Abstract
Stroke is a leading cause of death and disability but has limited therapeutic options. Thiazolidinediones (TZDs), agonists for the nuclear receptor, peroxisome proliferator-activated receptor (PPAR)γ, reduce infarct volume and improve neurologic function following transient middle cerebral artery occlusion (MCAO) in rats. Translation of these findings into clinical therapy will require careful assessment of dosing paradigms and effective time windows for treatment. Understanding the mechanisms by which TZDs protect the brain provides insight into how time windows for neuroprotection might be extended. We find that two TZDs, pioglitazone and rosiglitazone, significantly reduce infarct volume at doses similar to those used clinically (1 mg/kg for pioglitazone and 0.1 mg/kg for rosiglitazone). We also find that pioglitazone reduces infarction volume in a transient, but not a permanent MCAO model suggesting that reperfusion plays an important role in TZD mediated neuroprotection. Since PPARγ agonists reduce inflammation and oxidative stress, both of which are exacerbated by reperfusion, we hypothesized that TZDs would be most effective if administered prior to reperfusion. We administered TZDs three hours after MCAO and found that infarction volume and neurologic function are significantly improved in animals reperfused at three hours and fifteen minutes (after TZD treatment), but not in animals reperfused at two hours (before TZD treatment) when assessed either twenty-four hours or three weeks after MCAO. While TZDs reduce intercellular adhesion molecule (ICAM) expression to a similar extent regardless of the time of reperfusion, leukocyte entry into brain parenchyma is more dramatically reduced when reperfusion is delayed until after drug treatment. The finding that delaying reperfusion until after TZD treatment is beneficial despite a longer period of ischemia, is dramatic given the widely held view that duration of ischemia is the most important determinate of injury.
Introduction
The only FDA approved therapy for ischemic stroke is early reperfusion using thrombolytic medication. Although reperfusion is critical to restore blood flow to ischemic tissue, it is also associated with the induction of oxidative stress and a robust inflammatory response that can further exacerbate injury. Numerous agents targeting these processes are protective in animal models; however, translation to effective clinical therapy remains elusive. Treatment of stroke is particularly challenging because of the rapid pace of injury, and it is widely believed that the failure to translate laboratory findings into clinical therapy is due to the difficulty in administering drugs before irreversible injury occurs. Drugs with the most therapeutic potential will be those that can be given to patients quickly, preferably, those that can be administered prior to hospital evaluation. Understanding the time window for therapy will be critical to successful translation of neuroprotective therapy for stroke.
TZDs are PPARγ agonists that we have found reduce infarct volume and improve neurologic function following cerebral ischemia in rats (Sundararajan et al., 2005; Victor et al., 2006). These findings have been validated by several independent laboratories (Allahtavokoli et al., 2006; Luo et al., 2006; Pereira et al., 2006; Shimazu et al., 2005; Tureyen et al., 2007; Zhao et al., 2005). PPARγ forms a heterodimer with RXR and binds a PPAR response element (PPRE) in the promoter of target genes inducing gene expression. In addition, activated PPARγ suppresses inflammatory gene expression by transrepression of other transcription factors. In the presence of ligand, PPARγ binds small ubiquitin-like modifier (SUMO1) and stabilizes the co-repressor complex on the promoter of pro-inflammatory genes preventing the transcription factor, NFκB, from binding to the promoter and initiating pro-inflammatory gene expression (Straus and Glass, 2007). In ischemic stroke models, TZD-mediated neuroprotection is associated with reduced inflammatory infiltrate and pro-inflammatory gene expression (Allahtavokoli et al., 2006; Luo et al., 2006; Pereira et al., 2006; Shimazu et al., 2005; Sundararajan et al., 2005; Tureyen et al., 2007; Zhao et al., 2005). In addition, PPARγ agonists reduce the formation of superoxide anion in vascular endothelial cells and increase the expression of the free radical scavengers superoxide dismutase and catalase (Hwang et al., 2007; Shimazu et al., 2005). Reductions in both inflammation and oxidative stress likely contribute to PPARγ agonist mediated neuroprotection.
TZDs act as insulin sensitizers and two drugs, pioglitazone and rosiglitazone, are FDA approved for treatment of type 2 diabetes. The most serious side effect, congestive heart failure, occurs after several weeks of daily use and is reversed after discontinuation of the drug (Tang and Maroo, 2009). It is unlikely that congestive heart failure would be a consequence of a single dose of TZD. Importantly, both rosiglitazone and prostaglandin J2 (PGJ2), an endogenous PPARγ ligand, are beneficial in a rodent hemorrhage model (Zhao et al., 2007; Zhao et al., 2006) suggesting that PPARγ ligands might be given safely before differentiating cerebral ischemia and hemorrhage by CT scanning, thereby allowing TZDs to be given before hospital evaluation.
In the current study we explore optimal TZD dosing and the therapeutic time window of efficacy following MCAO using the suture model of proximal MCAO in rats. We confirm previous findings that pioglitazone is protective in transient but, not permanent ischemia. In addition, we formally test the hypothesis that TZDs are most effective when administered prior to reperfusion by administering TZDs 3 hours after MCAO and varying the time of reperfusion relative to MCAO. Outcome, assayed by both infarction volume and behavioral function, is improved in drug treated animals that are reperfused after drug treatment despite the longer duration of ischemia. Finally, we examined leukocyte infiltration, a feature of reperfusion injury using real time PCR to examine ICAM mRNA and immunohistochemistry to examine ICAM and myeloperoxidase protein.
Experimental Procedures
Rats
Male Wistar rats, 250–300g, were obtained from Charles River (Wilmington, MA). Animals were housed and cared for in the Animal Resource Center and allowed free access to food and water before and after surgery. All procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University and in accordance with the guidelines specified in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Transient Middle Cerebral Artery Occlusion
MCAO was performed using the suture method as described by Longa (Longa et al., 1989), modified by Kawamura (Kawamura et al., 1994) and previously described by ourselves (Sundararajan et al., 2005). All surgery was performed using continuously inhaled short acting anesthetics delivered by nose cone. Initial experiments used halothane, however, halothane was no longer available in the US when the experiments utilizing three week survival were conducted and, therefore, those experiments utilized isoflurane. The tail artery was cannulated to measure arterial blood pressure. A small hole was drilled in the skull using a dental drill at the intersection of the coronal and saggital sutures on the left over the region of the MCA. A probe holder (PH07-4; Perimed AB, Jarfalla, Sweden) was glued into place in order to ensure constant probe position over time. A laser Doppler probe (407; Perimed AB, Jarfalla, Sweden) was inserted into the probe holder and cerebral blood flow measured using the Periflux 5000 system and Perisoft software (Perimed AB, Jarfalla, Sweden). A vertical incision was made in the neck, the common carotid artery was tied with a reversible knot and the external carotid artery was isolated and ligated. A 4.0 nylon suture with a flame rounded tip was advanced from the external carotid artery into the internal carotid artery to occlude the MCA while cerebral blood flow was monitored. Following suture insertion, the laser Doppler probe was removed, although the probe holder was left in place. The wounds were closed and the inhaled anesthetic gas removed, allowing the animals to regain consciousness within a few minutes. At the time of reperfusion, which varied depending on the experiment, rats were reanesethetized, the neck wound was reopened, the laser Doppler probe reinserted into the probe holder and suture placement confirmed. Cerebral blood flow was measured and the suture was removed. The ligature on the common carotid artery was released. Reperfusion of the artery was confirmed visually in the neck and using cerebral blood flow within the brain. The laser dopper probe and the probe holder were removed, all wounds were closed and the animals allowed to recover. Arterial blood pressure and arterial blood gases were monitored and kept within normal parameters throughout the procedure. Temperature was maintained between 36.5 and 37.5°C during the procedure. In accordance with STAIR guidelines (Stroke Therapy Academic Industry Roundtable, 1999) only animals with drops of greater than 60% CBF at the time of MCAO were included in the analysis. In addition, animals with no increase in CBF at the time of suture removal (no reperfusion), infarction in the anterior cerebral artery (ACA) distribution (indicating improper suture placement) or obvious sub-arachnoid blood (due to vessel perforation) were excluded from analysis as they represent surgical failures. The number of animals excluded from analysis for surgical failures was equivalent in different treatment groups (Table 1).
Table 1.
Animals excluded from analysis for experiments with 21 day survival (75 animals lesioned)
| Non-surgical deaths | ||
| DMSO | 2 hour MCAO | 2 |
| DMSO | 3.25 hour MCAO | 5 |
| Pioglitazone | 2 hour MCAO | 1 |
| Pioglitazone | 3.25 hour MCAO | 1 |
| Less than 60% CBF drop at MCAO | ||
| DMSO | 2 hour MCAO | 1 |
| DMSO | 3.25 hour MCAO | 1 |
| Pioglitazone | 2 hour MCAO | 2 |
| Pioglitazone | 3.25 hour MCAO | 2 |
| No Reperfusion | ||
| DMSO | 2 hour MCAO | 1 |
| DMSO | 3.25 hour MCAO | 0 |
| Pioglitazone | 2 hour MCAO | 1 |
| Pioglitazone | 3.25 hour MCAO | 2 |
| Sub-arachnoid hemorrhage | ||
| DMSO | 2 hour MCAO | 2 |
| DMSO | 3.25 hour MCAO | 3 |
| Pioglitazone | 2 hour MCAO | 2 |
| Pioglitazone | 3.25 hour MCAO | 3 |
| ACA + MCA infarction | ||
| DMSO | 2 hour MCAO | 1 |
| DMSO | 3.25 hour MCAO | 0 |
| Pioglitazone | 2 hour MCAO | 1 |
| Pioglitazone | 3.25 hour MCAO | 1 |
Permanent Middle Cerebral Artery Occlusion
The procedure for permanent MCAO was identical to that of transient MCAO except that suture was not removed and the MCA, therefore, not reperfused.
Drug Treatment
Animals were treated with either DMSO 0.4 ml/kg, pioglitazone (Takeda Pharmaceuticals North America, Lincolnshire, IL) or rosiglitazone (GlaxoSmithKline Pharmaceuticals, Harlow, England) dissolved in 0.4ml/kg DMSO. All drug treatments were administered interperitoneally (IP). While initial experiments utilized a pretreatment paradigm in order to efficiently define optimal dosing and treatment protocols, subsequent experiments examining neuroprotection time windows utilized TZD treatment three hours after MCAO. Dose response studies tested pioglitazone (0.5, 1, 3.5 and 7 mg/kg) or rosiglitazone (0.05, 0.1, 0.35, 1 mg/kg) administered 24 hours before and at the time of MCAO. The maximally effective doses of 1mg/kg pioglitazone and 0.1mg/kg rosiglitazone were utilized in all other studies.
Prolonged Arterial Blood Pressure Monitoring
All animals had systemic blood pressure monitored by canulating the tail artery. A subset of animals was allowed to recover from anesthesia following canulation of the artery, while being restrained to avoid removal of the arterial line. Baseline blood pressure was measured and rats were then injected with either DMSO or rosiglitazone (0.1 mg/kg). Arterial blood pressure was then measured each hour for a total of five hours.
Prolonged Cerebral Blood Flow Monitoring
While all animals underwent monitoring for CBF with special attention paid to changes at the time of occlusion and the time of reperfusion (see description in transient MCAO procedure above), a subset of animals were monitored continuously over a five hour period. These animals had baseline CBF measured using the Perimed 5000 system and Perisoft software (Perimed AB, Jarfalla, Sweden) and were then injected with either DMSO or pioglitazone. The level of anesthesia was carefully controlled by continual monitoring of response to pain or other stimuli in order to minimize any effects of anesthesia on CBF.
Euthanasia and Tissue Preparation
Rats were anesthetized until breathing ceased. They then underwent intracardiac perfusion first with PBS and then with 4% paraformaldehyde. Brains were isolated and immersion fixed in 4% paraformaldehyde overnight. Tissue was cryoprotected in 30% sucrose in PBS. Approximately five 20μm frozen sections were collected every 1000μm beginning at the level of the forceps minor corpus callosum and extending through the hippocampus and were placed aside for infarct volume determination. At the level of the striatum, where the majority of the tissue in the sections is in the MCA distribution, 10μm sections were collected to be used for immunohistochemistry. These 10μm sections were collected so that adjacent sections were on separate slides.
Infarct Volume Determination
Twenty micron sections were stained with thionin and the area of infarction calculated using Bioquant computer software (R&M Biometrics, Nashville, TN) by subtracting the uninfarcted tissue in the ischemic hemisphere from the volume of the contralateral hemisphere. This method corrects for post-ischemic edema.
Immunohistochemistry
We examined the expression of ICAM, which mediates leukocyte infiltration and of myeloperoxidase, a marker of leukocytes, within the brains of treated animals since leukocytes are a potential source of reperfusion injury. Ten micron sections were labeled using either mouse anti-ICAM (Serotec, UK) or rabbit anti-myeloperoxidase (Dako Cytomation, Denmark) antibodies in 5% rat serum. Primary antibodies were visualized using either goat anti-mouse conjugated to Oregon Green or goat anti-rabbit conjugated to Oregon-Green (Molecular Probes, Eugene, OR) in 5% rat serum. Adjacent sections were incubated without primary antisera to control for non-specific binding of the secondary antiserum. Sections were viewed using a fluorescent microscope and images acquired and stored using Spot Advanced imaging software (Spot Image Corporation, Chantilly, VA). Some sections were also imaged using confocal microscopy. ICAM and myeloperoxidase immunolabeling was quantified from digital photographs of high powered fields (HPF; for ICAM each HPF represented 87.5μm2; for myeloperoxidase each HPF represented 350μm2) covering a representative section and was analyzed in Adobe Photoshop. For ICAM quantification a grid was placed over the images and the number of intersections by immunoreactive cells with the grid was counted. The average number of intersections per animal was plotted. All myeloperoxidase-IR cells were counted and the average number of immunoreactive cells per animal plotted. No adjustments were made to these images prior to counting the number of cells to ensure that all images were treated in the same manner. Images shown in the figures have been adjusted for contrast and brightness.
Neurologic Behavior
A battery of neurologic tests was utilized to assess neurologic function including the modified Neurological Severity Score (mNSS), the inclined plane and the cylinder test. All animals were randomly assigned to treatment group prior to the day of surgery by an investigator unaware of their performance during behavioral training. All animals underwent training for a minimum of three days prior to MCAO to familiarize them with the investigator and the behavioral assays. This also served to ensure normal performance on all tasks prior to MCAO, and in the case of the cylinder test, to establish baseline use of both forearms. Training and post-MCAO behavioral assessments were performed by the same investigator, who was not involved in surgery or drug administration. This investigator was blinded to treatment assessment throughout the period of behavioral assessments.
The mNSS examines a rat’s ability to walk correctly on both the floor and the balance beam, sense visual and tactile stimuli, as well as testing proprioception and reflex movements (Chen et al., 2001). A maximal score of 18 is awarded, although in our experience 24 hours after MCAO most rats with full MCA infarctions have a mNSS of approximately 12. In the inclined plane test rats walk on an incline and the angle at which they begin to slip and to fall are recorded (Yonemori et al., 1998). Normal animals tolerate an angle of approximately 40°, while unprotected, ischemic rats tolerate an incline of approximately 30° 24 hours after MCAO.
The cylinder test was used to assess preferential use of the affected versus the unaffected forepaw following MCAO (Schallert et al., 2000). Rats were observed in a glass cylinder (12 inch diameter) and the number of times they reared up and touched the glass using either the left forepaw first, the right forepaw first or both forepaws together, was recorded. They were observed for ten minutes or until they had touched the glass at least 10 times, whichever occurred first. In the case of severely affected rats, rearing was limited. Data points were included in analysis only if at least three rearing movements were made. All of the data points that were excluded due to an insufficient number of rears were from days 1–15, and the vast majority were from the first week following MCAO. Use of the right forepaw was calculated by using the number of times the rat touched the glass using the right forepaw only plus one half the number of times they touched the glass using both forepaws. Use following MCAO was corrected for any pre-existing bias by subtracting the average pre-MCAO use of the right forepaw from the post-MCAO use (Schallert et al., 2000).
Real Time PCR
Rat brains were removed immediately following euthanasia. A coronal slice, approximately 3mm thick, through the area of infarction was cut and homogenized in Trizol (Gibco BRL, Rockville, MD). The remaining tissue was placed in 2, 3, 5 triphenyl tetrazolium chloride (TTC; Sigma, St. Louis, MO) in order to confirm infarction. RNA isolation and cDNA synthesis, and PCR amplifications were performed as previously described (Sundararajan et al., 2005). Amplification was performed using an I-Cycler detection system (BioRad Laboratories, Hercules, CA) with SYBR Green I. ICAM primer (Integrated DNA Technology Inc. Coralville, IA) sequences were as follows: sense (5′GAG TCT CCC AGC ACC AGC AT 3′ sense (5′ ATT TAG GCA TGG TGG TTG ACA TT 3′). Beta actin primer (β-actin; Integrated DNA Technology Inc. Coralville, IA) sequences were: sense (5′ AGA GGG AAA TCG TGC GTG AC 3′) and anti-sense (5′ CCA TAG TGA TGA CCT GTC CGT 3′). Each PCR amplification was performed in triplicate wells, using the following conditions: 3 minutes at 95°C, 30 seconds at 95°C and 30 seconds at 60°C through 40 cycles, followed by 2 cycles of 30 seconds at 72°C and 1 minute at 60°C. Following amplification melt curve analysis ensured primer dimers were not present. Threshold cycle (Ct) values were calculated by determining the point at which emitted fluorescence exceeded the baseline plus ten times baseline standard deviation. Relative expression of the mRNA for ICAM was expressed as the ratio of the mRNA to the housekeeping gene, β-actin.
Statistical Analysis
Statistical analysis utilized the Mann-Whitney U test for most comparisons as the distribution of data was not normal. One way ANOVA was used to compare effects of TZDs and DMSO effects on blood pressure and cerebral blood flow over time. An alpha level of 0.05 was considered to be significant for all comparisons. All calculations were done using SPSS 17 software.
Results
Dose response curves for pioglitazone and rosiglitazone
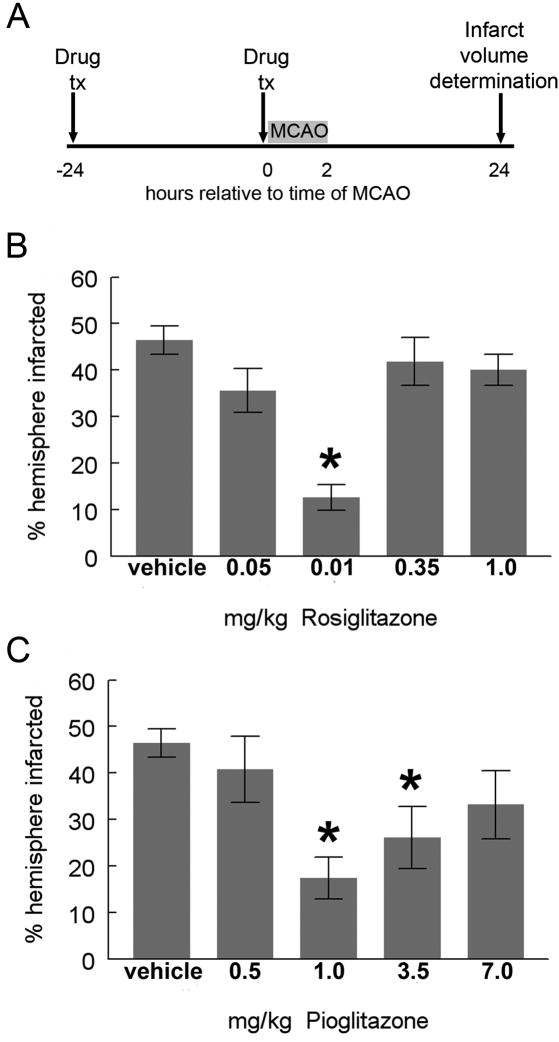
Several doses of pioglitazone and rosiglitazone were tested in a pre-treatment paradigm in order to most efficiently identify the optimal doses for neuroprotection. Subsequent experiments utilized more clinically relevant post-MCAO treatment times. Maximal reduction in infarction volume by pioglitazone was achieved using a dose of 1mg/kg and for rosiglitazone at 0.1mg/kg dissolved in DMSO and injected IP 24 hours before and at the time of MCAO (fig 1). These doses are consistent with the potency of the drugs (Young et al., 2007) and were used in all subsequent experiments. Although we found that pioglitazone 3.5 mg/kg also resulted in a significant reduction in infarction size, the degree of efficacy was less than that seen with the 1mg/kg dose. Similar loss of potency with high concentrations has been described in other disease models, although the basis of this effect is not clear (Diab et al., 2002; Lin et al., 2006).
Figure 1. Dose response curves for TZD effects on infarction volume.
Rats were injected with DMSO, pioglitazone or rosiglitazone 24 hours before and at the time of MCAO. Infarction volume was assayed 24 hours after MCAO (A). Rosiglitazone, 0.1 mg/kg, significantly reduced infarction volume at 24 hours, although rosiglitazone 0.05 mg/kg did not. Protection was lost with higher doses (B; Mann-Whitney U p=0.002; n=16 (vehicle); n=5 (0.05 mg/kg); n=4 (0.1 mg/kg), n=7 (0.35mg/kg and 1mg/kg). Pioglitazone at doses of 1.0 and 3.5 mg/kg significantly reduced infarction volume at 24 hours, although doses of 0.5 and 7 mg/kg were not significantly different from DMSO treated rats [C; Mann-Whitney U; p=0.001 for 1mg/kg and p<0.05 for 3.5mg/kg; n=16 (DMSO); n=8 (0.5 mg/kg); n=7 (1mg/kg); n=8 (3.5 mg/kg); n=5 (7mg/kg)]. Bars represent S.E.M.
TZDs did not affect blood pressure or cerebral blood flow
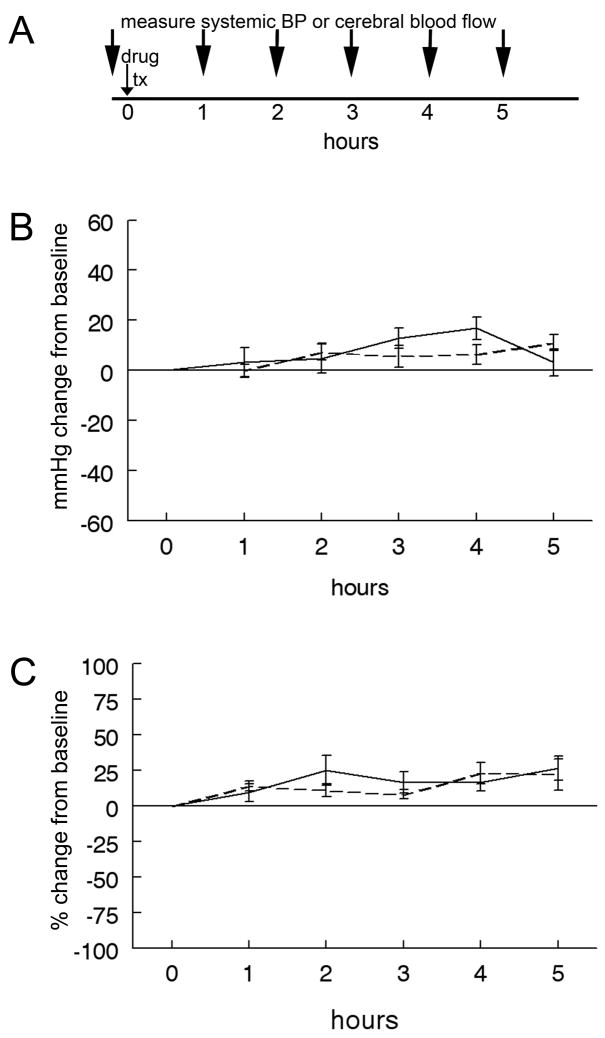
We characterized the effects of TZDs on blood pressure and cerebral blood flow over a prolonged period of time as changes in these parameters may influence infarction volume and complicate data interpretation. Separate groups of rats underwent sustained arterial blood pressure or CBF monitoring beginning before and continuing for five hours after injections of TZD. TZD treatment did not significantly change either parameter relative to vehicle injection (fig. 2).
Figure 2. Single doses of TZDs do not alter systemic blood pressure or cerebral blood flow.
Baseline arterial blood pressure was measured in awake rats at time 0. Rats were then injected IP with either DMSO (solid line) or rosiglitazone (0.1mg/kg) dissolved in DMSO (dashed line) and arterial blood pressure was measured every hour for a total of 5 hours (A). There was no significant change in the blood pressure in animals treated with rosiglitazone compared to those treated with DMSO (B; one way ANOVA p=0.6; n=5 in each group). Baseline cerebral blood flow was measured in anesthetized rats at time 0. Rats were then injected IP with either DMSO (solid line) or pioglitazone (1mg/kg) dissolved in DMSO and cerebral blood flow was measured every hour for a total of 5 hours (A). There was no significant change in cerebral blood flow in pioglitazone treated rats relative to DMSO treated rats (C; one way ANOVA p=0.4; n=4 in each group). Bars represent S.E.M.
Pioglitazone is protective in transient but not permanent ischemia
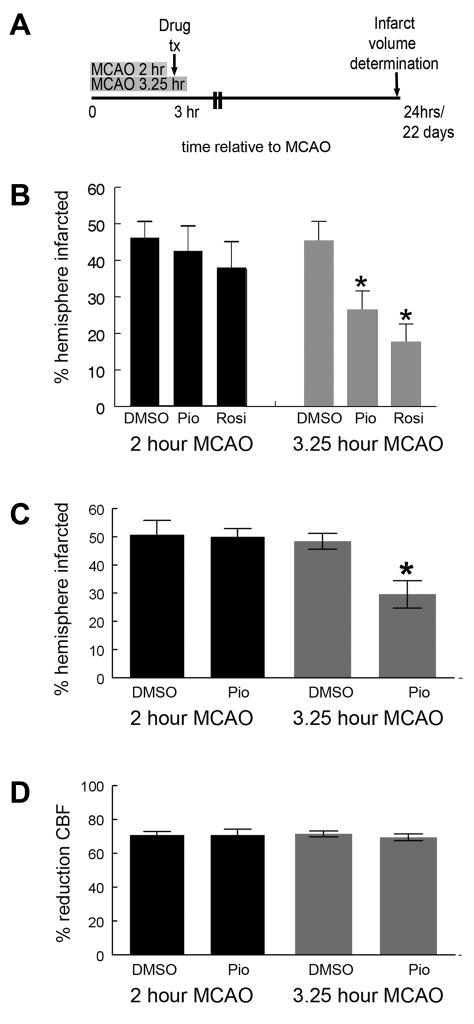
We examined the ability of pioglitazone to reduce infarction volume in both transient and permanent ischemia models. Rats were treated with pioglitazone 24 hours before and again at the time of MCAO. Pretreatment was used to maximize our ability to detect protection in a permanent ischemia model. While pioglitazone significantly reduced infarction volume during transient MCAO, it was not effective in the permanent ischemia model (fig 3).
Figure 3. Pioglitazone is protective in transient, but not permanent MCAO.
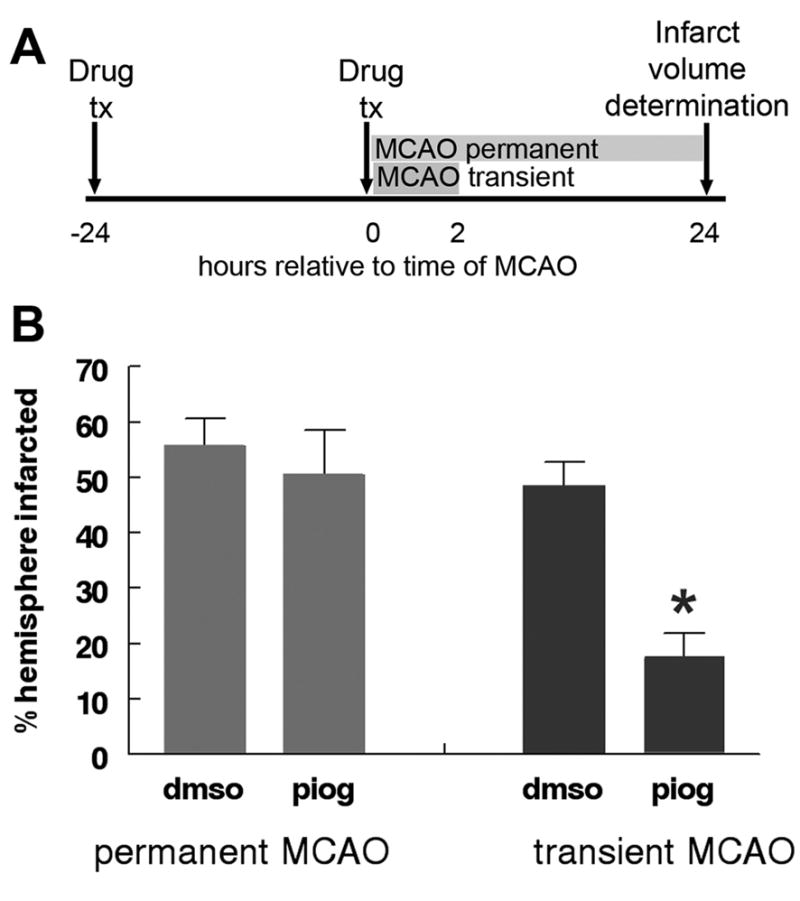
Pioglitazone (1mg/kg) IP or vehicle (DMSO) was administered to rats IP 24 hours before and again at the time of MCAO. Occlusion was maintained either permanently or for two hours. Animals were sacrificed at 24 hours and infarct volume calculated (A). Pioglitazone failed to effect infarction volume during permanent MCAO (B; Mann Whitney U p=0.9; n=4 (DMSO, permanent MCAO); n=3 (pioglitazone, permanent MCAO), but significantly reduced infarct volume relative to DMSO during transient MCAO (B; Mann Whitney U p<0.01; n=7 (DMSO, transient MCAO); n=7 (pioglitazone, transient MCAO). Bars represent S.E.M.
TZDs are most effective when administered prior to reperfusion
We tested the hypothesis that timing of drug treatment relative to reperfusion is an important determinate of TZD efficacy by treating rats 3 hours after MCAO with DMSO, rosiglitazone (0.1mg/kg) or pioglitazone (1mg/kg) IP and reperfusing the MCA either before or after drug treatment. One group of rats was reperfused one hour before drug treatment (2 hours after MCAO), while the second group of animals was reperfused fifteen minutes after drug treatment (3.25 hours after MCAO; fig 4A). When assayed 24 hours after MCAO, infarction volumes were smaller in rats treated with either rosiglitazone or pioglitazone at three hours and reperfused at 3.25 hours when compared to animals treated with DMSO or animals treated with pioglitazone or rosiglitazone after reperfusion (fig 4B).
Figure 4. Time window of TZD protection is increased if given before reperfusion, despite a longer duration of ischemia.
Rats underwent MCAO and were injected with DMSO, rosiglitazone (0.1mg/kg) or pioglitazone (1mg/kg) dissolved in DMSO three hours later. Rats were reperfused either two hours after MCAO/one hour before drug treatment or 3.25 hours after MCAO/15 minutes after TZD treatment (A). When assayed 24 hours after MCAO, rats reperfused before TZD treatment (2hr pio/rosi) had infarction volumes that were similar to DMSO injected rats (2hr or 3.25hr DMSO), however, rats reperfused after TZD treatment (3.25hr pio/rosi) had significantly smaller infarction volumes compared to either rats receiving DMSO or rats that received before reperfusion (2hr pio/rosi) [B; Mann Whitney U; p<0.05; n=4 (2hr DMSO); n=6 (2 hr pio); n=7 (2hr rosi), n=7 (3.25hr DMSO); n=7 (3.25hr pio); n=3 (3.25hr rosi)]. Infarction volume was also assayed 22 days after MCAO in separate animals injected with either DMSO or pioglitazone (1mg/kg). Rats reperfused before pioglitazone treatment (2hr pio) had similar infarction volumes to DMSO treated rats (2hr or 3.25hr DMSO), while rats reperfused after pioglitazone treatment (3.25hr pio) had significantly smaller infarction volumes compared to rats receiving DMSO (2hr or 3.25hr DMSO) or pioglitazone before reperfusion (2hr pio) (C; Mann Whitney U; p<0.05; n=10 (2hr DMSO); n=9 (2hr pio); n=13 (3.25hr DMSO); n=11 (3.25hr pio)]. There was no difference in cerebral blood flow reduction in any of the experimental groups (D; Mann Whitney U; p>0.50; n=10 (2hr DMSO); n=9 (2hr pio); n=13 (3.25hr DMSO); n=11 (3.25 hr pio). Bars represent S.E.M.
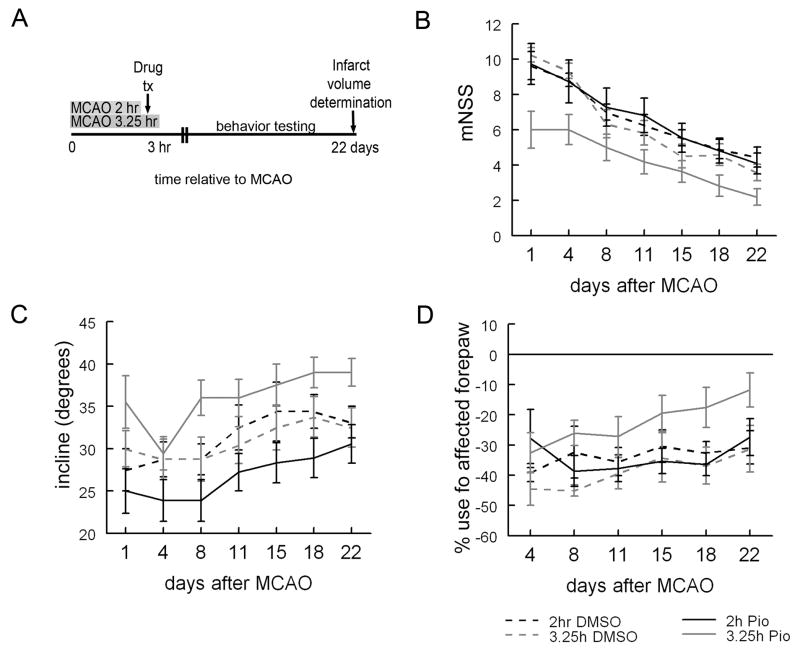
In order to ensure that the benefit seen at 24 hours represented actual neuroprotection and not a delay in infarct development, a similar experiment was conducted but with three week survival and infarction volume assayed at 22 days. During this time animals also underwent analysis of their neurologic function. Because of the number of animals and the labor involved in this experiment, only one TZD, pioglitazone, was tested. A total of 75 animals were lesioned for this experiment. We find that rats treated with pioglitazone three hours after MCAO and reperfused at 3.25 hours had significantly smaller infarction volumes than rats DMSO treated or pioglitazone treated animals that had been reperfused before drug treatment (fig 4C). Importantly, we demonstrate that the benefits in infarction volume are accompanied by significant improvements in neurologic function as assayed by the mNSS, performance on an inclined plane or by increased use of the affected right forelimb during exploratory behavior in a glass cylinder (fig 5). Importantly, while the number of surgically related deaths was similar in all experimental groups, there were several non-surgical deaths during the course of this protracted experiment and these occurred disproportionately in the DMSO treated group with the longer duration of ischemia (3.25 hours; Table 1).
Figure 5. Pioglitazone improves neurologic behavior when given before reperfusion.
Rats were trained on all functional tasks for at least three days prior to MCAO. On day 0 they underwent MCAO and were injected with DMSO or pioglitazone (1mg/kg) dissolved in DMSO 3 hours after MCAO. Rats were reperfused either 2 hours after MCAO/one hour before drug treatment or 3.25 hr after MCAO/15 minutes after drug treatment. All animals had functional testing twice weekly for 3 weeks (A). After 3 weeks, animals treated with either DMSO (2hr or 3.25hr DMSO) or pioglitazone after reperfusion (2hr pio) has similar mNSS (B), ability to tolerate an incline without falling (C) and use of the affected right forepaw (D). Three weeks after MCAO, rats treated with pioglitazone before reperfusion (3.25hr pio) had improved neurologic performance as evidenced by a lower average mNSS (B) and improved use of the affected right forepaw (D) relative to rats treated with DMSO (2 or 3.25hr DMSO) or pioglitazone after reperfusion (2hr pio). In addition, rats treated with pioglitazone before reperfusion (3.25hr pio) were able to tolerate a higher incline without falling relative to rats treated with DMSO before reperfusion (2hr DMSO) and rats treated with pioglitazone before reperfusion (2hr pio; p<0.05; Mann-Whitney U; C). Additionally, there was a trend towards improved ability to tolerate a higher degree of incline relative to rats treated with DMSO after reperfusion [3.25hr DMSO; p=0.056; Mann-Whitney U; n=11 (2hr DMSO); n=11 2hr pio; n=12 3.25hr DMSO; n=11 3.25hr pio)]. Bars represent S.E.M.
ICAM is reduced by TZDs to the same extent irrespective of time of reperfusion
ICAM mediates infiltration of leukocytes into brain parenchyma following injury. We first tested the ability of TZDs to suppress ICAM expression following MCAO. Rats were treated with DMSO, pioglitazone or rosiglitazone 24 hours before and again at the time of MCAO. Rats were sacrificed 24 hours after MCAO and immunohistochemistry and real time PCR were used to examine ICAM expression. There was a significant reduction in ICAM mRNA with both TZD treatments relative to DMSO treatment (fig 6). Both pioglitazone and rosiglitazone reduced ICAM mRNA to the same extent. In addition, we examined ICAM-IR in sections from animals treated with DMSO, pioglitazone or rosiglitazone three hours after MCAO and reperfused either one hour before TZD treatment or 15 minutes after TZD treatment. Animals treated with either pioglitazone or rosiglitazone 3 hours after MCAO had reduced ICAM-IR compared with DMSO treated animals, however, time of reperfusion did not significantly alter ICAM-IR in either DMSO, pioglitazone or rosiglitazone treated rats (fig 7).
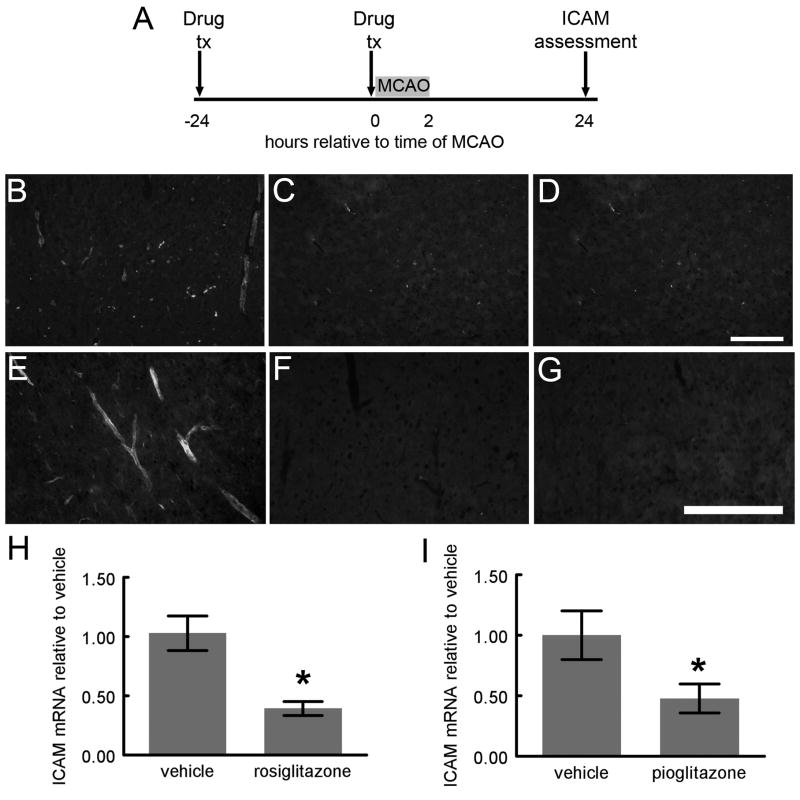
Figure 6. ICAM expression is reduced by TZD treatment prior to MCAO.
Rats treated with DMSO, rosiglitazone (0.1mg/kg) or pioglitazone (1mg/kg) 24 hours before and again at the time of 2 hour MCAO were sacrificed 24 hours after MCAO (A). Frozen sections of brain were incubated with mouse anti-ICAM antibody, and followed by secondary antisera conjugated to Oregon-green. While several ICAM-IR vessels are evident in sections from DMSO treated rats (B, E), few ICAM-IR vessels are seen in sections from rosiglitazone (C, F) and pioglitazone (D, G) treated rats. RNA from rats treated with either DMSO, rosiglitazone (0.1mg/kg), or pioglitazone (1mg/kg) injected 24 hours before and again at the time of 2 hour MCAO and sacrificed 24 hours later (A), was isolated and cDNA transcribed. Real time PCR showed significantly reduced ICAM mRNA in rosiglitazone (H) and pioglitazone (I) treated rats compared to DMSO treated rats [(p<0.05; Mann-Whitney U); H: n=3 (DMSO and rosiglitazone); I: n=4 (DMSO); n=5 (pioglitazone)]. Error bars represent S.E.M.
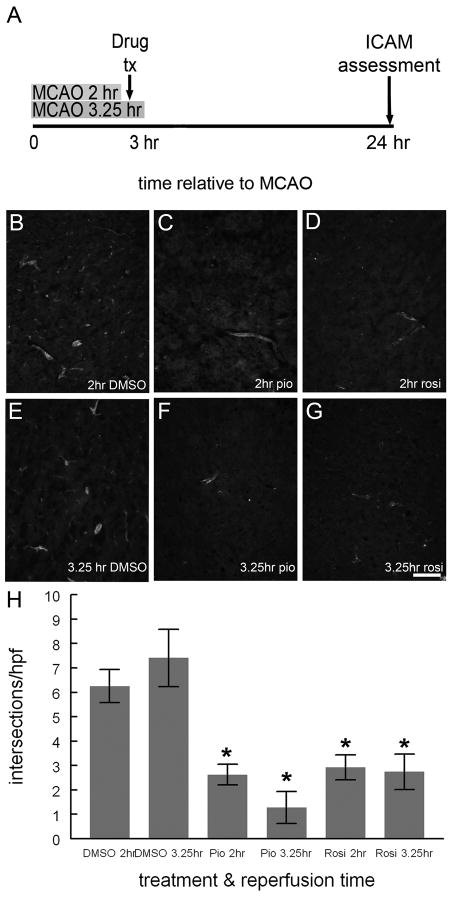
Figure 7. ICAM expression is reduced by TZD treatment 3 hours after MCAO.
Rats treated with DMSO, pioglitazone (1mg/kg) or rosiglitazone (0.1 mg/kg) dissolved in DMSO 3 hours after MCAO (A). Rats were reperfused either 2 hours after MCAO/one hour before drug treatment (A, B, C, D) or 3.25 hours after MCAO/15 minutes after drug treatment (A, E, F, G). Frozen sections of the rats’ brains were incubated with mouse anti-ICAM antibody followed by secondary antisera conjugated to biotin, which was visualized using avidin conjugated to Cy-3. While several ICAM-IR vessels are evident in sections from DMSO treated rats (B, E), few ICAM-IR vessels are seen in sections from pioglitazone (C, F) or rosiglitazone (D, E) treated rats. Scale bar equals 200μm. ICAM-IR was quantified by counting the number of times ICAM-IR intersected a grid overlying photomicrographs of each individual HPF (87.5μm2). There was dense ICAM-IR in sections from TZD treated rats relative to DMSO treated rats (p<0.05), but no significant difference between ICAM-IR in rats reperfused at either 2 or 3.25 hours for any drug treatment [(p>0.1); H; Mann-Whitney U; n=5 (2hr DMSO), n=4 (3.25hr DMSO); n=5 (2 hr pio), n=3 (3.25 pio); n=5 (2hr rosi); n=3 (3.25hr rosi); at least 775 HPF were examined per treatment group]. Bars represent S.E.M.
Leukocyte infiltration is more efficiently reduced when TZDs are given prior to reperfusion
Myeloperoxidase is a marker for leukocytes and is easily detected by enzyme assays or immunohistochemistry. A large number of myeloperoxidase-IR cells were distributed throughout the ischemic territory in DMSO treated rats. The average number of myeloperoxidase-IR cells/HPF/animal was significantly reduced in brains from pioglitazone and rosiglitazone treated animals relative to DMSO treated animals. Importantly, the reduction in myeloperoxidase-IR cells was significantly greater when animals were reperfused after TZD treatment than when animals were reperfused before drug treatment (fig 8).
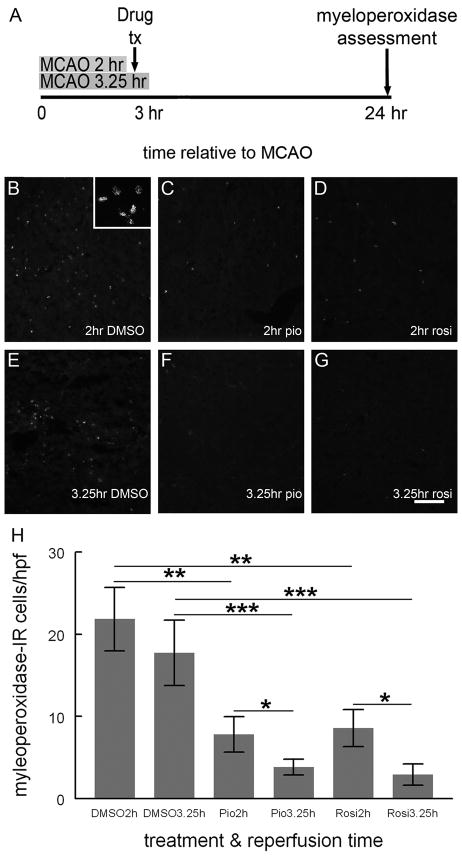
Figure 8. TZDs reduce Myeloperoxidase-IR cells, especially when given prior to reperfusion.
Rats treated with DMSO, pioglitazone (1mg/kg) or rosiglitazone (0.1mg/kg) dissolved in DMSO 3 hours after MCAO (A). Rats were reperfused either 2 hours after MCAO/one hour before drug treatment (A, B, C, D) or 3.25 hours after MCAO/15 minutes after drug treatment (E, F, G). Frozen sections of the rats’ brains were incubated with a rabbit anti-myeloperoxidase antibody followed by secondary antisera conjugated to Oregon-Green and the number of myeloperoxidase-IR cells/HPF (350μm2) counted. Inset of B shows high magnification image of myeloperoxidase-IR cells in brain from animals reperfused before treatment with DMSO. Myeloperoxidase-IR cells were most abundant in brains from DMSO treated rats, irrespective of the time of reperfusion (H). Rats treated with either pioglitazone or rosiglitazone and reperfused before TZD treatment had significantly less myeloperoxidase-IR cells than DMSO treated rats but more myeloperoxidase-IR cells than animals treated with either TZD and reperfused after TZD treatment [H; * p<0.05; ** p<0.02; ***p<0.01; Mann-Whitney U; n=5 (2hr DMSO); n=6 (3.25 hr DMSO); n=6 (2hr pio); n=5 (3.25hr pio); n= 6 (2hr rosi); n=5 (3.25hr rosi); at least 250 HPF were examined per treatment group]. Scale bar = 200μm. Error bars represent S.E.M.
Discussion
This study builds on our previous work demonstrating that TZDs reduce infarction volume and improve long term neurologic function in a rodent model of ischemia (Sundararajan et al., 2005; Victor et al., 2006) and specifically addresses optimal dosing paradigms including factors that influence the time window of efficacy. The current data indicate TZDs are protective at clinically relevant doses, independent of any effects on systemic blood pressure or cerebral blood flow. Importantly, reperfusion, and specifically, the timing of reperfusion relative to drug administration, influences the ability of pioglitazone to reduce infarction volume and improve neurologic function following MCAO. While the effects of TZDs on PPARγ activation and expression of individual gene products may not be altered by reperfusion itself, the implications of these changes may be very different depending on whether or not the tissue is reperfused. For example, the functional consequence of reduced ICAM expression is more dramatic if it occurs before reperfusion when activated leukocytes gain access to the endothelium. These observations are likely not unique to TZDs and may also apply to other potential therapies which target events occurring during reperfusion. While “pre-treatment” of ischemic injury may be impractical, “pre-treatment” of reperfusion injury is increasingly a viable clinical option given the use of thrombolytics and mechanical devices to reperfused arteries.
Effective Dosing of TZDs for Protection
The dose of TZDs used in these studies reflects what we found optimal in dose response studies. While the pioglitazone dose of 1mg/kg is similar to that used by other investigators, the rosiglitazone dose of 0.1mg/kg is ten to sixty times less concentrated than previously reported. Importantly, our findings are consistent with the known potency of these drugs and doses used to treat human diabetes (Barman Balfour and Plosker, 1999; Waugh et al., 2007; Young et al., 2007). One difference between our studies and others is that we dissolved TZDs in 100% DMSO, while several other laboratories have used 10–33% DMSO (Luo et al., 2006; Pereira et al., 2006). It is possible that dilution of the DMSO reduced rosiglitazone solubility requires a higher dose of drug. However, Tureyen and colleauges used water soluble potassium salts of TZD, and a higher dose of rosiglitazone was still required to protect the brain (Tureyen et al., 2007). Although rosiglitazone in lactate-buffered Ringer’s solution crosses the blood brain barrier, it is rapidly exported out of the brain via a p-glycoprotein (Festuccia et al., 2008). It is possible that rosiglitazone dissolved in DMSO is better able to cross the blood brain barrier or that it is not exported out of the brain in same manner. On the other hand, if TZDs primarily act on endothelium, then it should not be necessary to cross the blood brain barrier to be effective. Our finding that rosiglitazone and pioglitazone are effective at low doses indicates that individuals using TZDs to control diabetes might be protected from stroke. This is an important point since the drugs are used by thousands of patients on a chronic basis to treat a risk factor for stroke. These patients would be effectively “pre-treated” before the onset of ischemia and, therefore, would be in an excellent position to benefit from the neuroprotective actions of TZDs.
Potential mechanisms of TZD neuroprotection
Both rosiglitazone and pioglitazone are selective PPARγ agonists, but can weakly activate other PPAR isotypes, especially at high concentrations (Seimandi et al., 2005; Welch et al., 2003). While it is likely that the neuroprotective effects of pioglitazone and rosiglitazone on infarction volume and the effects of pioglitazone on improved neurologic function are secondary to PPARγ activation, we cannot rule out the possibility that these actions are mediated though interactions with other receptors.
TZD treatment in cerebral ischemia models is associated with reduced inflammation and oxidative stress (Culman et al., 2007; Kapadia et al., 2008). These are the most likely mechanisms for TZD mediated neuroprotection. We have previously found that TZDs reduce interleukin 1β, cyclooxygenase-2 and inducible nitric oxide synthase expression following MCAO (Sundararajan et al., 2005) and in this paper focus on changes in ICAM and leukocyte infiltration in response to TZD treatment since these may explain how the timing of reperfusion relative to drug treatment might differentially affect outcome. Leukocytes are activated within 30 minutes of ischemic onset (Garcia et al., 1994), begin to migrate into the brain within an hour of reperfusion and are present in significant numbers within six hours of reperfusion (Clark et al., 1994). In permanent ischemia leukocyte infiltration occurs at later times and is less robust (Clark et al., 1994). Activated leukocytes release proteases and reactive oxygen species (ROS) further injuring already compromised brain (Schaller and Graf, 2004). Interfering with leukocyte binding and infiltration is beneficial primarily in transient, but not permanent, ischemia (Frijns and Kappelle, 2002; Zhang et al., 1994).
Our data strongly suggest that TZDs act primarily on events occurring during reperfusion. The finding that pioglitazone is effective in transient, but not permanent, ischemia is similar to that reported by Greenberg and colleagues (Shimazu et al., 2005). They found that pretreatment of rats with high doses of oral pioglitazone (20mg/kg) was protective in transient, but not permanent MCAO. Rats undergoing transient ischemia undergo an additional surgery to remove the suture, requiring 5 to 10 minutes of anesthesia in addition to the approximately 45 to 60 minutes required for MCAO. It seems unlikely that this small increase in anesthesia time is sufficient to explain the reduction in infarction volume. A few reports have found rosiglitazone to be protective when administered after reperfusion (Luo et al., 2006; Tureyen et al., 2007), or in one case, in permanent ischemia (Pereira et al., 2006), however, these studies did not address differences between permanent and transient ischemia or address the timing of reperfusion. Interestingly, protection in each case was associated with reduced inflammation. This may be related to TZD actions on leukocyte infiltration at the infarct margin, primarily perfused by collateral circulation, or effects on microglia, the resident tissue macrophages of the brain. Such effects may be more evident at the higher doses utilized in these other studies.
The time required for suppression of inflammatory mediators is an important determinate in the ultimate efficacy of TZD treatment. PPARγ’s anti-inflammatory actions are largely mediated by transrepression and do not require new protein synthesis (Straus and Glass, 2007). Ischemia/reperfusion paradigms in other tissues demonstrate that PPARγ ligands suppress ICAM rapidly (Cuzzocrea et al., 2003; Wayman et al., 2002). In the heart this reduction was clearly evident three hours after treatment. In our experiments we gave TZDs fifteen minutes before reperfusion and, while it is unlikely that significant changes in ICAM expression were present at the time of reperfusion, the duration of contact between fully activated endothelium and systemic leukocytes would have been substantially shortened, providing a potential explanation for how the time of reperfusion could impact leukocyte infiltration without changing ICAM expression.
Although we have focused on the anti-inflammatory actions of TZDs, it is important to note that other potential mechanisms for PPARγ mediated neuroprotection have been suggested, including reduced oxidative stress and improved endovascular reactivity. PPARγ ligands upregulate Cu/Zn dismutase, an enzyme which plays an important role in regulating free radicals (Hwang et al., 2007; Maier et al., 2006; Shimazu et al., 2005; Tureyen et al., 2007). TZDs also increase endothelial release of nitric oxide resulting in vasodilatation thereby alleviating ischemia (Calnek et al., 2003; Cho et al., 2004). It is important to note that oxidative stress is also exacerbated during reperfusion (Kinouchi et al., 1991) and, and the effects of TZDs on oxidative stress may also be more effective when given prior to reperfusion.
Clinical Implications
There are several important implications in the data we describe. First, our dose response curves indicate that choice of TZD dose is critical in achieving protection in stroke models. We find that TZDs are protective at doses similar to those used in clinical practice and that the protective actions of TZDs are lost when the dose is increased beyond that. The choice of dose tested in a clinical trial will likely be crucial to the success of translating the promising protective effects of TZDs in animals into humans. The use of biomarkers of PPARγ activation may be helpful in determining optimal dosing regiments for clinical trial. Equally important, our data show that effective timing of drug administration depends not only on the time elapsed since artery occlusion but also the time relative to reperfusion. In clinical practice reperfusion can occur spontaneously, however, time of reperfusion is increasingly controlled by the use of thrombolytics and mechanical devices. To date, thrombolysis is the only FDA approved treatment for ischemic stroke. The practice has been to administer thrombolytics and then determine if patients are candidates for neuroprotective trials. Our findings indicate that this approach may not be optimal, especially for therapies targeting events during reperfusion.
It should be noted that there is controversy regarding the relevance of therapeutic time windows determined in animals to cerebral ischemia in humans, however, some studies suggest that the time window for therapy in rodents and humans are similar (Fisher et al., 2009). Importantly, time windows for treatment in both animals and humans are variable and depend on the degree of reduced perfusion, the presence of collateral circulation and metabolic rate of the brain during ischemia. Imaging techniques utilizing magnetic resonance imaging (MRI) or CT may be able to determine the volume of penumbra required at the time of treatment to salvage tissue in the rat. These data could potentially be used to identify the most appropriate time window in human trials.
While there is little data addressing how quickly TZDs reduce inflammation, our data suggest that it is important that the process begin before reperfusion. Recent data indicate that PPARγ activation is safe, and perhaps beneficial, following cerebral hemorrhage (Zhao et al., 2007; Zhao et al., 2006) indicating that TZDs could be given prior to CT scanning, perhaps by first responders. A clinical trial utilizing drug administration by paramedics for stroke has already demonstrated that such a scenario is feasible (Saver et al., 2004).
Acknowledgments
The authors would like to thank the technical assistance of Kimberly Deininger and Youzhi Kuang. In addition, the authors are indebted to the many helpful discussions with Drs. David Lust, Joseph LaManna and Dennis Landis, without whose insight the current study would not have happened. This work was funded by the NINDS/NIH (K08 NS-041594; SS) and GlaxoSmithKline (GL & SS) and Takeda Pharmaceuticals (SS).
Comprehensive List of Abbreviations
- ANOVA
Analysis of variance
- AP-1
Activator protein 1
- CBF
Cerebral blood flow
- CT
computed tomography
- Ct
cycle threshold
- Cu/Zn
copper/zinc
- DMSO
Dimethyl sulfoxide
- FDA
Federal Drug Administration
- HPF
High powered field
- ICAM
Intracellular adhesion molecule
- IP
Intraperitoneal
- -IR
Immunoreactivity
- HPF
high powered field
- MCAO
Middle cerebral artery occlusion
- mNSS
modified neurological severity score
- MRI
Magnetic Resonance Imaging
- NFκB
Nuclear factor κB
- PBS
Phosphate buffered saline
- PGJ2
15-deoxy-Δ(12,14)-prostaglandin J2
- PCR
polymerase chain reaction
- PPAR
Peroxisome proliferator-activated receptor
- PPRE
Peroxisome proliferator response element
- ROS
Reactive oxygen species
- RXR
Retinod X receptor
- S.E.M
Standard Error of the Mean
- STAIR
Stroke Academic Industry Roundtable
- STAT-1
Signal transducers and activator of transcription 1
- SUMO1
small ubiquitin-like modifier
- TTC
triphenyl tetrazolium chloride
- TZD
Thiazolidinedione
- US
United States
Footnotes
Conflict of interest
Gamboa: no conflict of interest
Blankenship: no conflict of interest
Niemi: no conflict if interest
Landreth: Dr. Landreth holds a patent for the use of PPARγ agonists in neurologic disease including stroke. Dr. Landreth has received a research grant from Glaxosmithkline.
Sundararajan: Dr. Sundararajan has received a research grants from Glaxosmithkline and Takeda Pharmaceuticals
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Allahtavokoli M, Shabanzadeh A, Sadr S, Parviz M, Djahanguiri B. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, reduces infarction volume and neurological deficits in an embolic model of stroke. Clin Exp Pharmacol Physiol. 2006;33:1052–1058. doi: 10.1111/j.1440-1681.2006.04486.x. [DOI] [PubMed] [Google Scholar]
- Barman Balfour J, Plosker G. Rosiglitazone. Drugs. 1999;57:921–930. doi: 10.2165/00003495-199957060-00007. [DOI] [PubMed] [Google Scholar]
- Calnek D, Mazzella L, Roser S, Roman J, Hart M. Peroxisome proliferator-activated receptor γ ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:52–57. doi: 10.1161/01.atv.0000044461.01844.c9. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg P, Li Y, Wang L, Lu M, Willing A, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Cho D-H, Choi Y, Jo S, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment troglitazone. J Biol Chem. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- Clark R, Lee E, White R, Jonak Z, Feuerstein G, Barone F. Reperfusion following focal stroke hastens inflammation and resolution of ischemic injured tissue. Brain Res Bull. 1994;35:387–392. doi: 10.1016/0361-9230(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR-gamma: therapuetic target for ischemic stroke. Trends Pharmacol Sci. 2007;28:244–249. doi: 10.1016/j.tips.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianoro A, Patel N, Di Paola R, Genovese T, Chatterjee P, Di Rosa M, Caputi A, Thiemermann C. Rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2, ligands of the peroxisome proliferator-activated receptor-γ (PPAR-γ), reduce ischemia/reperfusion injury of the gut. Br J Pharmacol. 2003;140:366–376. doi: 10.1038/sj.bjp.0705419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith J, Hussain R, Phanavanh B, Lovett-Racke A, Drew P, Racke M. Peroxisome proliferator-activated receptor-γ agonist 15-deoxy-Δ12,14-prostaglandin J2 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Festuccia W, Oztezcan S, Laplante M, Berthiaume M, Michel C, Dohgu S, Denis R, Brito M, Brito N, Miller D, Banks W, Bartness T, Richard D, Deshaies Y. Peroxisome proliferator-activated receptor-γ-mediated positive energy balance in the rat is associated with reduced symptahtetic drive to adipose tissues and thyroid status. Endocrinol. 2008:2121–2130. doi: 10.1210/en.2007-1553. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells D, Hurn P, Kent T, Savitz S, Lo E. Update of the Stroke Therapy Academic Industry Roundtable Preclinical Recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns C, Kappelle L. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Garcia J, Liu K, Yoshida Y, Lian J, Chen S, del Zoppo G. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) Am J Pathol. 1994;144:188–199. [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Kleinhenz D, Lassegue B, Griendling K, Dikalov S, Hart CM. Peroxisome proliferator-activated-γ ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol. 2007;288:C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi J, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Li Y, Shirasawa M, Nobuyuki Y, Fukasawa H. Reversible middle cerebral artery occlusion in rats using an intraluminal thread technique. Surg Neurol. 1994;41:368–373. doi: 10.1016/0090-3019(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Epstein C, Mizui T, Carlson E, Chen S, Chan P. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T-N, Cheung W-M, Wu J-S, Lin H, Chen J-J, Liou J-Y, Shyue S-K, Wu K. 15Δ-prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–487. doi: 10.1161/01.ATV.0000201933.53964.5b. [DOI] [PubMed] [Google Scholar]
- Longa E, Weinstein P, Carlson S. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore A, Zhang F, Hong Z, Wang S, Graham S, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- Maier C, Hsieh L, Crandall T, Narasimhan P, Chan P. A new approach for the investigation of reperfusion-related brain injury. Biochem Soc Trans. 2006;34:1366–1369. doi: 10.1042/BST0341366. [DOI] [PubMed] [Google Scholar]
- Pereira M, Hurtado O, Cardenas A, Bosca L, Castillo J, Davalos A, Vivancos J, Serena J, Lorenzo P, Lizasoain I, Moro M. Rosiglitazone and 15Δ-deoxy-delta 12,14-prostaglandin J2 cause potent neuroprotection after experimental stroke through noncompletely overlapping mechanisms. J Cereb Blood Flow Metab. 2006;26:218–229. doi: 10.1038/sj.jcbfm.9600182. [DOI] [PubMed] [Google Scholar]
- Saver J, Kidwell C, Eckstein M, Starkman S. Prehospital neuroprotective therapy for acute stroke results of the field administration for stroke therapy-Magnesium (FAST-MAG) pilot trial. Stroke. 2004;35:e106–e108. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004;24:351–371. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming S, Leasure J, Tillerson J, Bland S. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacol. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Seimandi M, Lemaire G, Pillon A, Perrin A, Carlavan I, Voegel J, Vignon F, Nicolas J, Balaguer P. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anals Biochem. 2005;344:8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Inoue I, Araki N, Asano Y, Sawada M, Furuya D, Nagoya H, Greenberg J. A peroxisome proliferator-activated receptor-γ agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- Straus D, Glass C. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable. Recommendations for standards regarding pre-clinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa J, Victor N, Wanderi E, Lust W, Landreth G. PPARγ ligands reduce inflammation and infarction size in transient focal ischemia. Neurosci. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Tang W, Maroo A. PPARγ agonists: safety issues in heart failure. Diabetes, Obesity and Metabol. 2009;9:447–454. doi: 10.1111/j.1463-1326.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen K, Satriotomo I, Jiang J, Feinstein D, Vemuganti R. Peroxisome proliferator-activated receptor-γ agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Victor N, Wanderi E, Gamboa J, Zhao X, Aronowski J, Lust W, Landreth G, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Waugh J, Keating G, Plosker G, Easthope S, Robinson D. Pioglitazone, a review of its use in type 2 diabetes mellitus. Drugs. 2007;66:85–109. doi: 10.2165/00003495-200666010-00005. [DOI] [PubMed] [Google Scholar]
- Wayman N, Hattori Y, McDonald M, Mota-Filipe H, Cuzzocrea S, Pisano B, Chatterjee P, Thiemermann C. Ligands of the peroxisome proliferator-activated receptors (PPAR-γ and PPAR-α) reduce myocardial infarct size. FASEB J. 2002;16:1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- Welch J, Ricote M, Akiyama T, Gonzalez F, Glass C. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. PNAS. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemori F, Yamaguchi T, Yamada H, Tamura A. Evaluation of motor deficit after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1998;18:1099–1106. doi: 10.1097/00004647-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Young P, Buckle D, Cantello B, Chapman H, Clapham J, Coyle P, Haigh D, Hindley R, Holder J, Kallender H, Latter A, Lawrie K, Mossakowska D, Murphy G, Roxbee Cox L, Smith S. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther. 2007;284:751–759. [PubMed] [Google Scholar]
- Zhang R, Chopp M, Li Y, Zaloga C, Jiang N, Jones M, Miyasaka M, Ward P. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurol. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta J, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Grotta J, Aronowski J. 15d-Prostaglandin J(2) activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006;26:811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci. 2005;22:278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]