Abstract
Objective
Previous findings suggest that phobic anxiety may pose increased risk of cardiac mortality in medically healthy cohorts. The present study evaluated whether phobic anxiety is associated with increased risk of cardiac mortality in individuals with established coronary heart disease (CHD) and examined the role of reduced heart rate variability (HRV) in mediating this risk.
Methods
We performed a prospective cohort study in 947 CHD patients recruited during hospitalization for coronary angiography. At baseline, supine recordings of heart rate for HRV were collected, and participants completed the Crown-Crisp phobic anxiety scale. Fatal cardiac events were identified over an average period of 3 years.
Results
Female CHD patients reported significantly elevated levels of phobic anxiety when compared with male patients (p <.001) and survival analysis showed an interaction between gender and phobic anxiety in the prediction of cardiac mortality (p =.058) and sudden cardiac death (SCD) (p=.03). In women, phobic anxiety was associated with a 1.6-fold increased risk of cardiac mortality (HR, 1.56; 95% CI, 1.15–2.11; p=.004) and a 2.0-fold increased risk of SCD (HR, 2.02; 95% CI, 1.16–3.52; p=.01) and was unassociated with increased mortality risk in men (p=.56). Phobic anxiety was weakly associated with reduced high frequency HRV in female patients (r=−.14, p=.02), but reduced HRV did not alter the association between phobic anxiety on mortality.
Conclusions
Phobic anxiety levels are high in women with CHD and may be a risk factor for cardiac-related mortality in women diagnosed with CHD. Reduced HRV measured during rest does not appear to mediate phobic anxiety-related risk.
Keywords: phobic anxiety, coronary disease, sudden death, heart rate variability
INTRODUCTION
Anxiety disorders are the most common DSM-IV disorder, with a one-year prevalence of 18% in U.S. adults (1). Phobic anxiety, characterized by an unreasonable fear when exposed to specific situations such as enclosed spaces, heights, or crowds, is the predominant complaint in approximately half of these individuals (2). Although the association between phobic anxiety and mortality in patients with documented coronary heart disease (CHD) has not been investigated, several epidemiological studies have reported an association between phobic anxiety and increased risk of fatal cardiac events in community dwelling men and women free of known cardiovascular disease (3–5), with relative risks ranging from 1.6 (95% CI: 1.2 to 2.1) in 72,359 female nurses participating in the Nurses’ Health Study (3) to 3.8 (95% confidence interval [CI]: 1.64 to 8.64) in 1457 British urban men aged 40–64 from the Northwick Park Heart study (5).
These adverse cardiac effects of phobic anxiety appear to be in part secondary to increased risk of sudden cardiac death (SCD), where relative risks range from 1.8 to 8.4, suggesting that aversive environmental stimuli related to phobic states may act to trigger arrhythmic events (3, 4). In support, we previously found that phobic anxiety was associated with increased risk of ventricular arrhythmias in CHD patients enrolled in the VAGUS (Very Anxious Group Under Scrutiny) study, a prospective observational study designed to evaluate the effects of phobic anxiety on risk in CHD patients enrolled during hospitalization for coronary angiography (6). We extend these findings in the current study by examining the association between phobic anxiety and cardiac mortality in the VAGUS cohort. In addition, because reduced heart rate variability (HRV) has been found in anxiety disorders (7–11, 11, 12) and may contribute to anxiety-related risk, a second aim of the study was to identify the role of reduced HRV in phobic anxiety-related risk. As the prevalence of anxiety disorders is roughly twice as high in women as in men (13, 14), we also examined whether there are gender differences in the effects of phobic anxiety on cardiac mortality.
METHODS
Study Design
The VAGUS (Very Anxious Group Under Scrutiny) study is a prospective observational study designed to evaluate the effects of phobic anxiety on risk in CHD patients. Patients were enrolled at Duke University Medical Center between April 1999 and June 2002 while undergoing diagnostic cardiac catheterization. At the time of enrollment, approximately half of the sample reported progressive angina and one-third reported unstable angina; the remainder of the patients reported stable angina. A psychological interview was administered to patients either on the same day as cardiac catheterization (two-thirds of patients) or 1–4 days following catheterization. The interview included the Crown-Crisp phobic anxiety scale, the Beck Depression Inventory (BDI), and demographic and medical history information.
Study Population
Patient charts were reviewed to determine demographic and clinical variables, including eligibility requirements and comorbid medical conditions associated with mortality. The primary inclusion criteria for the VAGUS study was a diagnosis of CHD, defined as either significant coronary artery disease (≥ 75% occlusion of one coronary artery found during cardiac catheterization) or defined as a documented history of prior myocardial infarction/cardiac revascularization procedure. The majority of patients in the current study (91%) met inclusion criteria based on the presence of significant coronary artery disease. Patients were excluded if medical chart review identified the presence of a pacemaker, myocardial infarction or revascularization in the previous 30 days, or if they were not in normal sinus rhythm. Further details of the inclusion and exclusion critera appear in previous publications (6). Of the 955 enrolled patients, eight patients were unable to complete the phobic anxiety scale due to time constraints. The study was approved by the Duke University Medical Center Institutional Review Board, and all patients gave written informed consent before participating in the research protocol.
Medical Comorbidity Index
The presence of each of 13 medical conditions was determined from medical chart review, and the cumulative burden of medical comorbidity was estimated from the sum of mortality weights, based on the weighting coefficients suggested by Charlson (14) (See Table 1). Following the approach employed by Charlson et al (15), the comorbidity index scores were transformed into a four-level ordinal scale on which categories “0”, “1”, “2”, and “3” correspond to comorbidity index scores of 0, 1–2, 3–4, and ≥ 5, respectively.
Table 1.
Comorbid conditions and weights used to derive the Charlson comorbidity index
| Comorbid Medical Condition | Charlson weight | N | % of sample |
|---|---|---|---|
| Diabetes without end-organ damage | 1 | 256 | 27 |
| Peripheral vascular disease | 1 | 230 | 24 |
| Congestive heart failure | 1 | 200 | 21 |
| Rheumatological disease | 1 | 176 | 19 |
| Chronic pulmonary disease | 1 | 158 | 17 |
| Cerebrovascular disease | 1 | 151 | 16 |
| Peptic ulcer disease | 1 | 98 | 10 |
| Diabetes with end-organ damage | 2 | 106 | 11 |
| Moderate/severe renal disease | 2 | 131 | 14 |
| Any tumor, leukemia, or lymphoma | 2 | 27 | 3 |
| Moderate/severe liver disease | 3 | 13 | 1 |
| Metastatic tumor | 6 | 3 | 0.3 |
| HIV-positive | 6 | 1 | 0.1 |
Myocardial infarction and dementia not collected because they were exclusion criteria
Assessment of Phobic Anxiety
Phobic anxiety was measured using the phobic anxiety subscale of the Middlesex Hospital Questionnaire (16). This eight-item scale has two to three levels of possible responses for each item ranging from 0 to 2, with a score ranging from 0 to 16. In six patients, data was missing on one item, and the total score was therefore estimated by dividing the estimated score by the fraction of questions answered. Validity studies have shown that the phobic anxiety subscale successfully discriminates phobic disorders from other diagnostic groups in psychiatric patients (17, 18), with phobic neuroses being associated with mean phobic anxiety scores of 9 in mixed-gender samples (17), and mean scores of 10 in all-female samples (18).
Assessment of Depressive Symptomatology
Depressive symptomatology was measured using the original version of the Beck Depression Inventory (BDI), a 21-item self-report rating inventory which measures characteristic symptoms of depression for a total depression score ranging from 0–72 (19). In this sample, BDI scores ranged from 0 to 46 (mean: 7.3, median: 6.0, standard deviation: 6.6).
Estimation of short-term HRV
Beat-by-beat systolic blood pressure and heart rate were collected using the Finapres noninvasive blood pressure monitor (model 2300; Ohmeda, Madison, WI), with the appropriate sized cuff applied to the middle finger of one hand. The time series was edited for artifacts, and R-R intervals were calculated from the Finapres-derived heart rate (60,000/heart rate), linearly interpolated, and resampled at a frequency of 4 Hz. The Finapres-derived beat-by-beat heart rate values are highly correlated (r2=0.998) with beat-by-beat heart rate estimated by electrocardiographic recordings (20), and HRV indices calculated using the Finapress-derived heart rate are highly correlated with those calculated using ECG-derived R-R intervals (21–23). A fast Fourier transform was applied to the interpolated data after detrending and application of a Hanning filtering window. Power spectra were derived for each 300 second file using the Welch algorithm, which ensemble averages successive periodograms (24). High frequency HRV was estimated from the R-R interval power summed across the frequency band associated with respiration (0.13 to 0.5 Hz). Raw power was log-transformed before analysis in order to normalize the values. In order to be acceptable for spectral analysis, data was required to have at least 80% of the segment free of artifact or ectopy. Data failed to meet these criteria in 47 patients.
End Point Ascertainment
All patients were contacted by mail at 6 months and annually thereafter up to 4 years after the initial assessment, in order to determine death. An end-point committee consisting of two cardiologists (MHS and CO) blinded to phobic anxiety scores, reviewed medical records and telephone transcripts in order to determine cause of death. A consensus was reached for each case. Sudden cardiac death was defined as either (1) witnessed death that was noted to occur within 15 minutes of observed collapse or new cardiac symptoms, without preceding circulatory failure or other modes of death; or (2) unwitnessed death that occurred within an observation period of 72 hours and known to occur in the absence of pre-existing circulatory failure or other modes of death. The mean duration of the follow-up period was three years, and two patients were lost to follow-up.
Analytic Methods
Multivariable Cox regression analysis was used to examine whether phobic anxiety predicted outcome after adjusting for a priori selected predictors of gender, age, LVEF, and Charlson comorbidity category. In the primary analysis, phobic anxiety was modeled as a continuous variable, standardized to a mean of 0 and a standard deviation of 1 (about 2.8 points on the original scale). The impact of additional clinical factors that could influence the association between anxiety and mortality (beta-blocking agents, antidepressant/antianxiety drugs and presence of an internal cardioverter defibrillator) was also evaluated in exploratory models. We also examined whether gender moderated the effect of anxiety by adding a gender by phobic anxiety interaction term to the model, and in subsequent analyses we used within-gender estimates from separate models for men and women.
We examined the contribution of depressive symptomatology to the phobic anxiety/cardiac mortality relationship by adding the standardized BDI score to (1) the primary model containing the adjustment variables, the standardized phobic anxiety score, and the phobic anxiety by gender interaction term; (2) the female-only model in the presence and absence of the standardized phobic anxiety score.
In order to test whether low HRV acts as a mediator of the effects of phobic anxiety, we added HRV to the model simultaneously with phobic anxiety. In supplementary analyses, we evaluated risk across quartiles of phobic anxiety, in order to facilitate comparisons with previous studies of the influence of phobic anxiety on risk (3–5).
RESULTS
Baseline Characteristics of Study Sample
Patients ranged in age from 29 to 90 years (mean age, 62 years), and approximately one-third (n= 289) were women. Approximately one-fifth were of minority race/ethnicity as determined by self-report (70% African-American, 23% American Indian, 5% Asian, and 2% Hispanic). Most patients (75%) had a history of CHD defined as prior myocardial infarction and/or previous coronary artery revascularization, and most patients were taking a β-blocker (80%). Approximately one-quarter of the sample was taking antidepressant and/or benzodiazepine medications. Selective serotonin reuptake inhibitors (SSRI) were the most common type of antidepressants (n=132), followed by the nonselective serotonin reuptake inhibitors (n=41) and tricyclic antidepressants (n=7). In addition, 19 patients were taking bupropion (in 9 of these patients the primary indication was smoking cessation), and 11 patients were taking a SSRI combined with an additional antidepressant.
Phobic anxiety scores ranged from 0 to 13 in the male patients (mean: 2.5, median: 2.0, standard deviation: 2.4) and from 0 to 16 in the female patients (mean: 4.1, median: 3.0, standard deviation: 3.2). There were no differences in phobic anxiety scores between patients evaluated on the day of coronary angiography compared with the patients evaluated on the days subsequent to cardiac catheterization, suggesting that the measurement of phobic anxiety was resistant to events occurring during hospitalization. Phobic anxiety was higher in women compared to men (p < .001), inversely related to age (r = −.13, p < .001) and directly related to body mass index (BMI) (r=.10, p=.002) and to overall medical comorbidity estimated using the Charlson comorbidity index (r=.07, p<.03). As shown in Table 2, patients with elevated phobic anxiety were more likely have a history of hypertension, diabetes, and congestive heart failure (CHF), and more likely to be taking diuretics, antidepressants, and antianxiety drugs. However, they were less likely to have 3-vessel coronary disease, possibly reflecting the preponderance of female patients in the group with elevated phobic anxiety, since female patients were less likely than male patients to have 3-vessel disease (p<.001).
Table 2.
Baseline characteristics for patients stratified by quartile split of phobic anxiety. Values represent the mean ± 1 S.D.
| Characteristic | Phobic Anxiety Score | ||||
|---|---|---|---|---|---|
| 0 or 1 | 2 | 3 or 4 | ≥5 | p-value† | |
| Demographic data and medical history | |||||
| Age, mean ± SD, y | 64±11 | 62±11 | 62±11 | 60±12 | <.001 |
| Gender, % female | 21 | 24 | 22 | 55 | <.001 |
| Minority, % | 17 | 22 | 18 | 27 | .02 |
| Obesity, % | 35 | 36 | 42 | 50 | .001 |
| Alcohol use | |||||
| None, % | 53 | 59 | 56 | 75 | <.001 |
| ≤ 1 drink/day, % | 42 | 32 | 38 | 22 | <.001 |
| > 1 drink/day, % | 5 | 9 | 6 | 4 | .38 |
| Education | |||||
| < High School, % | 25 | 28 | 33 | 42 | <.001 |
| High School, % | 31 | 23 | 20 | 32 | .82 |
| > High School, % | 44 | 49 | 47 | 26 | <.001 |
| Smoking History, % | 72 | 70 | 73 | 70 | .72 |
| Current Smokers, % | 21 | 21 | 12 | 29 | .097 |
| Hypertension history, % | 74 | 80 | 78 | 86 | .002 |
| Diabetes, % | 33 | 34 | 37 | 46 | .007 |
| MI history, % | 42 | 39 | 45 | 45 | .58 |
| ICD, % | 2 | 3 | 3 | 5 | .17 |
| CHF, % | 18 | 19 | 20 | 27 | .02 |
| Baseline assessments | |||||
| Coronary artery disease | |||||
| 0-vessel, % | 9 | 8 | 7 | 12 | .28 |
| 1-vessel, % | 26 | 24 | 23 | 31 | .31 |
| 2-vessel, % | 20 | 23 | 24 | 22 | .72 |
| 3-vessel, % | 45 | 45 | 46 | 36 | .046 |
| LVEF (%) | 54±15 | 54±15 | 54±15 | 55±15 | .85 |
| Clinic SBP (mm Hg) | 131 ± 17 | 130 ± 17 | 130 ± 17 | 129±18 | .43 |
| Clinic DBP (mm Hg) | 69±11 | 69±11 | 69±11 | 67±10 | .13 |
| Medications, % | |||||
| β-Blockers | 81 | 80 | 78 | 80 | .68 |
| Lipid lowering | 61 | 59 | 68 | 63 | .71 |
| Angiotensin blockers | 71 | 69 | 70 | 67 | .34 |
| Diuretics | 36 | 34 | 33 | 44 | .046 |
| Benzodiazepines | 7 | 14 | 12 | 16 | .001 |
| Antidepressants | 10 | 18 | 20 | 34 | <.001 |
| SSRIs | 5 | 11 | 16 | 24 | <.001 |
| Non-SSRIs | 6 | 8 | 6 | 13 | .01 |
Abbreviations: ICD. implantable cardioverter defibrillator; CHD, congestive heart failure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SSRI, selective serotonin reuptake inhibitor
Obesity was defined as ≥ 30 kg/m2
p-value reflects the contrast between groups with phobic anxiety in the highest and lowest quartile
Mortality Risk
During the follow-up period, 137 patients died (14%) and 70% of the deaths were attributable to cardiovascular causes (cardiac: 64%; vascular: 6%). Of the 88 cardiac deaths, approximately 40% (n=35) were classified as probable cases of SCD. Cardiac mortality was observed in 11% of the 289 females (n=32) and in 8.5% of the 656 males (n=56), and did not differ significantly between male and female patients (p=.22). SCD was identified as the most likely cause of death in 4% of the male patients (n=27) and in 3% of the female patients (n=8). As shown in Table 3, male CHD patients who suffered a fatal cardiac event during the follow-up period were significantly older, had a lower LVEF, and were more likely to be diagnosed with 3-vessel coronary artery disease and CHF, when compared with male CHD patients who were alive at the end of follow-up. In contrast, female CHD patients who died of cardiac causes during follow-up showed no differences in age or CHD severity, when compared with the female CHD patients who survived; however, like the male patients, they were more likely to show a reduced LVEF and a higher incidence of CHF.
Table 3.
Baseline clinical and demographic data stratified by cardiac death during follow-up. Values represent the mean ± 1 S.D.
| Male Patients | Female Patients | |||||
|---|---|---|---|---|---|---|
| Survivors | Deaths | p Value† | Survivors | Deaths | p Value†† | |
| Age (years) | 61±11 | 66±11 | <.001 | 63±12 | 63±12 | .968 |
| Minority, % | 18 | 14 | .492 | 28 | 44 | .059 |
| Obesity, % | 38 | 38 | .468 | 48 | 47 | .883 |
| Diabetes, % | 32 | 45 | .056 | 46 | 63 | .070 |
| Smoking history, % | 78 | 77 | .892 | 58 | 47 | .232 |
| % smoking | 20 | 12 | .197 | 25 | 33 | .472 |
| 3-vessel CAD, % | 45 | 71 | <.001 | 34 | 41 | .448 |
| ICD, % | 2 | 13 | <.001 | 2 | 19 | <.001 |
| LVEF (%) | 51±12 | 44±15 | <.001 | 54±10 | 45±17 | <.001 |
| CHF, % | 16 | 38 | <.001 | 25 | 63 | <.001 |
| Medications: | ||||||
| Beta-blockers | 81 | 75 | .239 | 79 | 72 | .358 |
| Diuretics | 29 | 54 | <.001 | 47 | 66 | .048 |
| Antidepressants | 16 | 20 | .517 | 29 | 38 | .334 |
| Benzodiazepines | 11 | 21 | .019 | 12 | 22 | .141 |
| Psychological Measures | ||||||
| Phobic anxiety score | 2.5 ± 2.3 | 2.3 ± 2.7 | .666 | 3.9 ± 3.2 | 5.7 ± 3.2 | .003 |
| BDI score | 6.7 ± 6.0 | 7.9 ± 7.0 | .164 | 8.2 ± 7.0 | 11.9 ± 9.5 | .008 |
| Parasympathetic Cardiac Control | ||||||
| HF power (ln msec2) | 4.9±1.1 | 4.7±1.1 | .180 | 5.1±1.2 | 4.6±1.4 | .030 |
Abbreviations: ICD. implantable cardioverter defibrillator; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; CHF, congestive heart failure; BDI, Beck Depression Inventory; HF, high frequency
p-value reflects the contrast between survivors and deaths in male patients
p-value reflects the contrast between survivors and deaths in female patients
Phobic anxiety was related to modest increases in risk of cardiac mortality in the VAGUS cohort (unadjusted hazard ratio [HR], 1.22; 95% CI, 1.01 to 1.47, p=.04) and this association was maintained after adjustment for the a priori selected variables of LVEF, gender, and medical comorbidity (HR, 1.23; 95% CI, 1.00–1.51, p=.05). There was an interaction between gender and phobic anxiety in the prediction of cardiac mortality following adjustment for the a priori selected adjustment variables (HR, 1.52; 95% CI, 0.99–2.34, p= .058). Phobic anxiety was unrelated to increased risk of SCD in the full sample (unadjusted HR, 1.11; 95% CI, 0.81 to 1.52, p=.52); however, as with cardiac mortality risk, there was an interaction between gender and phobic anxiety in the prediction of SCD (HR, 2.20; 95% CI, 1.06–4.53, p=.03). The gender-phobic anxiety interaction was not attenuated following adjustment for medications (beta-blocking agents and antidepressant/antianxiety drugs) and the presence of an ICD (cardiac mortality HR, 1.56; 95% CI, 1.01–2.40, p=.04; SCD HR, 2.09; 95% CI, 1.00–4.39, p=.05).
Within-Gender Analyses
Given the presence of the gender-phobic anxiety interaction in the primary model, we examined the association between phobic anxiety and cardiac risk in separate models for men and women. Phobic anxiety was associated with a 1.56-fold increased risk of cardiac mortality (95% CI, 1.15 to 2.11, p=.004) and a 2.02-fold increased risk of SCD (95% CI, 1.16–3.52; p=.01) in the female CHD patients, but was not reliably associated with increased risk of cardiac mortality or SCD in the male CHD patients (cardiac mortality HR, 0.92; 95% CI, 0.70 to 1.22, p=.56; SCD HR, 0.86; 95% confidence interval, 0.57–1.30; p=.48). The association between phobic anxiety and mortality risk in the women remained significant following adjustment for the a priori selected variables of LVEF, age and medical comorbidity (adjusted HR, 1.52; 95% CI, 1.10–2.10, p=.01) and following further adjustment for the presence of an ICD, beta-blocker use, and antidepressant/antianxiety drug use (fully adjusted HR, 1.49; 95% CI, 1.06–2.10, p=.02) (See Table 4). Similarly, the association between phobic anxiety and SCD remained significant after adjustment for LVEF, age, and medical comorbidity (adjusted HR, 1.96; 95% CI, 1.06–3.63, p=.03). The additional exploratory variables of ICD and medication use were not included in the model predicting SCD risk because of the relatively small number of SCD cases.
Table 4.
Cox proportional hazards regression analyses for cardiac mortality in male and female CHD patients
| Variable* | Men | Women | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age/10 yr† | 1.72 | 1.29–2.29 | <.001 | 1.07 | .78–1.48 | .67 |
| LVEF/15, %†† | 0.68 | 0.53–0.87 | .002 | 0.79 | .58–1.06 | .11 |
| Charlson category | 1.46 | 1.09–1.96 | .01 | 1.62 | 1.04–2.54 | .03 |
| Beta-blocker use | 0.73 | 0.39–1.37 | .33 | 0.71 | 0.32–1.59 | .40 |
| Antidepressant or Anxiolytic medication | 1.50 | 0.85–2.66 | .16 | 1.60 | 0.77–3.32 | .21 |
| Presence of ICD | 5.75 | 2.49–13.28 | <.001 | 6.56 | 2.56–16.81 | <.001 |
| Standardized phobic anxiety score | 0.99 | 0.74–1.33 | .96 | 1.49 | 1.06–2.10 | .02 |
Abbreviations: LVEF, left ventricular ejection fraction; CI, confidence interval; ICD, internal cardioverter defribrillator
Values for each variable in the model are adjusted for all other variables in the model
Age divided by 10 indicates that the HR values associated with age reflect a decade
LVEF divided by 15 indicates that the HR values associated with LVEF reflect a 15% change
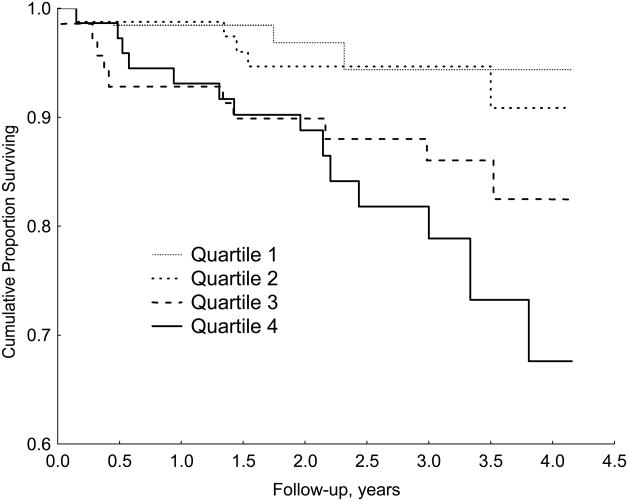
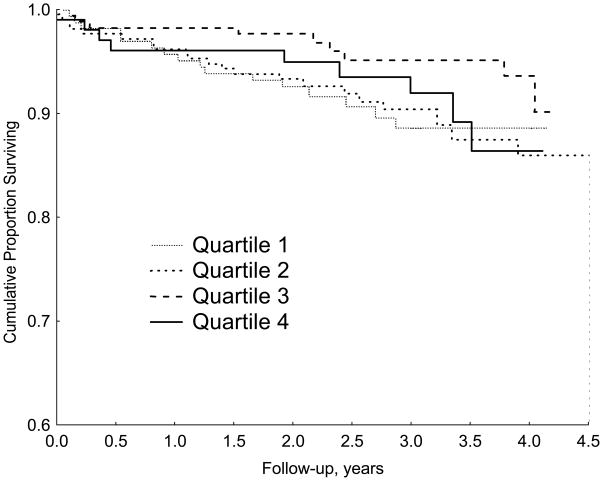
Analysis of the continuous phobic anxiety score suggested a linear association with cardiac mortality in a stepwise fashion. Because some readers may be relatively unfamiliar with interpreting associations between continuous predictor variables and responses, we also present the time-to-event results with phobic anxiety categorized by quartile, using the lowest quartile of phobic anxiety as the reference group (See Table 5). Scores on the phobic anxiety scale ranged from 7 to 16 (mean: 8.5±1.9) in the female CHD patients with phobic anxiety scores in the highest quartile and these patients were at a 2.6-fold increased risk of cardiac mortality compared with the remaining female patients (95% confidence interval, 1.30–5.27, p=.007), and at a 6.0-fold increased risk of SCD (95% confidence interval, 1.43–25.43, p=.01). Figures 1 and 2 compare the time course of survival in female and male CHD patients separated by quartile of the phobic anxiety score.
Table 5.
Hazard Ratios and 95% CI of 3-year Cardiac Mortality in male and female CHD patients
| Phobic Anxiety Quartile | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P for trend | |
| WOMEN | |||||
| No. of cases | 3 | 5 | 10 | 14 | |
| Unadjusted | 1.0 | 1.39 (0.33–5.83) | 3.10 (0.85–11.28) | 4.84 (1.39–16.90) | .002 |
| Multivariate 1† | 1.0 | 2.04 (0.47–8.84) | 3.28 (0.88–12.32) | 4.11 (1.11–15.22) | .007 |
| Multivariate 2†† | 1.0 | 2.34 (0.39–14.17) | 2.39 (0.62–9.15) | 3.08 (0.71–13.33) | .015 |
| MEN | |||||
| No. of cases | 16 | 22 | 9 | 9 | |
| Unadjusted | 1.0 | 0.98 (0.51–1.87) | 0.49 (0.21–1.10) | 0.86 (0.38–1.95) | .25 |
| Multivariate 1† | 1.0 | 1.10 (0.57–2.12) | 0.51 (0.22–1.16) | 1.06 (0.46–2.45) | .45 |
| Multivariate 2 | 1.0 | 1.07 (0.55–2.08) | 0.50 (0.22–1.18) | 1.06 (0.45–2.47) | .47 |
Abbreviations: CI, confidence interval
Adjusted for a priori selected variables of age, left ventricular ejection fraction, and medical comorbidity.
Adjusted for additional variables of beta-blocker use, antidepressant/antianxiety medication use, and presence of an internal cardioverter defibrillator
Figure 1.
Kaplan-Meier curves showing the association of quartile of phobic anxiety score on survival in female CHD patients.
Figure 2.
Kaplan-Meier curves showing the association of quartile of phobic anxiety score on survival in male CHD patients.
Depressive Symptomatology and Phobic Anxiety-Related Risk
We examined an additional model in which standardized BDI score was included in the primary full cohort model already containing the a priori selected adjustment variables, the continuous phobic anxiety score, and the phobic anxiety by gender interaction. BDI score was a significant predictor of cardiac mortality in this model, with a modest effect size (HR, 1.29, 95% CI 1.06–1.57, p = .01). BDI score was unrelated to risk of SCD (HR, 1.29, 95% CI 0.79–1.57, p = .54). The phobic anxiety by gender interaction in this model was not substantively changed from that observed in the primary model (cardiac mortality HR, 1.49; 95% CI, 0.96–2.30, p=.076; SCD HR, 2.28; 95% CI, 1.09–4.77, p=.03). BDI did not interact with gender in the prediction of cardiac mortality (HR, 1.09; 95% CI, 0.77–1.54, p=.64) or in the prediction of SCD (HR, 1.65; 95% CI, 0.91–3.01, p=.10).
Using within-gender models, among female patients BDI score was associated with a 1.30-fold increased risk of cardiac mortality (95% CI, 1.01 to 1.67, p=.04) and a trend towards an increased risk of SCD (HR, 1.47, 95% CI, 0.96–2.24, p=.08) after adjusting for the a priori selected predictors of age, LVEF, and medical comorbitidy. When phobic anxiety was included in this model, the magnitude of the effect of BDI score on cardiac mortality was reduced and nonsignificant (HR, 1.20; 95% CI, 0.91 to 1.58, p=.20). However, the magnitude of the effect of phobic anxiety score was also reduced and no longer significant in this model (phobic anxiety HR, 1.32; 95%CI, 0.92 to 1.89, p=.13).
Heart Rate Variability as a Mediator of Phobic Anxiety-Related Risk
In female CHD patients, high frequency HRV was inversely associated with phobic anxiety (r= −.14, p<.02), and was significantly related to risk of cardiac mortality, with a one log unit increase in HF HRV associated with a HR of .69 (95% CI, 0.49–0.97, p=.03). However, adding the HRV term to the adjusted Cox model did not alter the association between phobic anxiety and cardiac mortality (adjusted HR for standardized phobic anxiety score in the women cohort, 1.58; 95% CI, 1.09–2.28; p=0.015), suggesting that reduced HRV, measured under resting supine conditions, is unlikely to account for the association between phobic anxiety and risk.
DISCUSSION
The present findings of an association between phobic anxiety and an increased risk of cardiac mortality and SCD in CHD patients extends previous findings of an association between phobic anxiety and increased risk of cardiac mortality and SCD in men and women free of established cardiovascular disease. Elevated phobic anxiety was particularly common in female CHD patients, and a 50% increase in risk of cardiac mortality and a doubling of risk for SCD was observed for every unit increase in standardized phobic anxiety scores reported by the female patients. The prevalence of clinically significant phobic anxiety in this sample was relatively low, as evidenced by the observation of a median phobic anxiety score of 2 in the CHD patients, which is substantially less than the mean scores ranging from 9 to 10 shown in psychiatric patients with clinically significant phobic neurosis (17, 18). Thus, the restricted range of phobic anxiety scores in the sample may have attenuated our estimates or it’s associated hazard. As an adjunct to the analysis of phobic anxiety as a continuous score, we also examined phobic anxiety comparing risk among individuals with the highest anxiety to the remaining participants. Dichotomizing the sample of female CHD patients at the 75th percentile of the phobic anxiety scores (≥7), women in the upper quartile showed a doubling of risk for fatal CHD and a six-fold increased hazard of SCD when compared with the remaining female CHD patients. We note, however, that making such groups, especially with a limited number of events, can yield somewhat less reliable estimates compared to modeling the continuous phobic anxiety scores (25).
These findings extend previous findings in healthy women free of cardiovascular disease, where both phobic anxiety and panic disorder have been associated with increased risk of mortality. In the Women’s Health Initiative study of 3369 medically healthy community-dwelling postmenopausal women, panic disorder was associated with a 1.8 fold increased risk of all-cause mortality (26). Similarly, in the Nurses’ Health Study Cohort of 72,359 women with no history of cardiovascular disease, women with phobic anxiety scores greater than 3 were at a 1.3-fold increased risk of fatal CHD and a 1.6-fold increased risk of SCD compared with those reporting phobic anxiety scores less than 2 (3). The increased risk in the Nurses’ Health Study Cohort was attributed in part to an association between phobic anxiety and factors linked to the metabolic syndrome (hypertension, diabetes, and elevated cholesterol). In the present sample of women diagnosed with CAD, phobic anxiety was not related to either elevated clinic blood pressure or lipid profile, although significantly more women with elevated phobic anxiety reported a history of hypertension. The female CHD patients with high phobic anxiety were more likely to be overweight and to be diagnosed with diabetes and CHF; however, adjusting for medical comorbidity using the weighted Charlson index did not significantly attenuate the magnitude of the association between phobic anxiety and risk.
In contrast to the association between phobic anxiety and fatal CHD in female patients, phobic anxiety was unrelated to risk in male CHD patients. Self-reported phobic anxiety levels were significantly lower in males than in females in hospitalized CHD patients, and one interpretation of the lack of an association between phobic anxiety and cardiac risk in the male patients is that phobic anxiety may not have reached the threshold to produce adverse effects in these patients. It is also possible that phobic anxiety was underreported in the male patients due to situational pressure to be perceived as masculine. Phobic anxiety was typically assessed while patients were waiting for or recovering from cardiac catheterization, and in most cases a verbal interview was necessary due to the logistical limitations related to the cardiac catheterization. Previous studies have reported that men report experiencing more difficulty in expressing negative emotions than women, especially under situations where they would be perceived as weak or ineffectual (27) and fear of expressing symptoms may have been underscored by the presence of a spouse or significant other, which was more common in male than female CHD patients. Previous epidemiological studies of men free of baseline CHD measured phobic symptoms using mailed questionnaires, and in these studies phobic anxiety was found to be related to increased risk of cardiac mortality and SCD (4, 5).
It has been suggested that reduced HRV may be involved in the adverse effects of phobic anxiety on mortality. High levels of anxiety have been associated with reduced levels of high frequency HRV (7–12) and more specifically, phobic anxiety has also been found to be associated with reduced HRV measured during paced breathing (11). Our findings that phobic anxiety was associated with reduced HRV in female CHD patients are consistent with these earlier findings; however, reduced in HRV did not explain the increased risk of cardiac mortality associated with phobic anxiety in the current study of hospitalized CHD patients. Although these findings suggest that resting levels of HRV are not involved in the adverse cardiac effects of phobic anxiety, they do not rule out an effect of change in HRV associated with phobic triggers during naturalistic conditions.
There are a number of logistical issues related to assessing patients in the hospitalized setting that may have influenced anxiety and HRV. We cannot rule out an impact of procedure-related anxiety either on the day of cardiac catheterization, or on the subsequent day when revascularization procedures were often in the planning stages. However, we did not detect any differences in phobic anxiety or HRV in patients assessed on the day of cardiac catheterization compared with those assessed on the day following cardiac catheterization. Phobic anxiety is considered to be a stable measure (i.e., “trait”) of an individual’s tendency to respond to certain environmental situations (fear of heights, fear of crowded places, fear of using public transportation) as anxiety-provoking, and is considered to be relatively immune to stressors unrelated to the phobia. In contrast, we noted a stepwise increase in general (non-phobic) anxiety measured using the Hospital Anxiety and Depression Scale and the Speilberger state-trait anxiety inventory during the course of hospitalization (data not shown).
An additional limitation is that approximately 40% of the patients were evaluated for anxiety and HRV following Benadryl administration, which is routinely given to all patients prior to cardiac catheterization to produce sedation. When contrasting patients assessed before, during, and after Benadryl, we noted a significant increase in sleepiness measured on a 3-point sleepiness scale, followed by a reduction in sleepiness after Benadryl. In these three groups, there were no differences in phobic anxiety or HRV, suggesting that Benadryl had minimal effects on the variables of interest.
Scores on the BDI were moderately correlated with phobic anxiety scores and predictive of increased risk of cardiac mortality in the current cohort of female CHD patients. The findings that neither standardized BDI score nor standardized phobic anxiety score was predictive when both were included in the survival model suggests that these factors share a common mechanism in the prediction of cardiac risk. One limitation of this analysis is that the range of phobic anxiety scores encompassed by a one-unit change in a standardized score are relatively small and may not be indicative of the effects of clinically significant phobic anxiety (17, 18).
In summary, the present study found that elevated phobic anxiety is common in women with established CHD and is independently associated with increased risk of cardiac mortality and SCD. Reduced parasympathetic cardiac control, measured under supine conditions, does not appear to mediate the adverse effects of phobic anxiety in women. Ambulatory Holter monitoring studies designed to evaluate the HRV during naturalistic conditions are needed to determine whether phobic anxiety is associated with excessive reductions in HRV during the acute increases in phobic anxiety experienced under naturalistic conditions, and whether these HRV changes are involved in phobic anxiety-related risk.
Acknowledgments
This study was supported by funds from the National Institutes of Health, grant number HL060826 and HL070954.
Abbreviations
- CHD
coronary heart disease
- SCD
sudden cardiac death
- MI
myocardial infarction
- LVEF
left ventricular ejection fraction
- BMI
body mass index
- HRV
heart rate variability
- BDI
Beck Depression Inventory
- CHF
congestive heart failure
- ICD
internal cardioverter defibrillator
Footnotes
There are no conflicts of interest.
References
- 1.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of twelvemonth DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Archives of General Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:480–7. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 4.Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89:1992–7. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 5.Haines AP, Imeson JD, Meade TW. Phobic anxiety and ischaemic heart disease. British Medical Journal. 1987;295:297–9. doi: 10.1136/bmj.295.6593.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins LL, Blumenthal JA, Davidson JRT, Babyak MA, McCants CB, Jr, Sketch MH., Jr Phobic anxiety, depression, and risk of ventricular arrhythmias in patients with coronary heart disease. Psychosomatic Medicine. 2006;68:651–6. doi: 10.1097/01.psy.0000228342.53606.b3. [DOI] [PubMed] [Google Scholar]
- 7.Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry. 1997;41:627–9. doi: 10.1016/s0006-3223(96)00525-2. [DOI] [PubMed] [Google Scholar]
- 8.Yeragani VK, Balon R, Pohl R, Ramesh C, Glitz D, Weinberg P, Merlos B. Decreased R-R variance in panic disorder patients. Acta Psychiatrica Scand. 1990;81:554–9. doi: 10.1111/j.1600-0447.1990.tb05498.x. [DOI] [PubMed] [Google Scholar]
- 9.Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, Srinivasan K, Weinberg P. Decreased heart rate variability in panic disorder patients: a study of power-spectral analysis of heart rate. Psychiatry Research. 1993;46:89–103. doi: 10.1016/0165-1781(93)90011-5. [DOI] [PubMed] [Google Scholar]
- 10.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39:255–66. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 11.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Decreased heart rate variability in men with phobic anxiety (data from the normative aging study) American Journal of Cardiology. 1995;75:882–5. doi: 10.1016/s0002-9149(99)80680-8. [DOI] [PubMed] [Google Scholar]
- 12.Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosomatic Medicine. 1998;60:498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Wittchen HU, Zhao S, Kessler RC, Eaton WW. DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:355–64. doi: 10.1001/archpsyc.1994.03950050015002. [DOI] [PubMed] [Google Scholar]
- 14.Magee WJ, Eaton WW, Wittchen HU, McGonagle KA, Kessler RC. Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Archives of General Psychiatry. 1996;53:159–68. doi: 10.1001/archpsyc.1996.01830020077009. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Crown S, Crisp AH. A short clinical diagnostic self-rating scale for psychoneurotic patients: the Middlesex Hospital questionnaire. British Journal of Psychiatry. 1966;112:917–23. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- 17.Mavissakalian M, Michelson L. The Middlesex Hospital Questionnaire: a validity study with American psychiatric patients. British Journal of Psychiatry. 1981;139:336–40. doi: 10.1192/bjp.139.4.336. [DOI] [PubMed] [Google Scholar]
- 18.Crisp AH, Jones MG, Slater S. The Middlesex Hospital Questionnaire: a vailidity study. British Journal of Medical Psychology. 1978;51(3):269–80. doi: 10.1111/j.2044-8341.1978.tb02472.x. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatr. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Low PA, Opfer-Gehrking TL, Zimmerman IR, O’Brien PC. Evaluation of heart rate changes: electrocardiographic versus photoplethysmographic methods. Clinical Autonomic Research. 1997;7(2):65–8. doi: 10.1007/BF02267748. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco S, Gonzalez R, Jimenez J, Roman R, Medina V, Azpiroz J. Comparison of the heart rate variability parameters obtained from the electrocardiogram and the blood pressure wave. Journal of Medical Engineering & Technology. 1998;22(5):195–205. doi: 10.3109/03091909809032542. [DOI] [PubMed] [Google Scholar]
- 22.Giardino ND, Lehrer PM, Edelberg R. Comparison of finger plethysmograph to ECG in the measurement of heart rate variability. Psychophysiology. 2002;39:246–53. doi: 10.1017/S0048577202990049. [DOI] [PubMed] [Google Scholar]
- 23.McKinley PS, Shapiro PA, Bagiella E, Myers MM, De Meersman RE, Grant I, Sloan RP. Deriving heart period variability from blood pressure waveforms. Journal of Applied Physiology. 2003;95(11431):1438. doi: 10.1152/japplphysiol.01110.2002. [DOI] [PubMed] [Google Scholar]
- 24.Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short modified periodograms. IEEE Trans Audio Electroacoust. 1967;15:70–3. [Google Scholar]
- 25.Clinical Prediction Models: a Practical Approach to Development, Validation, and Updating. New York: Springer; 2009. [Google Scholar]
- 26.Smoller JW, Pollack MH, Wassertheil-Smoller S, Jackson RD, Oberman A, Wong ND, Sheps D. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the women’s health initiative observational study. Archives of General Psychiatry. 2007;64:1153–60. doi: 10.1001/archpsyc.64.10.1153. [DOI] [PubMed] [Google Scholar]
- 27.Shields SA. The role of emotion, beliefs and values in gender development. Review of personality and social psychology. 1995;15:212–32. [Google Scholar]