Abstract
Neurosteroids regulate GABA-A receptor plasticity. Neurosteroid withdrawal occurs during menstruation and is associated with a marked increase in expression of GABA-A receptor α4-subunit, a key subunit linked to enhanced neuronal excitability, seizure susceptibility and benzodiazepine resistance. However, the molecular mechanisms underlying the upregulation of α4-subunit expression remain unclear. Here we utilized the progesterone receptor (PR) knockout mouse to investigate molecular pathways of PR and the transcription factor early growth response factor-3 (Egr3) in regulation of the GABA-A receptor α4-subunit expression in the hippocampus in a mouse neurosteroid withdrawal paradigm. Neurosteroid withdrawal induced a threefold increase in α4-subunit expression in wild-type mice, but this upregulation was unchanged in PR knockout mice. The expression of Egr3, which controls α4-subunit transcription, was increased significantly following neurosteroid withdrawal in wild-type and PR knockout mice. Neurosteroid withdrawal-induced α4-subunit upregulation was completely suppressed by antisense Egr3 inhibition. In the hippocampus kindling model of epilepsy, there was heightened seizure activity, significant reduction in the antiseizure sensitivity of diazepam (a benzodiazepine insensitive at α4βγ-receptors) and conferral of increased seizure protection of flumazenil (a low-affinity agonist at α4βγ-receptors) in neurosteroid-withdrawn wild-type and PR knockout mice. These observations are consistent with enhanced α4-containing receptor abundance in vivo. Neurosteroid withdrawal-induced seizure exacerbation, diazepam insensitivity, and flumazenil efficacy in the kindling model were reversed by inhibition of Egr3. These results indicate that neurosteroid withdrawal-induced upregulation of GABA-A receptor α4-subunit expression is mediated by the Egr3 via a PR-independent signaling pathway. These findings help advance our understanding of the molecular basis of catamenial epilepsy, a neuroendocrine condition that occurs around the perimenstrual period and is characterized by neurosteroid withdrawal-linked seizure exacerbations in women with epilepsy.
Keywords: Allopregnanolone, diazepam, Egr3, GABA-A receptor, kindling, neurosteroid withdrawal, progesterone, progesterone receptor, α4-subunit, seizure
INTRODUCTION
Neurosteroids modulate the function of GABA-A receptors (Majewska et al., 1986; Belelli and Lambert, 2005). Neurosteroids such as allopregnanolone and pregnanolone are potent positive modulators of GABA-A receptors with anxiolytic and anticonvulsant properties (Harrison et al., 1987; Kokate et al., 1994; Reddy, 2003). Neurosteroids have a significant role in the pathophysiology of epilepsy, anxiety, stress and postpartum-depression (Smith et al., 1998a; Reddy et al., 2001; Purdy et al., 1991; Maguire and Mody, 2009; Shen et al., 2010). Women with catamenial epilepsy report an increase in seizures during the perimenstrual period (Herzog et al., 1997; Reddy, 2009). Ovarian cycle-linked fluctuations in progesterone and neurosteroids have been proposed to play a key role in catamenial epilepsy (Reddy et al., 2001; Maguire et al., 2005; Tuveri et al., 2008). Seizures decrease in the mid-luteal phase when serum progesterone levels are high and increase premenstrually when progesterone levels fall, causing the perimenstrual-type catamenial epilepsy. Progesterone is an anticonvulsant hormone. It protects against seizures by its conversion to neurosteroids (Reddy et al., 2004). Consequently, perimenstrual seizure exacerbations may be due to withdrawal of the antiseizure effects of neurosteroids (Reddy, 2009). Therefore, it is critical to understand how neurosteroid withdrawal causes this hyperexcitable state.
Neurosteroids regulate GABA-A receptor plasticity (Smith et al., 2007; Maguire and Mody, 2008; Reddy, 2009). GABA-A receptors are composed of five subunits from several classes (α1–6, β1–4, γ1–3, δ, ε, θ, ρ1–3) (Korpi et al., 2002). The major isoforms consist of 2α,2β,and1γ-subunits; the most common subtype being α1β2γ2. Neurosteroids modulate most receptor isoforms (Puia et al., 1990; Belelli et at., 2002), but are more sensitive at the δ-containing receptors (Belelli et al., 2002; Wohlfarth et al., 2002; Stell et al., 2003). Neurosteroids are well poised to assume a role in ovarian cycle-related control of GABA-A receptor composition (Follesa et al., 2000; Maguire et al., 2005; Reddy, 2009). The most striking finding following neurosteroid withdrawal is a marked increase in expression of the GABA-A receptor α4-subunit in the hippocampus (Smith et al., 1998a,b; Follesa et al., 1998), which is associated with a decrease in total GABA-gated current, an increase in seizure susceptibility, and alteration of several classes of modulatory drugs, including benzodiazepine resistance (Reddy and Rogawski, 2001; Smith and Gong, 2005). Together, these findings support a central role for α4-subunit changes in neurosteroid withdrawal-linked catamenial epilepsy.
The molecular mechanisms that regulate changes in α4-subunit expression remain unclear. Although the progesterone receptors (PRs), which mediate the physiological actions of progesterone, are not involved in the antiseizure effects of progesterone and neurosteroids (Reddy et al., 2004; 2005), it is unclear whether PRs mediate the neurosteroid withdrawal-induced α4-subunit expression. Upregulation of α4-subunit is also triggered by ethanol withdrawal (Devaud et al., 1997) or status epilepticus (Brooks-Kayal et al., 1998), suggesting a common mechanism. Indeed, a new mechanism has been identified for upregulation of the α4-subunit in animal models of epilepsy. The early growth response factor-3 (Egr3) has been found to regulate α4-subunit expression in epilepsy models (Roberts et al., 2005). Recent studies have identified the α4-promoter that is activated by Egr3, a member of the Egr family of transcription factors involved in synaptic plasticity (Eldredge et al., 2008). The Egr family consists of four proteins (Egr1–4) that share identical zinc finger DNA binding domains, and bind to a common Egr-response-element consensus sequence: GCG-T/GGG-GCG (O’Donovan et al.,1998). Specific signals activate Egr3, which in turn control the α4-gene transcription (Roberts et al., 2005; 2006). Progesterone activates the Egr family of transcription factors via a PR-dependent pathway (Mercier et al., 2001). Therefore, we hypothesize that neurosteroid withdrawal induces α4-subunit upregulation via activation of the Egr3 pathway.
In this study, we sought to determine the molecular pathways of PR and Egr3 in regulation of GABA-A receptor α4-subunit expression and seizure susceptibility following neurosteroid withdrawal. Our results show that PR-independent Egr3 pathways control neurosteroid withdrawal-induced α4-subunit upregulation in the hippocampus, and may suggest a molecular mechanism underlying changes in catamenial seizure susceptibility.
EXPERIMENTAL PROCEDURES
Animals
Female adult wild-type and PR knockout mice weighing 25 to 30 g were used in the study. The development of the PR knockout mouse strain and genotyping procedures have been described previously (Lydon et al., 1995; Reddy et al., 2004). A breeding colony was established at the Texas A&M University Laboratory Animal Facility. The strain was maintained on a C57BL6/129SvEv hybrid background. Mice were housed four to a cage with free access to food and water. The animals were cared for in strict compliance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Homozygous PR knockout pups were obtained from crosses between heterozygous PR knockout females and homozygous PR knockout males. For PR genotyping, genomic DNA was isolated from tails and analyzed by polymerase chain reaction amplification (Reddy et al., 2005). PR knockout mice lack PR binding activity for PR agonists in reproductive tissues (Lydon et al., 1995). We have previously confirmed by immunohistochemical studies that PR knockout mice do not express PR in the brain (Reddy et al., 2005). The stage of the estrous cycle was determined by microscopic analysis of the cellular profile in vaginal smears, as described previously (Maguire et al., 2005). All animal procedures were approved by the Institutional Animal Care and Use Committee.
Neurosteroid withdrawal paradigm
A state of neurosteroid withdrawal was induced in wild-type and PR knockout mice by an exogenous progesterone treatment protocol (Fig.1). To produce prolonged elevated levels of neurosteroids that more closely model the luteal changes in women, mice were treated with progesterone (25 mg/kg, s.c.), twice daily at 9 AM and 6 PM for 7 days. On the morning of the seventh day, mice were injected with the 5α-reductase inhibitor and neurosteroid synthesis blocker finasteride (50 mg/kg, i.p.) to produce an abrupt decline in neurosteroid levels to more closely model perimenstrual changes in women. Animals were tested 24 h after finasteride administration (neurosteroid withdrawal). The control group received vehicle injections (15% β-cyclodextrin, 0.2 ml, s.c.) twice daily for 7 days. Progesterone was administered rather than the neurosteroid allopregnanolone because it is known that elevated circulating levels of progesterone, such as those found during the luteal phase, are readily converted to neurosteroids in the brain regions that express neurosteroid synthesizing enzymes (Mellon et al., 2001; Agís-Balboa et al., 2006). The progesterone administration protocol results in a high physiological concentration of allopregnanolone in plasma, and an acute withdrawal was evident by a nearly complete decline in allopregnanolone 24 h after finasteride administration (see Fig.1B). This protocol is similar and consistent with standard progesterone treatment approaches used previously for induction of neurosteroid withdrawal (Smith et al. 1998a,b; Moran and Smith, 1998a,b), and also comparable to the pseudopregnancy model (Reddy et al., 2001; Reddy and Rogawski, 2001). Although it is not practical to replicate the actual endocrine milieu of the menstrual cycle in mouse models, this endocrine state may be physiologically similar to the perimenstrual period. We did not utilize the gonadectomy model because of potential problems of interpretation associated with complete deficiency of ovarian-derived hormones, and such animals need hormone replacements that may have variable effects on seizures depending on the age, dose, and duration of treatment (Scharfman et al., 2005; Shen et al., 2010).
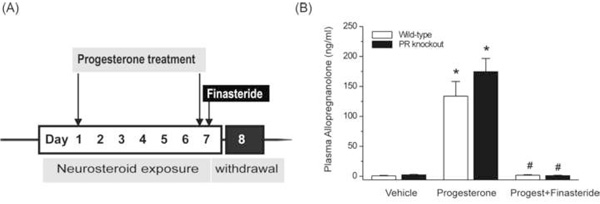
Fig.1. Neurosteroid withdrawal model in wild-type and PR knockout mice.
(A) Experimental protocol for the neurosteroid exposure and withdrawal model in mice. To mimic the prolonged exposure (like that of the luteal phase) followed by withdrawal (like that of menstruation) of progesterone, and therefore the “neurosteroid” allopregnanolone, mice were treated with progesterone (25 mg/kg, s.c.) twice daily for seven days. Then, they were injected on day 7 with a single dose of finasteride (50 mg/kg, i.p.), which is an irreversible 5α-reductase inhibitor that blocks the synthesis of progesterone-derived neurosteroid allopregnanolone. Thus, the dramatic decline in neurosteroid levels 24 h after finasteride (see Fig.1B) would create a state of acute neurosteroid withdrawal, which is proposed to partly model the neuroendocrine milieu commonly observed around the perimenstrual period in women. (B) Plasma levels of allopregnanolone in wild-type and PR knockout mice following treatment with vehicle, progesterone, and progesterone+finasteride (neurosteroid withdrawal). Plasma samples were collected from mice after chronic treatment with vehicle (15% cyclodextrin solution) or progesterone (25 mg/kg, s.c., twice daily for seven days), or progesterone (25 mg/kg, s.c., twice daily for seven days) and finasteride (50 mg/kg, i.p.) injection on day 7 for induction of neurosteroid withdrawal as outlined in Fig.1A. Each bar represents the mean ± SEM (n=6 mice per group). *p<0.01 vs. vehicle; #p<0.01 vs. progesterone-treatment group.
Determination of plasma allopregnanolone levels
Animals were anesthetized with isoflurane and ~0.5 ml carotid blood was collected in heparinized tubes. The plasma was separated by centrifugation at 12,000 × g for 10 min and stored at −20°C in 10 ml glass tubes coated with 7.5% EDTA solution. The concentration of allopregnanolone was analyzed by liquid chromatography-mass spectrometry as previously described (Reddy et al., 2004). Briefly, 0.2 ml plasma sample was added to a tube containing evaporated internal standard. The steroid and internal standard were extracted with 4 ml hexane. Each sample was analyzed using the APCI technique under acidic conditions. A standard curve was plotted using pure allopregnanolone in methanol mixed with 0.2 ml of blank mouse plasma.
TaqMan real-time PCR assay
The GABA-A receptor subunit mRNA expression was determined by the TaqMan real-time PCR assay from our lab, as described previously (Gangisetty and Reddy, 2009). Mice were anesthetized with isoflurane and the hippocampus was rapidly dissected for RNA isolation. The total RNA was extracted from the hippocampus using a Trizol reagent and cDNA was prepared using the Superscript II first-strand cDNA synthesis kit (Invitrogen Inc., Carlsbad, CA). The PCR primers and TaqMan probe specific for GABA-A receptor α4-subunit, Egr3, and GAPDH genes were designed using the Primer Express software (Applied Biosystems Inc., Foster City, CA). Primer set and probe for the α4-subunit were composed of the following sequences: forward, 5’-AGA-ACT-CAA-AGG-ACG-AGA-AAT-TGT-3’; reverse, 5’-TTC-ACT-TCT-GTA-ACA-GGA-CCC-C-3’; and sequence-specific TaqMan probe: 5’-6-FAM-ACG-CAG-CCT-GTT-GTC-ATA-ACC-ATC-CAG-C-TAMRA-3’. A set of primers and sequence specific TaqMan probes were designed and optimized for real-time PCR analysis of Egr3 mRNA: forward, 5’-GGA-GAC-GTG-GAG-GCC-ATG-TA-3’; reverse, 5’-ACA-CGG-GCT-CCG-AGT-AGA-GA-3’; and TaqMan probe, 5’-6-FAM-CCG-GCG-CTG-CCT-CCT-TAT-TCCA-TAMRA-3’. TaqMan PCR reactions were carried out in an AB 7500 fast real-time system (Applied Biosystems). Real-time PCR was performed with TaqMan Universal PCR Master Mix (Applied Biosystems), which contained AmpliTaq Gold DNA Polymerase, AmpErase, UNG, dNTPs with dUTP, and optimized buffer components. Each sample was run in triplicate design and each 25-µl reaction mixture consists of 12.5-µl TaqMan Universal PCR Master mix, 400 nM primers, and 300 nM TaqMan probe for the target genes as described previously (Gangisetty and Reddy, 2009). The real-time PCR run consisted first of 1 cycle of 50°C for 2 min, then 1 cycle of 95°C for 10 min, 50 cycles of 95°C for 15 s, and 60°C for 1 min. The target input amount for each target gene was normalized to GAPDH expression in the same samples to control for loading variability, and then expressed as a percent change with respect to mean control values in the same run.
Western blot analysis
Western blot analysis of α4-subunit was performed with modification of published protocols (Kralic et al., 2002; Kumar et al., 2002). In summary, an identical amount of protein (100 µg) was loaded onto a 10% Tris-HCl gel (Bio-Rad) and subjected to electrophoresis for 1.5 h at 110 V under denaturing conditions. The blot was then transferred to polyvinylidene fluoride membrane (Bio-Rad). Membranes were blocked in 5% nonfat milk at room temperature for 1 h. Membranes were then incubated with a rabbit polyclonal antibody specific for GABA-A receptor α4 subunit (1:1000 dilution) (Novus Biologicals, Littleton, Colorado), β-actin antibody (1:1000 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA) or the Egr3 antibody (1:200 dilution) (Santa Cruz) at 4°C for overnight. Membranes were washed three times with 1× mixture of Tris-buffered saline and Tween 20 (TBST) at room temperature for 20 min. The blots were incubated with anti-rabbit antibody (1:2500) conjugated to horseradish peroxidase (HRP) for 1 h at room temperature. Blots were washed three times for 20 min with 1× TBST. Immunoreactive α4 bands (67 kDa) were detected with the use of enhanced chemiluminiscence reagent (Perkin-Elmer, Shelton, CT), then membranes were stripped and reprobed with the mouse monoclonal antibody for β-actin. Protein bands were quantified using alpha imager software (Alpha Innotech, San Leandro, CA). All values were normalized to β-actin expression in the same samples to control for loading amount variability and then expressed as a percent change with respect to mean control values.
Hippocampus kindling model of epilepsy
To evaluate seizure sensitivity and protection efficacy of GABA-A receptor modulating agents during neurosteroid withdrawal, we utilized the hippocampus kindling model, which is a model of human temporal lobe epilepsy (Albright and Burnham, 1980). Kindling is the repetition of stimuli that initially evoke afterdischarges but not seizures (Goddard et al., 1969; McNamara et al., 1992). A mild focal, non-convulsant electrical stimulus to the hippocampus on a daily basis leads to development of a kindled state exhibiting electrographic and behavioral seizures. Once an animal has been kindled, the heightened response to the stimulus is permanent and seizures occur upon stimulation even after several months (McNamara et al., 1992). In mouse kindling, the focal EEG afterdischarge models complex partial seizures, while the behavioral motor seizure stages 4/5 models generalized seizures. Electrode implantation and stimulation procedures for mouse hippocampus kindling were performed as described previously (Reddy and Rogawski, 2002; 2010). Briefly, mice were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). A twisted bipolar stainless steel wire electrode (model MS303/1; Plastic One, Roanoke, VA) was stereotaxically implanted in the right hippocampus (2.9 mm posterior, 3.0 mm lateral, and 3.0 mm below dura) (Franklin and Paxinos, 1997) and anchored with dental acrylic to three jeweler's screws placed in the skull. A period of 7 to 10 days was allowed for recovery. The stimulation paradigm consisted of 1 ms-duration, bipolar, square current pulses delivered at 60 Hz for 1 s using a kindling stimulator (A-M Systems, Sequim, WA). The afterdischarge threshold was determined by stimulating at 5 min intervals beginning with an intensity of 25 µA and increasing in increments of 25 µA until an afterdischarge of at least 5 s was obtained. Stimulation on subsequent days used an intensity 125% of the threshold value. Seizure activity following each stimulation was rated according to the criterion of Racine (1972) as modified for the mouse: stage 0, no response or behavior arrest; stage 1, chewing or head nodding; stage 2, chewing and head nodding; stage 3, forelimb clonus; stage 4, bilateral forelimb clonus and rearing; stage 5, falling. The afterdischarge was recorded from the hippocampus electrode with a Grass CP511 preamplifier (Astro-Med, West Warwick, RI) and stored in digital form using Axoscope 8.1 (Axon Instruments, Foster City, CA). Afterdischarge duration was the total duration of hippocampus electrographic spike activity (amplitude > 2 × baseline) occurring in a rhythmic pattern at a frequency > 1Hz. The day of afterdischarge threshold determination was considered day 1 of kindling. Kindling stimulation was delivered daily until stage 5 seizures were elicited on three consecutive days. Stimulation was continued on a five-day per week schedule each afternoon. Mice were used for neurosteroid withdrawal paradigm when they consistently exhibited stage 5 seizures with stimulation, which is considered the “fully kindled” state.
Test drug administration and kindling protocol
To examine the ability of test drugs to suppress the expression of kindled seizures, fully-kindled animals were tested 24 h after the induction of neurosteroid withdrawal. Two different benzodiazepines, diazepam and flumazenil, were selected for pharmacological evaluation in neurosteroid-withdrawn animals. Benzodiazepines interact with GABA-A receptors containing either an α1-, α2-, α3- or α5- subunit along with β and γ2 subunits. Their specific recognition site occurs at the interface of the α and γ2 subunit (Mohler et al., 2002). Hence, benzodiazepine site agonists possess anxiolytic and antiseizure activities (Hansen et al., 2004). Benzodiazepines act either as agonists or antagonists with little or no intrinsic activity depending on their interaction with specific receptor isoforms. Diazepam is a benzodiazepine-site agonist at α1-, α2-or α3-containing GABA-A receptors with potent antiseizure activity, but is insensitive as an agonist at α4-containing GABA-A receptors expressed in the hippocampus (Smith et al., 1998b; Gulinello and Smith, 2003). Flumazenil is a benzodiazepine-site antagonist at α1-containing GABA-A receptors with little intrinsic activity for seizure protection, but acts as an agonist at α4-containing GABA-A receptors and causes seizure protection (File and Pellow, 1986; Smith et al., 1998b; Gulinello and Smith, 2003). In the kindling studies, animals were first verified to exhibit stimulation-induced stage 5 seizures twelve hours prior to drug testing. On the day of testing, animals were injected intraperitonially with diazepam (0.1–1 mg/kg) or flumazenil (1–10 mg/kg) 15 min before kindling stimulations. Control animals were injected similarly with vehicle (15% cyclodextrin). During each test session, the behavioral seizure score and the afterdischarge duration were noted. Control, non-withdrawal mice were tested at the diestrous/metestrous stage.
Antisense generation and administration
Antisense oligonucleotides were designed complementary to the mouse Egr3 mRNA using website software (IDT). The 21-mer antisense oligonucleotide, 5’-GGA-TGT-GAG-TGG-TGA-GGT-GGT-3’, does not show any significant homology with any other neural gene sequence identified in GenBank. A 21-mer missense oligonucleotide was also constructed as 5’-GAG-TAT-GAC-AGC- TAG-GCA-ACG-3’, and did not show any significant homology with any other neural gene sequences identified in GenBank. HPLC purified phosphorothioated Egr3 antisense (5 nmol) or Egr3 missense (5 nmol) oligonucleotides (IDT, San Diego, CA) were infused intracerebroventricularly (ICV) in a 5 µl volume of sterile saline. ICV injections were made via an indwelling 26-gauge stainless steel guide cannula 5 mm in length (model C315GS-5/SPC; Plastic One). At the time of antisense administration, the cannula was used to guide an injection needle into the left lateral ventricle. The guide cannula was implanted surgically at least 1 week prior to the infusions. The cannula was guided stereotaxically into the frontal horn of the left ventricle coordinates in mm from bregma: anteroposterior, −0.5; lateral, +1.5; dorsoventral, −1.0; and secured with dental acrylic to 1/8-inch machine screws implanted in the skull. The cannula was plugged with a 30-gauge stainless steel wire with dummy cap (model C315DCS-5/SPC; Plastic One) until the time of injection. Antisense oligos were administered via the implanted guide cannula using a blunt end 11.2-mm, 33-gauge internal needle (model C315IS-5/SPC; Plastic One) attached with polyethylene tubing to a 10-µl microsyringe. Infusions were delivered at a constant rate of 1 µl/min by a programmable infusion pump (Harvard Apparatus). At the end of the infusion, the cannula was left in place for an additional 5 minutes to minimize infusate backflow and to ensure better drug distribution. Cannulated mice were injected with antisense oligos on day 7, about 24 h prior to drug testing. Egr3 missense oligos were utilized as controls for the antisense group.
Data analysis
Group data are expressed as the mean± standard error of the mean (SEM). The α4 and Egr3 subunit expression was analyzed based on the relative quantification approach as described previously (Gangisetty and Reddy, 2009). The target gene expression in the samples was expressed as a percent change from control. The unpaired Student’s t-test was used to compare the effect of neurosteroid withdrawal on α4-subunit or Egr3 expression in wild-type and PR knockout mice. Differences in kindling seizure stage between groups were compared with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U-test. Comparison of means of the afterdischarge duration between groups was made with one-way analysis of variance, followed by unpaired two-tailed Student’s t-test. Comparison of the mean percentage inhibition of seizure stage and afterdischarge duration in fully kindled animals was made by Wilcoxon signed ranks test and paired two-tailed Student’s t-test, respectively. To construct dose-effect curves, diazepam and flumazenil were tested at several doses spanning the dose producing 50% protection (ED50) in the kindling model. ED50 values were determined by non-linear curve fitting using the Levenberg-Marquardt algorithm to a logistic equation where the maximum inhibition was assumed to be 100%. In all statistical tests, the criterion for statistical significance was p < 0.05.
Drugs
Stock solutions of progesterone (Steraloids Inc., Newport, RI) and other drugs for injection were made in 15% β-cyclodextrin in saline, and additional dilutions were made using sterile saline. β- Cyclodextrin alone, at concentrations as high as 50%, failed to affect kindled seizures (Reddy et al., 2004). Diazepam solution (Hospira, Lake Forest, IL) was diluted in sterile saline. Drug solutions were administered subcutaneously in a volume equaling 1% of the animal’s body weight. The dosage of diazepam was based on a dose-response curve generated in a preliminary study in fully kindled mice. The doses chosen were comparable to those previously found to be effective in the kindling model in mice (Hansen et al., 2004).
RESULTS
Perimenstrual-like neurosteroid withdrawal paradigm in mice
To create a model of the perimenstrual endocrine milieu to simulate the menstruation associated neurosteroid withdrawal, we utilized the exogenous progesterone treatment paradigm in female mice (Fig.1A). A state of prolonged neurosteroid levels was induced in wild-type and PR knockout mice by two daily injections of progesterone (25 mg/kg, sc) for 7 days. Neurosteroid withdrawal was induced by treatment with finasteride (50 mg/kg, ip), a 5α-reductase inhibitor that blocks the conversion of progesterone into allopregnanolone. Plasma allopregnanolone levels were measured by LC-MS assay. As shown in Fig.1B, plasma allopregnanolone levels were increased following progesterone treatment in wild-type (mean level, 133 vs. vehicle control, 1.7 ng/ml) and PR knockout mice (mean level, 174 vs. vehicle control, 2.4 ng/ml). The levels of allopregnanolone did not differ between wild-type and PR knockout mice at baseline or after progesterone treatment (Fig.1B). Plasma allopregnanolone was significantly reduced (~96%) 24 h after finasteride treatment in both genotypes. Thus, the elevated plasma allopregnanolone concentrations during progesterone treatment mimics the high levels of neurosteroids during the luteal phase of the menstrual cycle, whereas the finasteride-induced withdrawal is similar to the marked decrease in neurosteroid concentrations that occur before the start of the menstruation (Wang et al., 1996; Tuveri et al., 2008).
Changes in GABA-A receptor expression during neurosteroid exposure and withdrawal
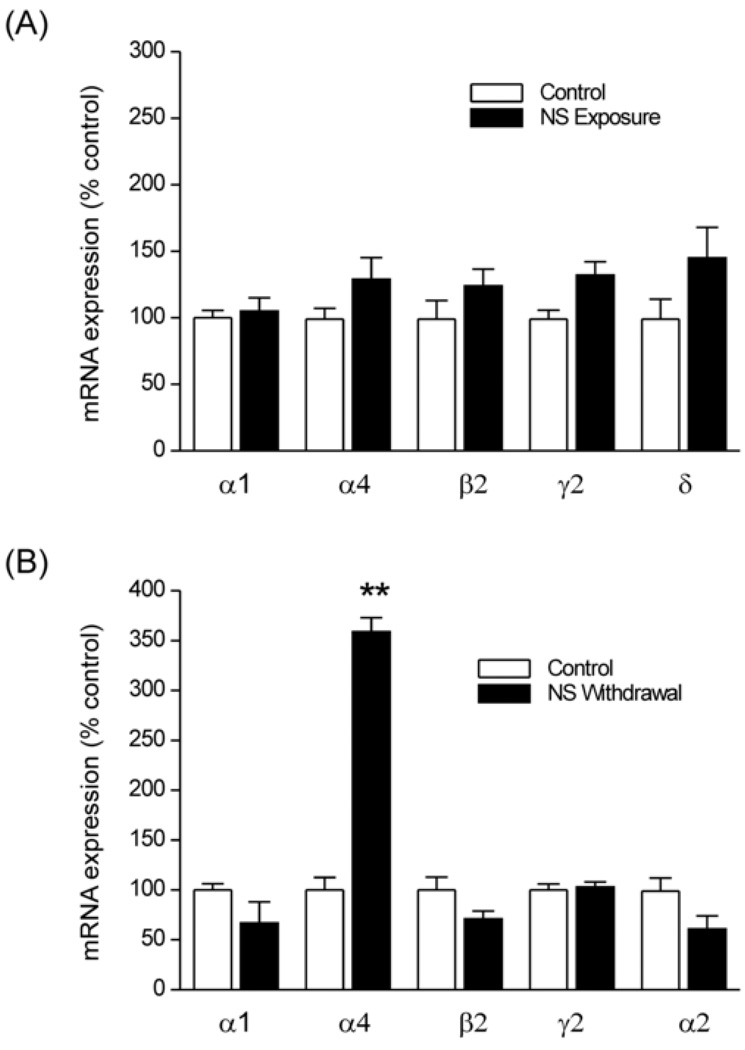
Using this mouse paradigm, we first determined the potential changes in GABA-A receptor subunit mRNA expression during neurosteroid treatment and withdrawal by TaqMan real-time PCR analysis in the hippocampus, which has previously been shown to exhibit neurosteroid-dependent plasticity (Smith et al., 1998a,b; Maguire et al., 2005; 2009; Maguire and Mody, 2007). In animals with persistently elevated neurosteroid levels, induced by treatment with progesterone, there was no significant change in levels of α1, β2, γ2 and δ subunit expression as compared to vehicle control (Fig.2A). The α4-subunit expression was moderately increased during neurosteroid exposure. Twenty-four hours after neurosteroid withdrawal, the levels of α4-subunit were significantly increased compared with its expression in vehicle- and progesterone-treated (non-withdrawal) animals (Fig.2B). In contrast, no changes in levels of α1, α2, β2, and γ2 subunit expressions were observed 24 h after neurosteroid withdrawal (Fig.2B). Overall, these findings indicate a marked increase in the expression of α4-subunit expression in the hippocampus after neurosteroid withdrawal in the mouse perimenstrual paradigm.
Fig.2. Changes in GABA-A receptor subunit expression in the hippocampus during neurosteroid exposure (A) and neurosteroid withdrawal (B).
TaqMan real-time PCR analysis of specific subunit mRNA expression in the hippocampus in wild-type mice. The target subunit expression was quantified in the hippocampus samples collected from control mice following 7 day treatment with vehicle (15% cyclodextrin solution), or neurosteroid exposure (by progesterone treatment) or neurosteroid withdrawal as described in Fig.1A. Total RNA was extracted from the hippocampus and cDNA was prepared for TaqMan PCR analysis. The TaqMan PCR data was normalized in every assay using GAPDH as an internal standard. The data represents the mean ± SEM (n = 8 mice per group). **p<0.01 vs. control group.
Neurosteroid withdrawal increases GABA-A receptor α4-subunit expression in the hippocampus via a PR-independent pathway
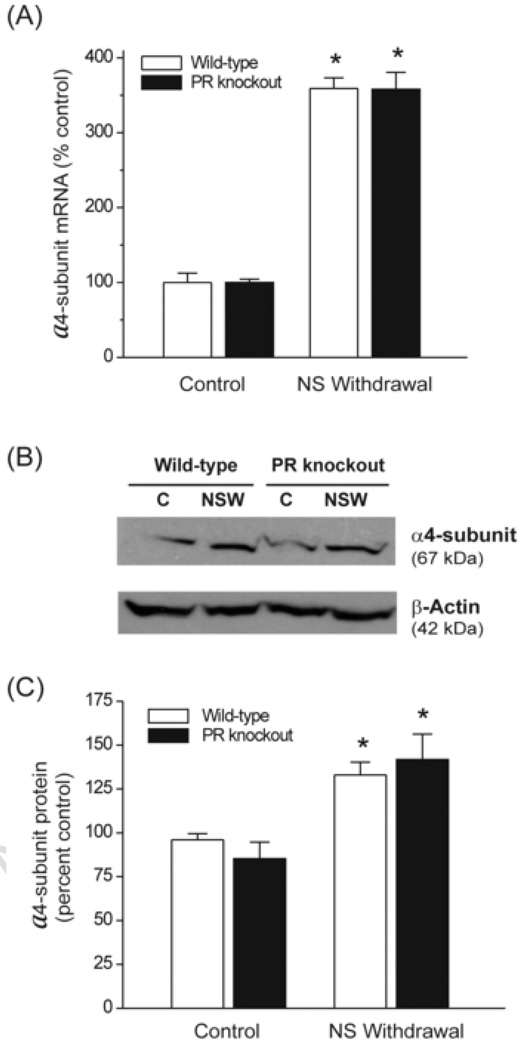
To determine whether the PR pathway is involved in regulating GABA-A receptor α4-subunit expression in response to neurosteroid withdrawal, we utilized female homozygous PR knockout mice, which lacks PR-A and PR-B receptor subtypes in the brain (Reddy et al., 2005), as a robust genetic model. Twenty-four hours after neurosteroid withdrawal, the abundance of α4-subunit mRNA in the hippocampus was increased by 360% in wild-type mice as compared with vehicle control (Fig.3A). Such an upregulation was undiminished in PR knockout mice (Fig.3A). In PR knockout mice, there was a 358% increase in α4-subunit mRNA at 24 h following neurosteroid withdrawal. Furthermore, no change in α4-subunit mRNA was observed in PR knockout animals treated from days 1 to 7 with progesterone and finasteride (a low neurosteroid condition), demonstrating that α4-subunit upregulation requires acute withdrawal from chronically elevated neurosteroids and that it does not require the presence of PRs. To determine whether changes in α4-subunit mRNA levels are translated to α4 protein abundance, we determined the α4 protein levels in the hippocampus 24 h following neurosteroid withdrawal. As shown in Fig.3B, Western blot analysis indicated significant increase in total α4 protein in both wild-type and PR knockout mice as compared to non-withdrawal control animals. The levels of β-actin control protein did not differ between treatment conditions or genotype. Consistent with changes in α4-subunit mRNA levels, levels of α4 protein were increased by almost 1.5-fold 24 h after neurosteroid withdrawal in wild-type and PR knockout mice (Fig.3C). Taken together, these observations suggest that neurosteroid withdrawal induced upregulation of α4-subunit expression is not mediated by the PR pathway.
Fig.3. Neurosteroid withdrawal causes upregulation of GABA-A receptor α 4 subunit expression in the hippocampus through a PR-independent pathway.
(A) TaqMan real-time PCR analysis of α4-subunit mRNA expression in the hippocampus in wild-type and PR knockout mice. The α4-subunit expression was quantified in the hippocampus samples collected from control mice following treatment with vehicle (15% cyclodextrin solution) and mice following neurosteroid withdrawal protocol as described in Fig.1A. (B) Western blot analysis of α4-subunit levels in the hippocampus. Representative immunoblots of α4-subunit and p-actin levels in control (C) and neurosteroid withdrawn (NSW) wild-type and PR knockout mice. (C) Densitometric quantification of α4-subunit protein expression. The integrated density values of each band were analyzed and the α4-subunit data was normalized with β-actin. The data represent the mean ± SEM (n = 6–8 mice per group). *p<0.01 vs. control group.
Neurosteroid withdrawal increases GABA-A receptor α4-subunit expression in the hippocampus through an Egr3-dependent pathway
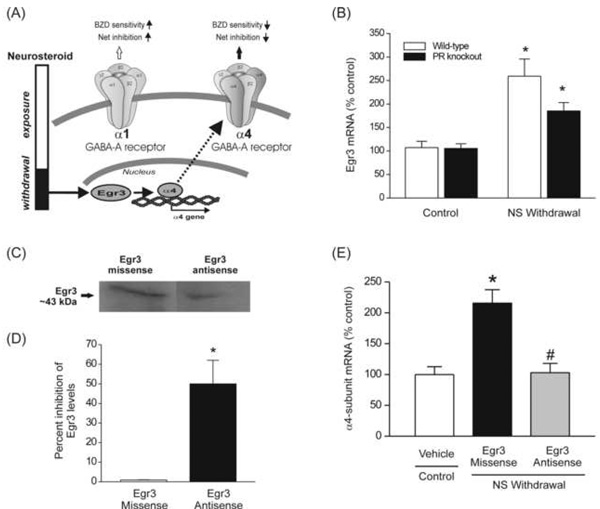
Since the Egr3 pathway can regulate α4-subunit expression in the hippocampus, we hypothesized that neurosteroid withdrawal results in activation of Egr3 and thereby causes upregulation of the GABA-A receptor α4-subunit (Fig.4A). Therefore, we sought to directly identify changes in Egr3 following neurosteroid withdrawal and its role in the changes underlying α4-subunit expression. Progesterone activates the Egr family of transcription factors via the PR pathway (Mercier et al., 2001). Therefore, we focused on both PR-dependent and PR-independent Egr3 pathways using the PR knockout mouse model. As shown in Fig.4B, the levels of Egr3 mRNA in the hippocampus increased 2.5-fold after neurosteroid withdrawal in wild-type mice. Although PRs are not involved in α4 upregulation, progesterone activation of PR may alter Egr3 transcription. To determine this possibility, we investigated the changes in Egr3 expression in PR knockout mice. There were no significant differences in Egr3 mRNA levels between the control wild-type and PR knockout mice. Neurosteroid withdrawal induced upregulation of Egr3, however, it was undiminished in PR knockout mice (Fig.4B). These data indicate that neurosteroid withdrawal enhances Egr3 expression in the hippocampus via a PR-independent pathway. To ascertain whether Egr3 upregulation increases α4-subunit expression, we used the antisense inhibition of Egr3 mRNA expression to selectively block the Egr3 pathway. To demonstrate knockdown of Egr3 in the hippocampus after ICV administration of Egr3 antisense oligonucleotides, we compared the total Egr3 protein in the hippocampus of normal wild-type mice treated with Egr3 missense or Egr3 antisense oligos. Mice were treated with either 5 nmol of Egr3 missense or 5 nmol Egr3 antisense oligos and protein analysis was performed about 14 hours later. As shown in Fig.4C, antisense treatment markedly reduced Egr3 expression when compared to missense control. Analysis of optical density shows that antisense treatment significantly (p<0.05) decreased total Egr3 levels in the hippocampus (Fig.4D). To determine whether blocking Egr3 upregulation seen during neurosteroid withdrawal prevents an increase in α4-subunit expression, we carried out ICV treatment of mice with Egr3 antisense or missense oligos and α4-subunit mRNA analysis was performed about 14 h later, when the mice had 24 h of neurosteroid withdrawal. As shown in Fig.4E, unlike Egr3 missense, Egr3 antisense treatment completely prevented the neurosteroid withdrawal-induced upregulation of α4-subunit expression. Thus, upregulation of α4-subunit during neurosteroid withdrawal is mediated by the Egr3 pathway.
Fig.4. Neurosteroid withdrawal causes upregulation of GABA-A receptor α4-subunit expression in the hippocampus through an Egr3-dependent pathway.
(A) A working model indicating neurosteroid withdrawal signaling pathway for Egr3, which is hypothesized to regulate GABA-A receptor plasticity. The α4 promoter is activated by Egr3, which in turn is upregulated by neurosteroid withdrawal, and therefore an Egr3-mediated increase in α4 expression may underlie the reduced net inhibition and benzodiazepine (BZD)-insensitivity. (B) Neurosteroid withdrawal causes an increase in Egr3 transcription in wild-type and PR knockout mice. Egr3 mRNA was quantified in hippocampal samples collected from mice following treatment with vehicle or neurosteroid withdrawal as described in Fig.1. The data represents the mean ± SEM (n = 8). *p<0.01 vs. control group. (C) Representative immunoblots of Egr3 levels in the hippocampus in neurosteroid-withdrawn wild-type mice following ICV infusion of Egr3 antisense oligos. Treatment with Egr3 antisense during neurosteroid withdrawal results in lower Egr3 expression as compared to that in mice treated with Egr3 missense. (D) Densitometric quantification of changes in Egr3 expression in mice after Egr3 antisense or missense treatment (*p<0.05, n=4–6 for each group). (E) Antisense Egr3 inhibition prevents the neurosteroid withdrawal-induced upregulation of α4-subunit expression. Hippocampal samples were collected from wild-type mice following ICV infusion of antisense or missense oligos on day-7 of withdrawal protocol. Data represent mean ± SEM (n = 6 –8 mice per group). *p<0.01 vs. control; #p<0.01 vs. missense group.
Neurosteroid withdrawal increases seizure susceptibility in the hippocampus kindling model of epilepsy
To determine whether the cellular changes that occur during neurosteroid withdrawal, such as enhanced α4-subunit-containing GABA-A receptors in the hippocampus, are associated with heightened seizure susceptibility, we analyzed the stimulation-evoked seizure activity in animals undergoing neurosteroid withdrawal in the hippocampus kindling model of epilepsy. Mice were subjected to once-daily kindling via implanted electrode in the dentate gyrus region (Fig.5A) until they exhibited stage 5 seizures for 3 consecutive days, which is considered the “fully kindled” state (Fig.5B). Fully-kindled mice were then subjected to neurosteroid withdrawal protocol as described in Fig.1A. Four parameters were assessed as indices of seizure propensity: (a) threshold current for generalized seizures; (b) stimulation-induced electrographic afterdischarges, (c) behavioral seizure intensity measured as per the Racine scale (Racine, 1972), and (d) duration of generalized seizures. Kindling seizures are classified as partial (stage 1–3) and generalized (stage 4–5). Partial seizures typically occur if the network excitability is confined to the ipsilateral hippocampus and generalized seizures are more likely to occur if the excitability spreads rapidly to the contralateral hippocampus and cortex (McNamara et al., 1992; Reddy and Rogawski, 2010). Consistent with heightened excitability, there was a significant decrease in the threshold current to induce generalized seizures at 24 h after neurosteroid withdrawal (mean ADT value, 166 and 95 µV for control and withdrawal, respectively) (Fig.5B). The mean duration of the individual generalized seizures was longer in withdrawal (37 ± 4 s, n=8) than in control animals (25 ± 2 s, n=8) (Fig.5C). The total duration of afterdischarges were similar in control and withdrawal animals at 46 ± 5 s and 49 ± 6 s, respectively (Fig.5E). The number of animals exhibiting generalized seizures at 50% ADT current was significantly higher after neurosteroid withdrawal than in the control group (Fig.5D). This response was moderately higher 12 h after neurosteroid withdrawal, reached maximum values by 24 h after neurosteroid withdrawal, and returned to control level by 48 h after withdrawal (Fig.5D), indicating a transitory period for seizure exacerbation following neurosteroid withdrawal. Such an increase in seizure susceptibility was undiminished in PR knockout mice, as evident from a significant reduction in the threshold for generalized seizures (mean ADT value, 137 and 64 µV for control and withdrawal, respectively) and an increased number of animals displaying seizures at 50% ADT current following withdrawal (12 and 98 percent for control and withdrawal, respectively). These findings are consistent with previous reports that demonstrated a decreased seizure threshold and an increase in seizure intensity in rats following withdrawal from neurosteroids (Smith et al., 1998a; Reddy et al., 2001).
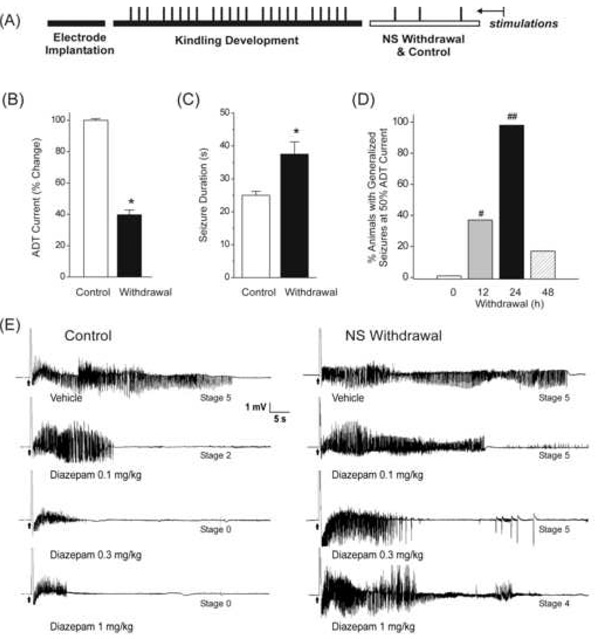
Fig.5. Increased seizure activity and reduced diazepam protection against electrographic seizure afterdischarges during neurosteroid withdrawal in the mouse hippocampus kindling model of epilepsy.
(A) Experimental protocol for kindling and withdrawal paradigm. Mice were stimulated until they had exhibited stage 5 seizures and then they were subjected to neurosteroid withdrawal as described in Fig.1A. (BCD) Changes in seizure activity during neurosteroid withdrawal in wild-type mice in the hippocampus kindling model. There is a decreased threshold to induce generalized seizures (B), increased seizure duration (C), and time-course of the percent of animals exhibiting generalized seizures (stage 4/5) at 50% of regular ADT current (D). Each bar represents the mean ± SEM of data from six to ten animals. *p<0.05 vs. control (non-withdrawn) group; #p<0.05, ##p<0.01 vs. control (non-withdrawn) group. (E) Representative EEG traces illustrating reduced diazepam inhibition of afterdischarge in fully-kindled neurosteroid-withdrawn mice as compared to that in fully-kindled control (non-withdrawal) mice. Traces show depth recordings from a right hippocampus stimulating/recording electrode. The arrow at the bottom indicates kindling stimulation that was followed by an artifact or a period of blanking surrounding the 1s stimulus. The slow negative wave following stimulation was digitally subtracted in each trace. Behavioral seizure stages are indicated on the trace. Diazepam (0.1– 1 mg/kg, i.p.) was administered 15-min prior to the stimulation; control trace was obtained with vehicle treatment.
Neurosteroid withdrawal reduces diazepam protection against hippocampus kindled-seizures
To confirm whether the elevated α4-subunit expression during neurosteroid withdrawal alters the sensitivity of GABA-A receptor modulators, we assessed the effect of diazepam on seizure activity in the hippocampus kindling model. As might be expected with increased α4βγ receptor expression, treatment with diazepam, a benzodiazepine selective for α1/2/3/5βγ receptors but relatively insensitive at α4βγ receptors, at 24 h after neurosteroid withdrawal, resulted in decreased antiseizure efficacy, evident from a lack of reduction in seizure stage and lack of suppression of electrographic afterdischarges in wild-type mice (Fig.5E). In contrast, diazepam (0.1–1 mg/kg, ip) produced a dose-dependent suppression of afterdischarges in control (non-withdrawal) animals, with nearly complete suppression at the highest dose tested (Fig.5E). As shown in Fig.5E, diazepam-treated control animals initially showed bursts of spikes that progressively decrease in amplitude and duration in a dose-dependent fashion, in contrast with the neurosteroid-withdrawn mouse, which displayed longer synchronous bursts with high amplitude discharges resembling epileptiform patterns (Fig.5E). This pattern is consistent with insensitivity to diazepam as observed in other models of neurosteroid withdrawal (Moran and Smith, 1998a; Reddy and Rogawski, 2000).
Pharmacological characterization of neurosteroid withdrawal-induced diazepam insensitivity in the hippocampus kindling model of epilepsy
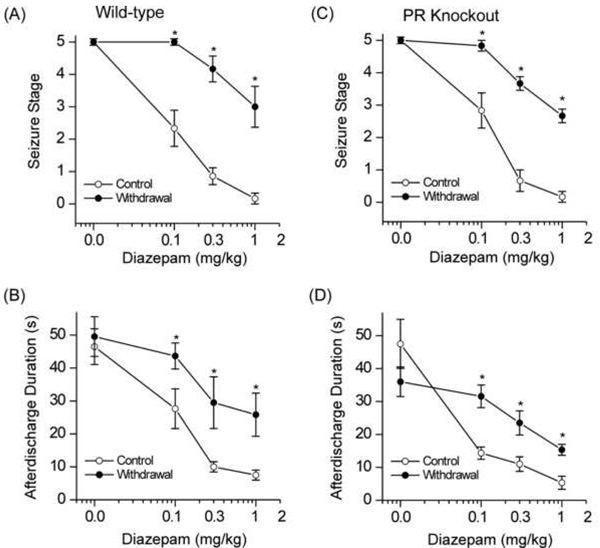
To further investigate if neurosteroid withdrawal is associated with alterations in the antiseizure profile of benzodiazepines, diazepam was characterized pharmacologically in wild-type mice at 24 after neurosteroid withdrawal (Fig.6AB). Diazepam was examined for its protective potency, tested behaviorally for the ability to suppress kindled-seizures and electrographically to reduce afterdischarge duration following kindling stimulations. Diazepam produced a dose-dependent suppression of behavioral seizure activity (Fig.6A) and afterdischarge duration with significant effects at 0.1, 0.3 and 1 mg/kg when compared with vehicle control (Fig.6B), confirming diazepam protection against hippocampus kindling-induced seizures. At the highest dose tested, behavioral seizures were nearly completely suppressed by diazepam. In contrast, mice undergoing neurosteroid withdrawal had significantly decreased seizure protection by diazepam (Fig.6AB). At 1 mg/kg dose, diazepam produced an average of a 95% and 40% decrease in seizure expression in control and withdrawal groups, respectively, and the same dose produced a mean of 83% and 46% reduction in the afterdischarge duration in control and withdrawal animals. The estimated ED50 values of diazepam for suppression of kindled seizures in control and withdrawal groups are 0.11±0.01 and 1.11±0.18 mg/kg, respectively. Taken together, these results are consistent with the notion that neurosteroid withdrawal causes relative insensitivity to diazepam due to increased α4βγ GABA-A receptor expression in the hippocampus. Moreover, electrophysiological studies confirm a striking reduction (~95%) of lorazepam potentiation of GABA-A-gated currents in CA1 neurons after neurosteroid withdrawal (Smith et al., 1998b).
Fig.6. Neurosteroid withdrawal reduces the antiseizure sensitivity of diazepam in wild-type and PR knockout mice in the hippocampus kindling model of epilepsy.
(AB) Dose-response curve for diazepam (0.1–1 mg/kg, i.p.)-induced suppression of behavioral seizures (A) and afterdischarge duration (B) in control and neurosteroid- withdrawn wild-type mice. Diazepam is a benzodiazepine-site agonist at α1/2-containing, but not α4-containing GABA-A receptors. The diazepam-insensitivity in mice undergoing neurosteroid withdrawal was consistent with increased α4-containing GABA-A receptor abundance in vivo. (CD) Dose-response curve for diazepam (0.1–1 mg/kg, i.p.)-induced suppression of behavioral seizure stage (C) and afterdischarge duration (D) in control and neurosteroid-withdrawn PR knockout mice. Vehicle or diazepam was injected intraperitonially 15 min before kindling stimulations or at other time after diazepam treatment as indicated in the time-course studies. In all graphs, each point represents the mean ± SEM of data from six to ten animals. *p<0.05 vs. diazepam-treated control (non-withdrawn) group.
To confirm the specificity of the diazepam effect in the kindling model, we pretreated a subgroup of mice with flumazenil, a reference benzodiazepine-site antagonist that blocks diazepam’s potentiation of GABA-A receptors and seizure protection. As expected, pretreatment with flumazenil (10 mg/kg, ip), 30-min prior to diazepam administration, completely blocked the antiseizure effect of 1 mg/kg diazepam (6 of 6 mice showed stage 4/5 seizures) when compared to diazepam control (6 of 6 mice showed stage 0/1 seizures). Flumazenil alone has no significant effect on seizure susceptibility (5 of 5 mice showed stage 5 seizures).
Neurosteroid withdrawal-induced diazepam insensitivity occurs via a PR-independent pathway
To verify the absence of a role of PRs in changes underlying diazepam insensitivity observed during neurosteroid withdrawal, diazepam was characterized pharmacologically in PR knockout mice. The neurosteroid withdrawal-induced diazepam insensitivity in the kindling model was preserved in PR knockout mice (Fig.6CD). The seizure stage and afterdischarge duration were nearly 40% suppressed by diazepam in PR knockout mice undergoing withdrawal as compared to 100% inhibition in the control animals. The estimated ED50 values of diazepam for suppression of kindled seizures in PR knockout control and withdrawal groups are 0.13±0.01 and 1.67 ±0.25 mg/kg, respectively. Furthermore, the potency and efficacy of diazepam did not differ between the wild-type and PR knockout mice during neurosteroid withdrawal (Fig.6A–D). These results further support the concept that PRs do not contribute to neurosteroid withdrawal induced diazepam insensitivity.
Neurosteroid withdrawal confers increased antiseizure efficacy to flumazenil in the kindling model of epilepsy
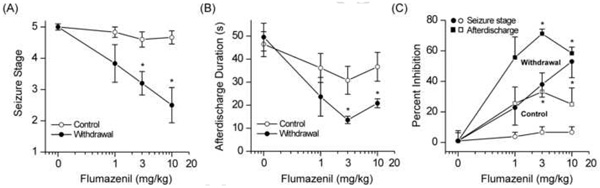
The efficacy of the benzodiazepine antagonist flumazenil (RO15–1788) was tested in wild-type mice 24 h after neurosteroid withdrawal (Fig.7). Control and neurosteroid-withdrawn mice were tested in the kindling model with three doses of flumazenil (1, 3 and 10 mg/kg, ip). At these doses, flumazenil had no effect on the behavioral seizures (Fig.7A) and afterdischarges (Fig.7B) in control mice. In contrast, after neurosteroid withdrawal, flumazenil produced dose-dependent suppression of behavioral seizures (Fig.7A) and afterdischarge duration (Fig.7B) with significant effects at 3 and 10 mg/kg when compared with vehicle treatment. Kindled-seizures were partially (53%) suppressed by flumazenil even at the highest dose tested (Fig.7C). These data suggest that neurosteroid withdrawal confers benzodiazepine agonist-like efficacy to flumazenil, possibly due to increased α4βγ GABA-A receptor expression in the hippocampus. These findings are consistent with significant (58%) enhancement of GABA-A-gated current in CA1 neurons by flumazenil after neurosteroid withdrawal (Smith et al., 1998a,b).
Fig.7. Neurosteroid withdrawal confers agonist-like antiseizure effects to flumazenil in wild-type mice in the hippocampus kindling model of epilepsy.
Dose-response curve for flumazenil (1–10 mg/kg, i.p.) inhibition of behavioral seizures (A), afterdischarge duration (B), and percent inhibition (C) in control and neurosteroid-withdrawn mice. Flumazenil is a benzodiazepine-site antagonist at α1/2-containing GABA-A receptors with little intrinsic activity, but it acts as an agonist at α4-containing GABA-A receptors. Flumazenil was largely devoid of antiseizure activity in control (non-withdrawal) animals. The flumazenil’s agonist-like antiseizure efficacy in mice undergoing neurosteroid withdrawal was consistent with increased α4-containing GABA-A receptor abundance in vivo. Vehicle or flumazenil was injected intraperitonially 15 min before kindling stimulations. Each point represents the mean ± SEM of data from six to eight animals. *p<0.05 vs. flumazenil-treated control (non-withdrawn) group.
Inhibition of Egr3 pathway prevents the neurosteroid withdrawal-induced seizure exacerbation in the kindling model of epilepsy
To investigate the effects of Egr3 inhibition on seizure susceptibility, we measured seizure activity in response to an ICV infusion of 5 nmol Egr3 antisense or missense oligos given at the onset of neurosteroid withdrawal. Cannulated mice were first subjected to hippocampus kindling and were utilized for withdrawal studies when they developed a fully-kindled state with consistent stage 5 seizures. In mice undergoing neurosteroid withdrawal, there was a significant increase in the threshold current for generalized seizures in antisense (111 ± 14 µA) than missense (63 ± 9 µA) groups, and there was a significant decrease in the percent of animals displaying generalized seizures at 50% ADT current (98% and 58% for missense and antisense, respectively). The mean duration of generalized seizures in vehicle control (non-withdrawal) animals was 34 ± 4 s (n=8). However, the duration of the generalized seizures was longer in missense-treated (45 ± 4 s, n=8) than in antisense-treated (32 ± 3 s, n=8) animals, suggesting a decrease in neurosteroid withdrawal-induced seizure exacerbation by Egr3 inhibition.
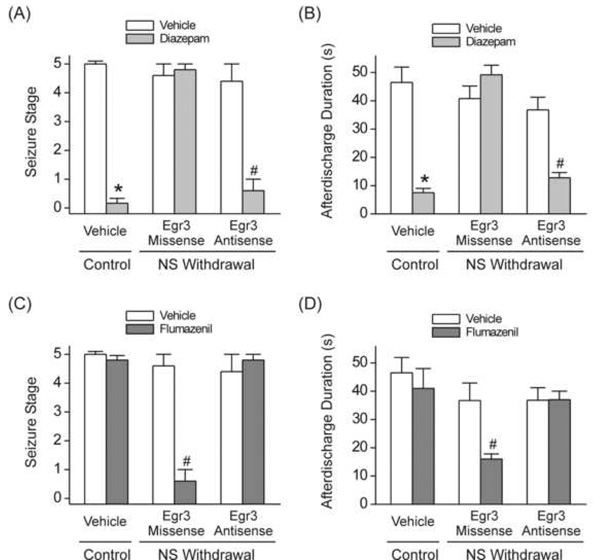
Inhibition of Egr3 pathway reverses the neurosteroid withdrawal-induced diazepam insensitivity and flumazenil efficacy in the kindling model of epilepsy
To ascertain whether the changes in the antiseizure efficacy of diazepam and flumazenil during the neurosteroid withdrawal result from specific alterations in Egr3-mediated α4βγ-GABA-A receptors, we evaluated the effects of diazepam and flumazenil in wild-type mice treated with Egr3 antisense oligos. We examined the effects of diazepam and flumazenil in mice confirmed to exhibit stage 5 seizures following stimulation at regular threshold current. Mice were treated with Egr3 antisense or missense (5 nmol, ICV) at 24 h prior to diazepam testing or 24 h before withdrawal testing. The diazepam insensitivity observed following the neurosteroid withdrawal was prevented by prior ICV administration of Egr3 antisense oligos, as assessed by significant suppression of behavioral seizures (Fig.8A) and afterdischarges (Fig.8B) to a level comparable to that observed in the control group. However, in missense-treated neurosteroid-withdrawn mice, diazepam has no significant protection against kindled-seizures as compared to vehicle controls (Fig.8AB). Furthermore, we tested the effect of flumazenil in wild-type mice following Egr3 antisense inhibition. Cannulated mice were treated with Egr3 antisense or missense (5 nmol, ICV) at 24 h prior to flumazenil testing or 24 h before withdrawal testing. The flumazenil efficacy to protect against kindled-seizures during neurosteroid withdrawal was significantly blocked by Egr3 antisense treatment, as displayed by lack of suppression of behavioral seizures (Fig.8C) and afterdischarges (Fig.8D). Overall, these results indicate that withdrawal from neurosteroids exacerbate seizures, decrease the antiseizure response to diazepam, and confer seizure protection to flumazenil as a direct result of increases in the α4-subunit of the GABA-A receptor via the Egr3 pathway.
Fig.8. Antisense Egr3 inhibition prevents the neurosteroid withdrawal-induced diazepam insensitivity and flumazenil efficacy in protecting against kindling-induced seizures.
(AB) Treatment with Egr3 antisense oligos in wild-type mice undergoing neurosteroid withdrawal results in restoration of diazepam sensitivity in protecting against kindled seizures to levels similar to that in non-withdrawal control animals. Diazepam (1 mg/kg) inhibition of behavioral seizures (A) and afterdischarge duration (B) is significantly increased in mice after intraventricular administration of Egr3 antisense but not Egr3 missense oligos on day-7 of withdrawal protocol. (CD) Treatment with Egr3 antisense oligos in mice undergoing neurosteroid withdrawal results in reversal of flumazenil efficacy in protecting against kindled seizures in wild-type mice. Flumazenil (10 mg/kg) inhibition of behavioral seizures (C) and afterdischarge duration is significantly decreased in mice treated with Egr3 antisense oligos on day-7 of withdrawal protocol. Each point represents the mean ± SEM of data from six to eight animals. *p<0.05 vs. diazepam- or flumazenil-treated (non-withdrawal) control group; #p<0.05 vs. diazepam- or flumazenil-treated neurosteroid withdrawal-missense group. AD, afterdischarge duration.
DISCUSSION
The current study present the first direct evidence distinguishing between the potential roles of two signaling pathways of PR and Egr3 in regulation of GABA-A receptor α4-subunit expression in a mouse model of neurosteroid withdrawal. The principal findings of the study are: (i) Neurosteroid withdrawal induced increase in α4-subunit expression is similar in wild-type and PR knockout mice; (ii) Neurosteroid withdrawal induces expression of Egr3 to a similar extent in wild-type and PR knockout mice; (iii) Antisense inhibition of Egr3 expression blocks the neurosteroid withdrawal-induced upregulation of the α4-subunit; (iv) Withdrawal from neurosteroids increases seizure activity in the kindling model; (v) Neurosteroid withdrawal causes a marked reduction in the antiseizure sensitivity of diazepam in wild-type and PR knockout mice, but confers increased seizure protection of flumazenil; and (vi) Antisense inhibition of Egr3 expression prevents the neurosteroid withdrawal-induced diazepam insensitivity and flumazenil efficacy against kindling seizure protection. Taken together, these observations indicate that neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of PR-independent Egr3 signaling pathway (see Fig.4A). Because neurosteroid withdrawal is linked to increased seizure susceptibility, these findings are highly relevant to the molecular basis of perimenstrual catamenial epilepsy and may provide new therapeutic approaches for preventing or inhibiting these seizures in women at risk. Changes in GABA-A receptor subunit composition are also evident in the pathogenesis of perimenstrual anxiety (Smith et al., 2007), alcoholism (Grobin et al., 2001), stress (Orchinik et al., 1995), and postpartum depression (Maguire and Mody, 2008).
Progesterone and neurosteroid regulation of GABA-A receptor plasticity
Catamenial epilepsy affects about 70% of women with epilepsy. There are currently no specific approved treatments to prevent seizure exacerbations in this condition. Understanding the molecular mechanisms underlying the cyclical seizure exacerbations is a prerequisite for designing targeted treatments for catamenial epilepsy. In perimenstrual catamenial epilepsy, the most common form, there is emerging evidence to suggest that progesterone withdrawal plays a key role in seizure exacerbations that occur around the time of menstruation (Backstrom 1976; Bonucelli et al., 1989; Rogawski and Reddy, 2004; Scharfman and McLusky, 2006). Progesterone has anticonvulsant properties (Selye, 1942; Herzog, 1995). These actions result from its conversion to the neurosteroid allopregnanolone, which is a potent GABA-A receptor modulator with antiseizure properties (Kokate et al., 1999; Frye et al., 2002; Reddy et al., 2004). During the menstrual cycle, circulating progesterone levels are low in the follicular phase, but rise in the midluteal phase for approximately 10 to 11 days, before declining in the late luteal phase. Circulating allopregnanolone levels parallel those of its parent progesterone (Tuveri et al., 2008). Although the dynamics of brain allopregnanolone during the menstrual cycle have not been studied, it is likely that local synthesis of GABA-A receptor modulating neurosteroids occur in regions relevant to epilepsy such as the cortex, hippocampus, and amygdala (Ebner et al., 2006; Agis-Balboa et al., 2006; Mukai et al., 2008). The results from the present study demonstrate a striking increase in seizure activity following neurosteroid withdrawal in a widely used animal model of epilepsy, the hippocampus kindling model. The seizure exacerbation was maximal 24 h after withdrawal and declined to control value at 48 h following withdrawal onset. We have previously published similar results obtained after neurosteroid withdrawal in the pseudopregnancy model (Reddy et al., 2001), and a transitory increase in the frequency of spontaneous seizures in epileptic rats (Reddy and Zeng, 2007a). Such heightened seizure susceptibility is consistent with previous reports in related models of neurosteroid withdrawal (Smith et al., 1998a; Moran and Smith, 1998a,b). The basis for the enhanced seizure susceptibility in perimenstrual catamenial epilepsy is not completely understood, but may be due to the withdrawal of neurosteroids around the time of menstruation.
The GABA-A receptor composition undergoes dynamic plasticity in response to physiological signals, the hormonal (neurosteroid) milieu, and exogenously administered agents such as benzodiazepines or ethanol (Smith et al., 2007). The precise changes in brain GABA-A receptor subunit composition occurring during the human menstrual cycle or in animal models of catamenial epilepsy have not been determined. There is strong evidence that ovarian cycle-linked fluctuations in neurosteroids modulate the GABA-A receptor plasticity (Maguire et al., 2005). Our observation that neurosteroid withdrawal increases the α4-subunit expression in the hippocampus of female mice is consistent with previous studies demonstrating that prolonged exposure to progesterone followed by withdrawal in female rats causes increased expression of the α4-subunit in the hippocampus and amygdala (Smith et al., 1998a,b; Gulinello et al., 2001). At 24 h following neurosteroid withdrawal, there was a 3-fold upregulation in α4-subunit transcription. Similar increases in α4-subunit levels have been observed previously in response to withdrawal from allopregnanolone (Concas et al., 1998; Smith et al., 1998b; Follesa et al., 2000) and synthetic neurosteroids (Mascia et al., 2002). Enhanced α4-subunit expression is associated with a compensatory reduction in α1 expression (Shen et al., 2005). We did not observe significant changes in α1, β2 and γ2 subunits. There is a transitory rise in α4-subunit expression during neurosteroid exposure (Gulinello et al., 2001). However, periods of exposure to neurosteroids longer than 72 hours followed by withdrawal is required for a dramatic rebound rise in α4-subunit expression. Although α4 can coassemble with γ2 to form synaptic GABA-A receptors, it preferentially coassembles with δ to form perisynaptic/extrasynaptic GABA-A receptors (Sur et al., 1999). The key consequence of the incorporation of the normally low abundance α4-subunit into synaptic GABA-A receptors is that currents generated by these receptors have accelerated decay kinetics, so that there is less total charge transfer, which likely results in reduced net synaptic inhibition and a state of hyperexcitability (Smith and Gong, 2005). The increase in α4 when coassembled with either γ2 or δ subunits in the hippocampus may result in benzodiazepine-insensitive receptors (Shen et al., 2005; Maguire et al., 2005). Therefore, when neurosteroids are withdrawn at the time of menstruation, the α4-subunit is upregulated and synaptic inhibition is diminished, resulting in enhanced excitability, which, among other effects, causes a predisposition to catamenial seizures. The specific molecular mechanism by which neurosteroid withdrawal leads to α4-subunit upregulation and seizure susceptibility remains unclear.
Role of PR pathway in neurosteroid regulation of α4-subunit expression
There is strong evidence that the perimenstrual form of catamenial epilepsy is due to the withdrawal of progesterone that occurs at the time of menstruation (Moran and Smith, 1998a; Smith et al., 1998b). The reproductive functions of progesterone are mediated via its interaction with PRs, expressed in A and B forms encoded by the same gene (Conneely et al., 2003). The PRs are expressed widely in the brain, including areas relevant to epilepsy such as the hippocampus, amygdala, and neocortex (Brinton et al., 2008). We have previously demonstrated that the seizure protection conferred by progesterone is not due to its interaction with PRs (Reddy et al., 2004), and PRs are not required for the anxiolytic effects of allopregnanolone and related neurosteroids (Reddy et al., 2005; Reddy and Zeng, 2007b). The key observation in this study is that the neurosteroid withdrawal-induced upregulation of α4-subunit transcription is undiminished in PR knockout mice, thus clearly proving that the PR is not involved in neurosteroid withdrawal regulation of α4-subunit expression. However, these findings apply only to the specific withdrawal paradigm used in this study. These results do not apply to other subunits as we did not verify the extent to which PR affects their expression in the hippocampus.
We observed that neurosteroid withdrawn mice were strikingly less sensitive to the antiseizure effects of diazepam in the kindling model than non-withdrawal control animals. PR knockout animals were also less sensitive to the protective actions of diazepam during neurosteroid withdrawal. These observations are highly consistent with the enhanced abundance of α4-subunit in vivo in the hippocampus. Receptors containing the α1/2/3/5-subunits in combination with any of the β-subunits and the γ2-subunit are most prevalent in the brain (Mohler et al., 2002). These receptors are sensitive to benzodiazepine modulation. However, receptors containing the α4-subunit are generally expressed at a very low abundance in the dentate gyrus and thalamus (Pirker et al., 2000), but are benzodiazepine insensitive (Whiting et al., 2000; Mohler et al., 2002). Diazepam exhibits reduced protection (insensitivity) against seizures during neurosteroid withdrawal. Because diazepam had similar antiseizure potencies in wild-type and PR knockout mice, we conclude that the PR does not contribute to its insensitivity observed following neurosteroid withdrawal. The benzodiazepine site profile of α4βγ-receptors is characterized by a low-affinity for flumazenil, which is a standard antagonist at α1 βγ-preceptors. Flumazenil is inactive in the kindling and other animal epilepsy models (Albertson and Walby, 1986; Hansen et al., 2004). We observed that neurosteroid withdrawal conferred increased protection to flumazenil against hippocampus kindled-seizures. The submaximal efficacy despite high doses of flumazenil suggest partial protection, which is consistent with the possibility of increased abundance of α4-subunit in vivo in the hippocampus. Similar increases in α4-subunit and its pharmacological properties have been described after withdrawal from progesterone (Smith et al., 1998b) and ethanol (Devaud et al., 1997).
Although the PR knockout mouse represents a robust genetic model for studies of PR functions, there are some caveats akin to constitutive transgenic models. The main concern is that the PR knockout mouse model may have development and/or compensatory changes due to lack of PRs from the embryonic stages. However, PR knockout mice grow normally to adulthood and respond normally to progesterone and neurosteroids except for some reproductive defects (Lydon et al., 1995; Reddy et al., 2004). Serum levels of progesterone, estrogen and gonadotropins in PR knockout mice are similar to wild-type mice (Chappell et al., 1997). Histological analysis showed normal architecture of hippocampal, amygdala, neocortex and hypothalamic regions in PR knockout mice (Reddy et al., 2005). PR knockout mice are deficient in PRs in the brain, but compensatory changes in levels of progesterone or neurosteroids, and hippocampal architecture are not evident.
Novel role of Egr3 pathway in neurosteroid regulation of α4 subunit expression
Egr3 is a member of early growth response genes expressed throughout the brain. It is proposed to play an important role in synaptic plasticity (Gallitano-Mendel et al., 2007). Egr3 binds to the GABA-A receptor α4 promoter and regulate its transcription (Roberts et al., 2005). Consequently, it has been shown to be activated in the hippocampus in response to prolonged seizures (Yamagata et al., 1994; Roberts et al., 2005). We studied changes in Egr3 expression in the mouse model of neurosteroid withdrawal. Our results showed that neurosteroid withdrawal is associated with a dramatic rise in Egr3 levels in the hippocampus. Furthermore, such an upregulation is also evident in PR knockout mice, providing strong evidence that neurosteroid withdrawal activates the Egr3 pathway in a PR-independent fashion. In the current study, reducing Egr3 levels in mice by antisense RNA treatment prevented the increase in α4-subunit in response to neurosteroid withdrawal. These findings are consistent with a role for Egr3 as a transcription regulator of α4-subunit. This mechanism has been identified for α4-subunit expression following neural injury by status epilepticus (Roberts et al., 2005). Moreover, our results demonstrated that antisense suppression of the Egr3 pathway prevents the pharmacological properties of withdrawal such as (a) increased seizure activity; (b) the diazepam insensitivity observed behaviorally and electrographically in the kindling model; and (c) the increased seizure protection of flumazenil in the kindling model. Overall, these findings suggests that specific Egr3-mediated increases in α4-subunit may account for these withdrawal properties of neurosteroids in the mouse paradigm.
Although our study is conclusive on the above findings, there are certain caveats that must be considered in regard to the interpretation of the findings. First, the results apply only to the perimenstrual-type withdrawal model used in this study. Second, our results are limited to the hippocampus, which is a critical region for epilepsy, while other limbic structures, such as the amygdala, are also important for seizure susceptibility (Fritsch et al., 2009). Third, the GABA-A receptor functional changes were not directly measured in neurons from neurosteroid-withdrawn animals. Nevertheless, our pharmacological observations are highly consistent with prior electrophysiological studies demonstrating that neurosteroid withdrawal accelerates decay kinetics and thereby reduces net synaptic inhibition (Smith and Gong, 2005), decreases lorazepam potentiation of GABA currents (Smith et al., 1998a,b), and increases flumazenil potentiation of GABA-gated currents in CA1 neurons (Smith et al., 1998a,b).
The upregulation of the transcription factor Egr3 is likely composes only a part of the cascade of signaling events that are initiated by neurosteroid withdrawal. The hyperexcitability may be an important factor for stimulation of Egr signaling. The Egr3 signaling pathway has been shown to affect GABA-A receptor subunit expression following specific signals (Roberts et al., 2006). Egr3 knockout mice have 50% less α4-subunit and also display abnormalities in nervous system development (Eldgredge et al., 2008). The Egr pathways regulate a myriad of genes with diverse functions in a cell-and region-specific fashion, but there is little information about the upstream and downstream components of the Egr3 pathway. Egr3 may serve as a signal effector for endogenous steroids, nerve growth factor and brain-derived neurotrophic factor (Mercier et al., 2001; Lund et al., 2008), which are known to affect GABA-A receptor expression.
In conclusion, these results demonstrate the role of the PR and Egr3 pathways in neurosteroid withdrawal regulation of the GABA-A receptor α4 subunit expression in the hippocampus and seizure activity in an animal model of epilepsy. The upregulation of Egr3-linked α4 subunit following neurosteroid withdrawal may represent a molecular mechanism underlying changes in neuronal excitability, drug sensitivity, and heightened seizure susceptibility observed in conditions associated with neurosteroid withdrawal, such as that in women with perimenstrual catamenial epilepsy.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health, NINDS grant R01 NS051398 (to D.S.R.). We thank Dr. A. Leslie Morrow for advice with Western blot studies.
ABBREVIATIONS
- ADT
afterdischarge threshold
- GABA
γ-aminobutyric acid
- ED50
effective dose that produce seizure protection in 50% of animals
- Egr3
early growth response factor-3
- ICV
intracerebroventricular
- PR
progesterone receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson TE, Walby WF. Effects of the benzodiazepine antagonists RO-15-1788, CGS-8216 and PK-11195 on amygdaloid kindled seizures and the anticonvulsant efficacy of diazepam. Neuropharmacology. 1986;25:1205–1211. doi: 10.1016/0028-3908(86)90137-1. [DOI] [PubMed] [Google Scholar]
- Albright PS, Burnham WM. Development of a new pharmacological seizure model: effects of anticonvulsants on cortical- and amygdala-kindled seizures in the rat. Epilepsia. 1980;21:681–689. doi: 10.1111/j.1528-1157.1980.tb04321.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA-A receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–106. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA-A receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lydon JP, Conneely OM, O'Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Folesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA-A receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Ebner MJ, Corol DI, Havlíková H, Honour JW, Fry JP. Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology. 2006;147:179–190. doi: 10.1210/en.2005-1065. [DOI] [PubMed] [Google Scholar]
- Eldredge LC, Gao XM, Quach DH, Li L, Han X, Lomasney J, Tourtellotte WG. Abnormal sympathetic nervous system development and physiological dysautonomia in Egr3-deficient mice. Development. 2008;135:2949–2957. doi: 10.1242/dev.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Pellow S. Intrinsic actions of the benzodiazepine receptor antagonist Ro 15–1788. Psychopharmacology. 1986;88:1–11. doi: 10.1007/BF00310505. [DOI] [PubMed] [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABA-A receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci. 1998;10:2905–2912. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA-A receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. first edition. Academic Press; 1997. [Google Scholar]
- Fritsch B, Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Rogawski MA, Braga MF. Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis. Neuroscience. 2009;163:415–429. doi: 10.1016/j.neuroscience.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Walf A, Harney J. Progesterone reduces pentylenetetrazol-induced ictal activity of wild-type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;43 Suppl. 5:14–17. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, Wozniak DF, Milbrandt J. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007;148:633–643. doi: 10.1016/j.neuroscience.2007.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. The optimization of TaqMan real-time RT-PCR assay for transcriptional profiling of GABA-A receptor subunit plasticity. J Neurosci Methods. 2009;181:58–66. doi: 10.1016/j.jneumeth.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Fritschy JM, Morrow AL. Chronic ethanol administration alters immunoreactivity for GABA-A receptor subunits in rat cortex in a region-specific manner. Alcohol Clin Exp Res. 2001;24:1137–1144. [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA-A receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Sperling BB, Sanchez C. Anticonvulsant and antiepileptogenic effects of GABA-A receptor ligands in pentylenetetrazol-kindled mice. Pro Neuro-Psychopharmacol Biol Psychiatr. 2004;28:105–113. doi: 10.1016/j.pnpbp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the GABA-A receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288:679–684. [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Korpi ER, Gründer G, Lüddens H. Drug interactions at GABA-A receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABA-A receptor alpha1 subunit knockout mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABA-A receptors containing α1 and α4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochemistry. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABA-A receptor transcription by activation of the JAK/STAT pathway. Science Signal. 2008;1:1–14. doi: 10.1126/scisignal.1162396. (41, ra9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABA-A receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA-A receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA-A receptor plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABA-A receptor plasticity. Psychoneuroendocrinology. 2009;34 Suppl 1:S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA-A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Biggio F, Mancuso L, Cabras S, Cocco PL, Gorini G, Manca A, Marra C, Purdy RH, Follesa P, Biggio G. Changes in GABA-A receptor gene expression induced by withdrawal of, but not by long-term exposure to, ganaxolone in cultured rat cerebellar granule cells. J Pharmacol Exp Ther. 2002;303:1014–1020. doi: 10.1124/jpet.102.040063. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Morrisett R, Nadler JV. Recent advances in understanding mechanisms of the kindling model. Adv Neurol. 1992;57:555–560. [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Mercier G, Turque N, Schumacher M. Early activation of transcription factor expression in Schwann cells by progesterone. Mol Brain Res. 2001;97:137–148. doi: 10.1016/s0169-328x(01)00311-4. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Therap. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Moran MH, Goldberg M, Smith SS. Progesterone withdrawal. II: insensitivity to the sedative effects of a benzodiazepine. Brain Res. 1998a;807:91–100. doi: 10.1016/s0006-8993(98)00781-1. [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith SS. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998b;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Mukai Y, Higashi T, Nagura Y, Shimada K. Studies on neurosteroids XXV : Influence of a 5α-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol Pharm Bull. 2008;31:1646–1650. doi: 10.1248/bpb.31.1646. [DOI] [PubMed] [Google Scholar]
- O’Donovan KJ, Wilkens EP, Baraban JM. Sequential expression of Egr-1 and Egr-3 in hippocampal granule cells following electroconvulsive stimulation. J Neurochem. 1998;70:1241–1248. doi: 10.1046/j.1471-4159.1998.70031241.x. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Weiland NG, McEwen BS. Chronic exposure to stress levels of corticosterone alters GABA-A receptor subunit mRNA levels in rat hippocampus. Mol Brain Res. 1995;34:29–37. doi: 10.1016/0169-328x(95)00118-c. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA-A receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;14:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Therap. 2000;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:303–310. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulates GABAA receptor function and seizure susceptibility. J Neurosci. 2002;42:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010;89:254–260. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Zeng YC. Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J. 2007a;21:885.14. [Google Scholar]
- Reddy DS, Zeng YC. Differential anesthetic activity of ketamine and the GABAergic neurosteroid allopregnanolone in mice lacking progesterone receptor A and B subtypes. Methods Find Exp Clin Pharmacol. 2007b;29:659–664. doi: 10.1358/mf.2007.29.10.1147766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- Roberts DS, Raol YH, Bandyopadhyay S, Lund IV, Budreck EC, Passini MA, Wolfe JH, Brooks-Kayal AR, Russek SJ. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA-A receptor α4 subunit expression. Proc Natl Acad Sci USA. 2005;102:11894–11899. doi: 10.1073/pnas.0501434102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Reddy DS. Neurosteroids: endogenous modulators of seizure susceptibility. In: Rho JM, Sankar R, Cavazos JE, editors. Epilepsy: Scientific Foundations of Clinical Practice. New York: Marcel Dekker; 2004. pp. 319–355. [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Rigoulot MA, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol. 2005;196:73–86. doi: 10.1016/j.expneurol.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (metrazol) J Lab Clin Med. 1942;27:1051–1053. [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α.4βδ GABA-A receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH. Neurosteroid administration and withdrawal alter GABA-A receptor kinetics in CA1 hippocampus of female rats. J Physiol. 2005;564(Pt 2):421–436. doi: 10.1113/jphysiol.2004.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA-A receptor α4-subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α -pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABA-A receptor α.4-subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA-A receptors: Focus on the α4 and δ subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and γ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Whiting P, Wafford KA, McKernan RM. Pharmacologic subtypes of GABA-A receptors based on subunit composition. In: Martin DL, Olsen RW, editors. GABA in the Nervous System: The View at Fifty Years. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 113–126. [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA-A receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Kaufmann WE, Lanahan A, Papapavlou M, Barnes CA, Andreasson KI, Worley PF. Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learn Mem. 1994;1:140–152. [PubMed] [Google Scholar]