. 2008;65:422–433. doi: 10.1002/cm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid M, Jaedicke A, Du TG, Jansen RP. Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr Biol. 2006;16:1538–1543. doi: 10.1016/j.cub.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura A, Fujii R, Watanabe Y, Okabe S, Fukui K, et al. Myosin-Va facilitates the accumulation of mRNA/protein complex in dendritic spines. Curr Biol. 2006;16(23):2345–2351. doi: 10.1016/j.cub.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Ali MY, Lu H, Bookwalter CS, Warshaw DM, Trybus KM. MyosinV and Kinesin act as tethers to enhance each others' processivity. Proc Natl Acad Sci USA. 2008;105:4691–4696. doi: 10.1073/pnas.0711531105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beningo KA, Lillie SH, Brown SS. The yeast kinesin-related protein Smy1p exerts its effects on the class V myosin Myo2p via a physical interaction. Mol Biol Cell. 2000;11:691–702. doi: 10.1091/mbc.11.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao TT, Chang W, Masters SE, Mooseker MS. Myosin-Va binds to and mechanochemically couples microtubules to actin filaments. Mol Biol Cell. 2004;15:151–161. doi: 10.1091/mbc.E03-07-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodges AR, Bookwalter CS, Krementsova EB, Trybus KM. A nonprocessive class V myosin drives cargo processively when a kinesin- related protein is a passenger. Curr Biol. 2009;19:2121–2125. doi: 10.1016/j.cub.2009.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sotelo-Silveira JR, Calliari A, Cárdenas M, Koenig E, Sotelo JR. Myosin Va and kinesin II motor proteins are concentrated in ribosomal domains (periaxoplasmic ribosomal plaques) of myelinated axons. J Neurobiol. 2004;60:187–196. doi: 10.1002/neu.20015. [DOI] [PubMed] [Google Scholar]
- 38.Stafford P, Brown J, Langford GM. Interaction of actin- and microtubule-based motors in squid axoplasm probed with antibodies to myosin V and kinesin. Biol Bull. 2000;199:203–205. doi: 10.2307/1542899. [DOI] [PubMed] [Google Scholar]
- 39.Espindola FS, Banzi SR, Calabria LK, Custódio RJ, Oliveira RA, et al. Localization of myosin-Va in subpopulations of cells in rat endocrine organs. Cell Tissue Res. 2008;333:263–279. doi: 10.1007/s00441-008-0630-8. [DOI] [PubMed] [Google Scholar]
- 40.Kierszenbaum AL, Tres LL, Rivkin E, Kang-Decker N, van Deursen JM. The acroplaxome is the docking site of Golgi-derived myosin Va/Rab27a/b- containing proacrosomal vesicles in wild-type and Hrb mutant mouse spermatids. Biol Reprod. 2004;70:1400–1410. doi: 10.1095/biolreprod.103.025346. [DOI] [PubMed] [Google Scholar]
- 41.Hayasaka S, Terada Y, Suzuki K, Murakawa H, Tachibana I, et al. Intramanchette transport during primate spermiogenesis: expression of dynein, myosin Va, motor recruiter myosin Va, VIIa-Rab27a/b interacting protein, and Rab27b in the manchette during human and monkey spermiogenesis. Asian J. 2008;Andro10:561–568. doi: 10.1111/j.1745-7262.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 42.Nascimento AA, Amaral RG, Bizario JC, Larson RE, Espreafico EM. Subcellular localization of myosin-V in the B16 melanoma cells, a wild-type cell line for the dilute gene. Mol Biol Cell. 1997;8:1971–1988. doi: 10.1091/mbc.8.10.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, Pan CY, Zheng BH, Xiang L, Yang WX. Invertebr Reprod Dev. In press; 2010. Immunocytochemical studies of the acroframosome during spermiogenesis of the caridean shrimp Macrobrachium nipponense (Crustacea, Natantia). [Google Scholar]
- 44.Blessing CA, Ugrinova GT, Goodson HV. Actin and ARPs: action in the nucleus. Trends Cell Biol. 2004;14:435–442. doi: 10.1016/j.tcb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Chen M, Shen X. Nuclear actin and actin-related proteins in chromatin dynamics. Curr Opin Cell Biol. 2007;19:326–330. doi: 10.1016/j.ceb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Pederson T, Aebi U. Nuclear actin extends, with no contraction in sight. Mol Biol Cell. 2005;16:5055–5060. doi: 10.1091/mbc.E05-07-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pranchevicius MC, Baqui MM, Ishikawa-Ankerhold HC, Lourenço EV, Leão RM, et al. Myosin Va phosphorylated on Ser1650 is found in nuclear speckles and redistributes to nucleoli upon inhibition of transcription. Cell Motil Cytoskeleton. 2008;65:441–456. doi: 10.1002/cm.20269. [DOI] [PubMed] [Google Scholar]
- 48.Kurtz K, Martínez-Soler F, Ausió J, Chiva M. Histones and nucleosomes in cancer sperm (Decapod: Crustacea) previously described as lacking basic DNA-associated proteins: a new model of sperm chromatin. J Cell Biochem. 2008;105:574–584. doi: 10.1002/jcb.21857. [DOI] [PubMed] [Google Scholar]