Abstract
Background
Melanoma rates are rising among young women, possibly due to increasing ultraviolet radiation to previously protected body sites. Therefore, we examined melanoma incidence trends by age, gender, and body site. Descriptive methods were complemented with the age-period-cohort parameters net drift and longitudinal age trend.
Methods
Case and population data were obtained from the Surveillance, Epidemiology, and End Results 9 Registries Database (1975-2006). Net drift summarized the average annual percentage change in log-linear rates per year of calendar-time (or year of diagnosis). Longitudinal age trend summarized the average annual percentage change by attained age at diagnosis. Early-and late-onset melanomas have low and high longitudinal age trends, respectively.
Results
There were 105,829 melanomas diagnosed in the SEER 9 Registries. The overall age-adjusted incidence rate (IR)for melanoma was 17.7/100,000 person-years. Age specific IRs were greater among women than men prior to age 40 years. Among women, Irs decreased for all anatomic sites relative to the trunk. The highest net drift occurred in truncal lesions among women (net drift = 3.8%/year of calendar time; 95% CI = 3.5%-4.0%). The lowest longitudinal age trends also were observed for truncal lesions among women (longitudinal age trend = 5.4%/year of attained age; 95% CI = 5.1-5.7).
Conclusions
Though melanoma Irs overall have risen for decades, the combination of high net drift and low longitudinal age trend demonstrate that melanomas are rising preferentially on the trunk among young women.
Impact
Future surveillance and analytic studies should consider melanoma effect modification by age, gender, and body site.
Keywords: Epidemiology, melanoma, skin cancer
Introduction
A continuous increase of cutaneous malignant melanoma incidence rates has been observed in the United States over the last four decades (1). The underlying causes of these rising melanoma trends are widely debated, though most authors attribute them to environmental risk factors and changes in sun exposure behavior (2). If the long-standing increases are caused by changes in sun-related behavior between generations, site-specific surveillance may be the most effective way of following these changes (3). It also has been recognized that anatomic sites of melanomas vary by gender with the majority of melanomas occurring on the trunk and lower extremity among men and women, respectively (4). Given that an increase in tan-seeking ultraviolet radiation exposure might place anatomic sites usually covered by clothing at greater relative risk for melanoma, i.e., trunk among women (5),we further assessed melanoma incidence trends by age, gender and anatomic body site.
Materials and Methods
Case and population data among white women and men were obtained from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 9 Registries Database (6). SEER’s 9 registries include five states (Connecticut, Hawaii, Iowa, New Mexico, and Utah) and four metropolitan areas (Atlanta, Detroit, San Francisco-Oakland, and Seattle-Puget Sound), covering approximately 10% of the United States population (6). Demographic and tumor characteristics included gender, age at diagnosis, and anatomic body site. We created sixteen 4-year age groups (ages 15-18, 19-22, …, 75-78 years) and eight 4-year time periods (1975-1978, 1979-1982, …, 2003-2006). Anatomic sites were defined using the International Classification of Diseases for Oncology-3rd edition (ICD-O-3) (7) as previously described (8). Anatomic body sites included skin of the face, head, and neck (C44.0-44.4), trunk (C44.5, including back, abdomen, and chest), upper extremity (C44.6), lower extremity (C44.7), and all “other or unknown” body sites combined into a single category. Combining of the “other or unknown” category had no statistically significant effects on the results (data not shown).
We used SEER*Stat 6.5.2 (6) to obtain age standardized (2000 US population) invasive melanoma incidence rates (IR)and to assess statistical significance at the 95% confidence level. Relative risks for anatomic body sites were expressed as incidence rate ratios (IRR) where face/head/neck, upper extremity, and/or lower extremity were compared to the trunk as the referent site with an assigned IRR of 1.0. For example, IRR face/head/neck:trunk <1.0 showed that Irs were greater for the trunk than face/head/neck. IRR face/head/neck:trunk:trunk >1.0 showed that Irs were greater for the face/head/neck than trunk. Secular trends in the IRRs were expressed as percentage change (%CH) from the earliest to the most recent 4-year time period (1975-1982 to 1999-2006). All statistical tests were two-sided.
We used MATLAB 2009b (9) to calculate median ages at diagnosis, as well as age-period-cohort parameters to simultaneously assess age-specific incidence rates that were adjusted for calendar period and birth cohort effects. Two very useful and estimable age period cohort parameters for descriptive studies are the net drift (10) and longitudinal age trend (11). Net drift is the sum of the linear trends in the period and cohort effects for all age groups combined. It estimates the average annual percentage change in the logarithm of the incidence rates over time. Net drift is conceptually similar to the estimated annual percentage change of the age standardized incidence rate. Longitudinal age trend is another type of drift parameter that is defined as the sum of the linear trends in the age and period effects. It estimates the average annual percentage change in the logarithm of the incidence rates per year of attained age. Early-onset and late-onset cancers have low and high longitudinal age trends, respectively (12).
Results
There were 105,829 microscopically confirmed invasive cutaneous melanomas diagnosed in SEER’s 9 Registries Databases from 1975 through 2006. The overall age-adjusted IR was 17.7/100,000 person-years (p-y). The mean age at diagnosis was lower in women than men (53.0 vs. 57.9, respectively, p<0.001) (Table 1). Age-specific IRs were greater among women than men prior to age 40 years (IR=6.9 vs. 4.6/100,000 p-y respectively; IRR=1.5, 95% CI 1.4-1.6, p<0.001).
Table 1.
Cutaneous malignant melanoma from SEER 9 Registries Database, 1975-2006
| All cases | Females | Males | |||||
|---|---|---|---|---|---|---|---|
| Total n | 105,829 | 48,183 | 57,646 | ||||
| % of total cases | 100% | 45.5% | 54.5% | ||||
| Rate | 17.7 | 15.2 | 21.4 | ||||
| Mean age | 55.7 | 53.0 | 57.9 | ||||
| All cases | Females | Males | IRRFemale:Male (95% CI) | ||||
| Variable | N | Rate | N | Rate | N | Rate | |
| Age at Diagnosis | |||||||
| <20 years | 1,036 | 0.6 | 628 | 0.7 | 408 | 0.5 | 1.6 (1.4, 1.8) |
| 20-29 years | 6,598 | 6.8 | 4,212 | 8.9 | 2,386 | 4.8 | 1.8 (1.7, 1.9) |
| 30-39 years | 13,987 | 14.6 | 8,069 | 17.0 | 5,918 | 12.3 | 1.3 (1.3, 1.4) |
| 40-49 years | 18,635 | 22.5 | 9,225 | 22.3 | 9,410 | 22.8 | 1.0 (0.9, 1.0) |
| 50-59 years | 19,941 | 30.4 | 8,395 | 25.3 | 11,546 | 35.8 | 0.7 (0.6, 0.7) |
| 60-69 years | 19,040 | 39.5 | 7,016 | 27.4 | 12,024 | 53.2 | 0.5 (0.5, 0.6) |
| 70-79 years | 16,614 | 49.6 | 6,177 | 31.7 | 10,437 | 74.7 | 0.4 (0.4, 0.5) |
| 80+ years | 9,978 | 53.3 | 4,461 | 35.4 | 5,517 | 90.1 | 0.3 (0.3, 0.4) |
| Anatomic site | |||||||
| Face/head/neck | 20,643 | 3.5 | 6,701 | 2.0 | 13,942 | 5.5 | 0.3 (0.3, 0.4) |
| Trunk | 35,155 | 5.9 | 12,003 | 3.9 | 23,152 | 8.3 | 0.4 (0.4, 0.5) |
| Upper Extremity | 24,584 | 4.1 | 12,328 | 3.9 | 12,256 | 4.5 | 0.9 (0.8, 0.9) |
| Lower Extremity | 20,589 | 3.4 | 15,391 | 4.9 | 5,198 | 1.9 | 2.6 (2.6, 2.7) |
| Othera | 4,858 | 0.8 | 1,760 | 0.6 | 3,098 | 1.2 | 0.5 (0.4, 0.5) |
Legend: Rates are per 100,000 and age-adjusted to the 2000 US Standard Population (Census P25-1130) Incidence rates and confidence intervals were derived from SEER*Stat software program 6.52. Relative risks were expressed as incidence rate ratios (IRRs), where a given characteristic was compared to a referent characteristic with an assigned IRR of 1.0.
Confidence intervals (δ method) are 95% for IRR (incidence rate ratios).
“Other” includes tumors coded as skin, not otherwise specified in SEER
The most common anatomic sites were the lower extremity in women (IR = 4.9 per 100,000 p-y; 95% CI = 4.8-5.0) and the trunk in men (IR = 8.3 per 100,000 p-y; 95% CI = 8.2-8.4) (Table 1). Notwithstanding these overall rates, the frequency distributions of melanomas shifted over time by gender and anatomic site (Table 2). Among women, incidence rates decreased for all anatomic sites relative to the trunk with negative percentage changes in the IRR site:trunk ranging from negative 11.8% for IRR upper extremity:trunk to negative 24.0% for IRR face/head/neck:trunk. Conversely, among men, incidence rates generally increased for anatomic sites relative to the trunk with the exception of the lower extremity (i.e., IRR lower extremity:trunk = negative 6.8%).
Table 2.
Temporal trends for cutaneous malignant melanoma by sex and anatomic site, using SEER 9 Registries Database(1975-2006)
| 1975-1982 | 1999-2006 | 1975-1982 to 1999-2006 | |||||
|---|---|---|---|---|---|---|---|
| N | Rate | IRRsite:trunk (95% CI) | N | Rate | IRRsite:trunk (95% CI) | Percentage change in IRRsite:trunk (95% CI) | |
| FEMALES | |||||||
| Trunk | 1,582 | 2.3 | 1.0 | 4,667 | 5.3 | 1.0 | NA |
| Face/head/neck | 1,038 | 1.5 | 0.7 (0.1,0.7) | 2,475 | 2.6 | 0.5 (0.5,0.5) | -24.0% (-20.1,-28.7) |
| Upper extremity | 1,737 | 2.6 | 1.1 (1.0,1.2) | 4,741 | 5.3 | 1.0 (0.8,1.0) | -11.8% (-11.4, -12.3) |
| Lower extremity | 2,286 | 3.3 | 1.4 (1.4,1.5) | 5,747 | 6.5 | 1.2 (1.2,1.3) | -16.1% (-15.6, -16.7) |
| MALES | |||||||
| Trunk | 2,914 | 4.8 | 1.0 | 8,988 | 11.1 | 1.0 | NA |
| Face/head/neck | 1,479 | 2.7 | 0.6 (0.5,0.6) | 5,936 | 7.9 | 0.7 (0.7,0.7) | 24.6% (23.6, 25.2) |
| Upper extremity | 1,338 | 2.2 | 0.5 (0.4,0.5) | 5,213 | 6.6 | 0.6 (0.6,0.6) | 27.3% (26.1, 28.5) |
| Lower extremity | 699 | 1.2 | 0.3 (0.2,0.3) | 2,087 | 2.5 | 0.2 (0.2,0.2) | -6.8% (-6.6, -7.2) |
Legend : Rates are per 100,000 and age-adjusted to the 2000 US Standard Population (Census P25-1130).
Incidence rates and confidence intervals were derived from SEER*Stat software program 6.52.
Relative risks were expressed as incidence rate ratios (IRRs), where a given characteristic was compared to a referent characteristic with an assigned IRR of 1.0.
Confidence intervals (δ method) are 95% for IRR (incidence rate ratios) and percentage change Percentage changes were calculated using 1 year for each end point.
Abbreviations: IRR, incidence rate ratio; NA, not applicable
The age period cohort net drift and longitudinal age trend for melanoma by gender and anatomic site are shown in Figures 1A and 1B, respectively. The greatest secular trends occurred for truncal lesions among women with the highest net drifts (Figure 1A); i.e., net drift =3.8% per year of calendar time; 95% CI = 3.5%-4.0%. In a sensitivity analysis that was stratified by age groups <40 and 40+ years, we observed similar increases for trunk melanomas among women ages <40 years but not among women ages 40+ years. Longitudinal age trends were generally lower for women than men (Figure 1B), consistent with the well-acknowledged predominance of early-onset melanomas among women.8 Indeed, the lowest longitudinal age trends were observed for truncal lesions among women, i.e., longitudinal age trend = 5.4% per year of attained age; 95% CI = 5.1-5.7.
Figure 1.
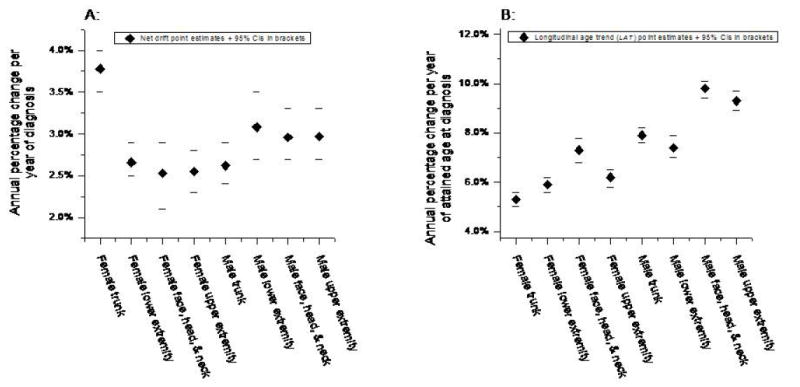
Age-period-cohort parameters for invasive cutaneous melanoma by gender and anatomic body site (SEER 9; 1975-2006). A: Net drift point estimates and 95% confidence intervals (in brackets). Net drift is a summary measure of the overall linear trend in the period + cohort effects. It is closely related to the estimated annual percentage change in the age-standardized incidence rate, see text for details. B: Longitudinal age trend point estimates and 95% confidence intervals (in brackets). Longitudinal age trend is a summary measure for the overall linear trend in the age + period effects. Low longitudinal age trends represent tumors that develop early in life; high longitudinal age trends reflect tumors that develop late in life.
Discussion
Despite the long-term increases in melanoma incidence rates, there has been only one recent trend analysis by anatomic site at a single large academic center in the United States (13). Though it is well-established that the majority of melanomas occur on the trunk and lower extremities for men and women, respectively (4),our results further demonstrated that melanoma of the trunk in women increased between the time periods 1975-1982 and 1999-2006 over 9 SEER Registries in the United States at a greater rate than for other sites in women, while the opposite was observed for men. The increase in trunk melanoma in women could be secondary to changes in sun exposure from various cultural, environmental, or behavioral factors. As described earlier, changes in the net drift suggest differential secular trends, whereas differences in longitudinal age trends suggest differential age-related biological or natural history effects. Hence, the combination of the high net drift and low longitudinal age trend of female trunk melanoma in Figure 1A and 1B demonstrate that melanomas are rising preferentially on the trunk among young women.
Past studies in the United States (13) and other countries have also shown the increasing prevalence of trunk melanoma in women (14) and younger patients (15, 16). Melanomas developing at different body sites are thought to be associated with distinct patterns of sun exposure, and the different anatomical distribution of lesions in men and women has been attributed to gender-specific patterns of sun-exposure (15). Melanomas of the head and neck are associated with chronic sun exposure, whereas trunk melanomas are thought to be associated with intermittent patterns of sun exposure, supporting the hypothesis that melanomas may arise through divergent causal pathways (8, 17). Some authors have suggested that susceptibility of melanocytes to malignant transformation might be site dependent, which could help explain why the relevance of known risk factors is not uniform by body site (18).The variable age distribution of risk by gender and anatomic site, particularly for trunk melanoma, might also support the hypothesis of a possible modulator role of sex hormones in female trunk melanoma (16). Estrogens are known to increase the number of melanocytes and modify their melanin content (19). However, one would need to hypothesize that sex hormones are changing over time; hence, the relationship between sex hormones and trunk melanoma should be analyzed further.
The rising melanoma incidence rates of the trunk in young women could also be partially caused by changes in clothing patterns. Young females are frequently wearing bikinis on the beach, and summer female casual clothes often leave part of the back and front of the trunk exposed to the sun (16). Finally, the increase in melanomas of the trunk in women could also be secondary to changes in sun exposure or a result of increased tanning behavior. It is noteworthy that usage of tanning beds, recently classified by the International Agency of Research on Cancer as a cause of melanoma (20) is most prevalent among young women (21, 22).
This analysis has important limitations. First, like most population-based registry studies, our analyses lacked information on individual risk factors, particularly a history of ultraviolet radiation exposure and characterization of nevus pattern, and had the potential of incomplete data collection. It is also widely recognized that trends in melanoma incidence are affected by underreporting (23) and surveillance issues. Melanoma has a longer reporting delay (up to 7 to 10 years) than other cancer sites because of the difficulties associated with reporting a cancer that is increasingly diagnosed in a non-hospital setting (24). Since there is a delay, the melanoma estimates for recent years are minimal levels of risk and may rise as data collection for recent years is completed. Case finding is therefore, an important aspect of data quality control for the SEER program, and SEER has adjusted data collection activities for melanoma to try to minimize errors. Age period cohort models share in all of the intrinsic limitations of standard descriptive studies. Additionally, since age, period, cohort are collinear (cohort = period – age), it is impossible to determine the independent effects for age, period, and cohort, giving rise to the so-called non-identifiability issue. Nonetheless, the parameters net drift and longitudinal age trend can be identified in the restricted age period cohort model, following the method of Holford (25). Thus, using these two estimable parameters, we have demonstrated statistically significant evidence for rising trunk melanomas in young women in the United States.
In conclusion, we present evidence for changing melanoma anatomic trends in the United States. Trunk melanomas among younger women in the United States are increasing relative to all other anatomic body sites, suggesting different trends in carcinogenic exposures or etiologic differences by melanoma anatomic site. Future surveillance and analytic studies on melanoma should include gender and anatomic site.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute. The SEER Program is operated by the National Cancer Institute Surveillance Research Program.
FINANCIAL SUPPORT: This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Footnotes
CONFLICT OF INTEREST: The authors state no conflict of interest.
References
- 1.Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–8. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tucker MA. Is sunlight important to melanoma causation? Cancer Epid Biomark Prev. 2008;17:467–8. doi: 10.1158/1055-9965.EPI-07-2743. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong BK, Kricker A. How much melanoma is caused by sun exposure? Melanoma Research. 1993;3:395–401. doi: 10.1097/00008390-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Garbe C, Leiter U. Melanoma epidemiology and trends. Clinics in Dermatology. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Bulliard JL, Cox B. Cutaneous malignant melanoma in New Zealand: Trends by anatomical site, 1969-1993. Int J Epidemiol. 2000;29:416–23. [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology and End Results (SEER) Program SEER*Stat Software. Version 6.52. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; [August 2009]. www.seer.cancer.gov. [Google Scholar]
- 7.Percy C, Fritz A, Ries L. Conversion of neoplasms by topography and morphology from the International Classification of Diseases for Oncology, Second Edition (ICD-O-2) to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) Bethesda, MD: Cancer Statistics Branch, DCCPS, Surveillance, Epidemiology and End Results Program, National Cancer Institute; 2001. [Google Scholar]
- 8.Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer. 2009;115:4176–85. doi: 10.1002/cncr.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [February 3, 2010];MATLAB Version 7.9.0.529 (R2009b) www.mathwarks.com.
- 10.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med. 1987;6:449–67. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 11.Robertson C, Gandini S, Boyle P. Age-period-cohort models: A comparative study of available methodologies. J Clin Epidem. 1999;52:569–83. doi: 10.1016/s0895-4356(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 12.Reimers LL, Anderson WF, Rosenberg PS, Henson DE, Castle PE. Etiologic heterogeneity for cervical carcinoma by histopathologic type, using comparative age-period-cohort models. Cancer Epidemiol Biomarkers Prev. 2009;18:792–800. doi: 10.1158/1055-9965.EPI-08-0965. [DOI] [PubMed] [Google Scholar]
- 13.Clark LN, Shin DB, Troxel AB, Khan S, Sober AJ, Ming ME. Association between the anatomic distribution of melanoma and sex. J Amer Acad Derm. 2007;56:768–73. doi: 10.1016/j.jaad.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Thorn M, Bergstrom R, Adami HO, Ringborg U. Trends in the incidence of malignant melanoma in Sweden, by anatomic site 1960-1984. Am J Epidemiol. 1990;132:1066–77. doi: 10.1093/oxfordjournals.aje.a115749. [DOI] [PubMed] [Google Scholar]
- 15.Elwood JM, Gallagher RP. Body site distribution of cutaneous malignant melanoma in relationship to patterns of sun exposure. Int J Cancer. 1998;78:276–80. doi: 10.1002/(SICI)1097-0215(19981029)78:3<276::AID-IJC2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Gomez B, Argones N, Gustavsson P, Lope V, Lopez-Abente G, Pollan M. Do sex and site matter? Different age distribution in melanoma of the trunk among Swedish men and women. Br J Dermatol. 2008;158:766–72. doi: 10.1111/j.1365-2133.2007.08429.x. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172–7. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 18.Green A. A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control. 1992;3:513–6. doi: 10.1007/BF00052747. [DOI] [PubMed] [Google Scholar]
- 19.Jee SH, Lee SY, Chiu HC, et al. Effects of estrogen and estrogen receptor in normal human melanocytes. Biochem Biophys Res Commun. 1994;199:1407–12. doi: 10.1006/bbrc.1994.1387. [DOI] [PubMed] [Google Scholar]
- 20.El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. WHO International Agency for Research on Cancer Monograph Working Group. Lancet Oncology. 2009;10:751–2. [Google Scholar]
- 21.Lazovich D, Forster J. Indoor tanning by adolescents: prevalence, practices and policies. Eur J Cancer. 2005;41:20–7. doi: 10.1016/j.ejca.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116–22. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- 23.Cockburn M, Swetter SM, Peng D, Keegan TH, Deapen D, Clarke CA. Melanoma underreporting: why does it happen, how big is the problem, and how do we fix it? J Am Acad Dermatol. 2008;59:1081–5. doi: 10.1016/j.jaad.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–45. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 25.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39:311–24. [PubMed] [Google Scholar]


