Abstract
Objectives
To evaluate the efficacy and safety of drug-eluting stents (DES) when compared with bare metal stents (BMS) in patients with moderate to severe calcified coronary lesions.
Background
Calcified coronary lesions present unique technical challenges during percutaneous coronary intervention (PCI) and it is not known if drug eluting stents (DES) are as safe and as effective in the presence of calcium, as randomized trials typically exclude this common patient subset.
Methods
We evaluated patients with PCI of a single calcified lesion enrolled across five recruitment waves in the NHLBI Dynamic Registry between 1997 and 2006. Patients were divided into two groups based on the stent type- BMS and DES. The primary efficacy outcome was the need for repeat revascularization at 1 year and the primary safety outcome was a composite of death and myocardial infarction at 1 year.
Results
Among the 1537 patients included in the analysis, 884 (57%) underwent PCI with BMS and 653 (43%) with DES. DES use was associated with a significant reduction in the risk of repeat revascularization (10.0% vs. 15.3%; p = 0.003) with no significant higher risk of primary safety outcome (9.3% vs. 10.5%; p = 0.45) when compared to the BMS group. In a propensity score adjusted analysis, DES use was associated with a significantly lower risk in repeat revascularization (HR = 0.57, 95% CI 0.40-0.82; p = 0.002) and no significant difference in the risk of death and myocardial infarction (HR = 0.78; 95% 0.53-1.15; p = 0.20) compared to BMS group.
Conclusion
In this large multicenter registry of patients with a moderate to severe calcified coronary lesion, use of DES compared to BMS was associated with significant reduction in the risk of repeat revascularization without any increase in death and myocardial infarction.
Keywords: calcified lesion, percutaneous coronary intervention, stents
INTRODUCTION
Calcified coronary lesions present unique challenges for percutaneous coronary intervention (PCI) with a smaller final lumen diameter and less acute lumen gain with stenting, when compared to non-calcified lesions (1). Furthermore, there is a risk for stent under expansion, a lower procedural success rate and a more frequent rate of acute complications, such as acute dissection, as well as a greater propensity for restenosis (1,2).
Drug eluting stents (DES) have revolutionized the field of interventional cardiology by preventing or delaying neointimal hyperplasia and thereby effectively lowering the rate of restenosis following coronary intervention (3). However, data on efficacy of DES in the presence of calcium are limited. The pilot and pivotal studies of DES have routinely excluded this patient subset, though in the real-world setting DES continues to be used on an off-label basis for this lesion subset. Despite the dramatic early efficacy of DES in reducing restenosis compared with bare metal stents (BMS) (4,5), there is concern that DES might lead to higher rates of stent thrombosis (6). Moreover, given the technical challenges in treating calcified lesions with greater propensity for under-deployment of stents, the safety of DES in this setting is not well defined.
The objectives of the present study were to assess the efficacy and safety of DES in comparison to BMS in a real-world cohort of patients undergoing PCI for a moderate to severe calcified lesion, enrolled in the National Heart, Lung and Blood Institute (NHLBI) sponsored Dynamic Registry.
METHODS
The NHLBI Dynamic Registry is a multicenter registry of 17 centers in North America. Recruitment of consecutive patients undergoing PCI was completed during pre-specified time intervals or “waves”. Informed consent was obtained from each patient and the institutional review board at each center approved the study. Five recruitment waves of approximately 2000 patients each have been enrolled and followed over the past 10 years to examine trends in PCI (wave 1: 1997 to 1998, 2524 patients; wave 2: 1999, 2105 patients; wave 3: 2001 to 2002, 2047 patients; wave 4: 2004, 2112 patients; and wave 5: 2006, 2178 patients). Data on baseline demographic, clinical, angiographic, procedural characteristics and discharge medication during the index PCI were collected for each patient. Patients were followed for the occurrence of death, myocardial infarction, stroke, need for coronary artery bypass grafting (CABG) and repeat PCI during index hospitalization and at 1 month, 6 month and 1 year post-intervention. Follow-up rates at 1 year were at least 90% for all waves. If patients underwent subsequent PCI, vessel-specific and lesion-specific data were collected whenever possible to determine target-vessel revascularization.
Study Population
For this analysis, consecutive patients who received PCI with a stent, for the treatment of a single moderate to severe calcified lesion during index PCI were included. Lesions were reported as “calcified” if the operator identified calcification on fluoroscopy. Of the 10,966 patients enrolled, 3133 (29%) patients had calcified lesions. Of these, 1161 patients who had PCI attempted to more than one lesion were excluded from this study to avoid a contamination effect of non-calcified lesions. During the recruitment of patients enrolled in waves 1-3 (1997 to 2002), only BMS were available while DES were available for use in patients recruited in waves 4 and 5 (2004 and 2006). However only a minority of patients in the most recent recruitment waves received BMS, and because of the evidence of a large selection bias (i.e., more cardiogenic shock in patients with bare-metal stents), patients in waves 4 and 5 who received BMS alone or BMS and DES were excluded to minimize ambiguity in the results. Thus, the total study population consisted of 1537 patients- BMS group from waves 1-3 (n=884) and DES group from waves 4-5 (n=653) (Figure 1).
Figure 1.
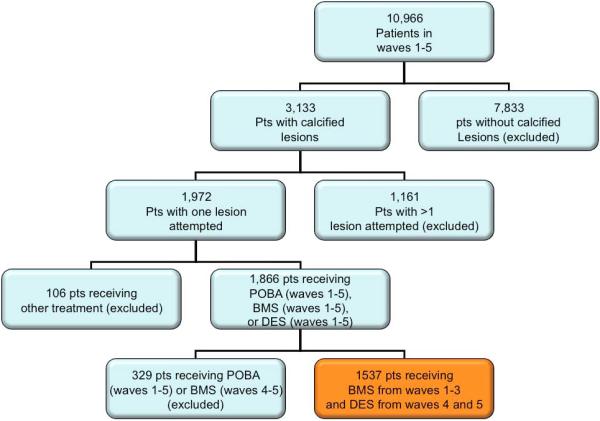
Patient selection.
Outcomes
We evaluated primary safety outcome of death (all-cause) or myocardial infarction and primary efficacy outcome of repeat revascularization (PCI or CABG). Secondary outcomes were individual components of the primary outcomes considered separately.
Repeat revascularization was defined as the combined end point of repeat PCI or CABG during the follow-up period. Repeat PCI was defined as any non-staged repeat PCI in the follow-up period, and included revascularization of target lesions and target vessels. The outcomes were evaluated at 30-day and 1-year follow-up. In-stent thombosis was defined as recurrent ischemia with angiographic documentation of total stent occlusion, i.e., definite stent thrombosis based on ARC criteria. Angiographic success was defined as attempted lesions with post PCI stenosis <50% and the difference post – pre stenosis >=20%. Procedural success was defined as angiographic success without death, Q-wave MI or emergency CABG.
Statistical Analysis
The study cohort was initially divided into two groups based on the treatment strategy of the index PCI- BMS group and DES group. Patient baseline demographic characteristics, cardiac risk factors, cardiac presentation, periprocedural medications, angiographic and procedural characteristics were compared between device groups with the use of Wilcoxon rank-sum test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Cumulative 30-day and one-year incidence rates of primary and secondary outcomes were estimated by the Kaplan–Meier method and tested by the log-rank statistic.
To adjust for the bias inherent to the selection of device, propensity scores were used. Propensity analysis aims to identify patients with similar probabilities of device type on the basis of observed clinical characteristics (7,8). With the use of a multivariable logistic regression model that includes basic risk parameters as the independent variables, the probability of a patient being assigned to the device group was determined. The following baseline clinical characteristics were considered in a multivariate probit model to define a propensity score -1. Baseline risk factors- age, sex, prior percutaneous procedures, prior CABG, prior myocardial infarction, cerebrovascular, renal, peripheral vascular disease, cancer, diabetes, heart failure, hypertension, hypercholesterolemia. 2. Presentation details- reason for revascularization, cardiogenic shock, acuity. 3. Lesion characteristics- vessel disease, any total occlusions, number of significant lesions, lesion location, lesion length, diameter stenosis, TIMI flow before procedure, evidence of thrombus, high-risk lesion, final percent stenosis, and 4. Medications within 24 hours of the procedure- aspirin, clopidogrel/ticlopidine and usage of glycoprotein IIb/IIIa. The risk of primary and secondary outcomes was evaluated after adjusting for the propensity score.
All statistical analyses were performed with the use of SAS software, version 9.1. A two-sided P-value of 0.05 or less indicated statistical significance.
RESULTS
Among the 1537 patients with a single moderate to severe calcified lesion included in this study, 884 (51%) patients underwent PCI with BMS and 653 (37%) patients with DES.
Baseline characteristics
Baseline demographic, clinical, angiographic and procedural characteristics are summarized in tables 1-3. When compared to patients receiving BMS, the DES group were older, had a higher prevalence of cardiac risk factors, including diabetes, hypertension, hypercholesterolemia, prior CABG surgery, prior PCI procedures, as well as a higher prevalence of severe non-cardiac concomitant disease (cerbrovascular, renal and peripheral vascular disease) (Table 1). The percentage of patients with prior myocardial infarction was lower in patients receiving DES. The frequency of DES patients presenting with stable/unstable angina was lower but a higher percentage of these patients were treated with a thienopyridine or a glycoprotein IIb/IIIa receptor antagonist at the time of PCI. Triple vessel disease was more common among patients treated with DES compared to BMS (Table 2). The majority of treated lesions were in the left anterior descending coronary artery in both groups. When compared to lesions treated in the BMS group, the intervened lesions in the DES patients were more often type C, smaller in mean reference vessel size, longer in mean length with lower use of rotational atherectomy and lower in final mean percentage stenosis (Table 3). Of note, intravascular ultrasound was used in 15% of patients.
Table 1.
Demographics and Baseline Risk Factors
| Parameter | BMS (N=884) |
DES (N=653) |
P-Value |
|---|---|---|---|
| Mean age (yrs) | 66 | 67 | 0.03 |
| Female, % | 39.9 | 36.3 | 0.15 |
| White, % | 86.2 | 80.2 | 0.01 |
| Mean Body Mass Index (kg/m2) | 28.4 | 29.0 | 0.16 |
| Diabetes, % | 27.4 | 36.1 | <0.001 |
| Hypertension, % | 65.5 | 83.1 | <0.001 |
| Hypercholesterolemia, % | 62.8 | 81.0 | <0.001 |
| Never Smoked, % | 34.2 | 33.0 | 0.88 |
| Prior Myocardial Infarction, % | 33.8 | 26.9 | 0.004 |
| Known Heart Failure, % | 11.9 | 12.7 | 0.67 |
| Prior Coronary Artery Bypass Surgery, % | 13.1 | 22.4 | <0.001 |
| Prior Percutaneous Procedure(s), % | 23.7 | 33.7 | <0.001 |
| Severe Non-Cardiac Concomitant Disease, % |
37.1 | 44.4 | 0.004 |
| Cerebrovascular, % | 6.7 | 10.6 | 0.006 |
| Renal, % | 5.2 | 10.6 | <0.001 |
| Peripheral Vascular Disease, % | 7.3 | 11.1 | 0.01 |
| Pulmonary, % | 9.4 | 10.0 | 0.71 |
| Cancer, % | 8.8 | 9.2 | 0.80 |
| Other, % | 12.8 | 13.1 | 0.89 |
Denotes significance across groups. BMS = bare metal stent; DES = drug eluting stent.
Table 3.
Procedural Characteristics
| Parameter | BMS (N=884) |
DES (N=653) |
P-Value |
|---|---|---|---|
| Lesion location, % | 0.16 | ||
| Left Main Coronary Artery | 1.6 | 2.1 | |
| Left Anterior Descending Artery | 45.2 | 43.5 | |
| Left Circumflex Artery | 17.4 | 20.7 | |
| Right Coronary Artery | 31.9 | 31.5 | |
| Graft | 3.8 | 2.1 | |
| TIMI Grade 0/1 pre-procedure | 14.5 | 11.5 | 0.02 |
| ACC/AHA Lesion Classification, % | <0.001 | ||
| A | 6.7 | 6.5 | |
| B1 | 21.8 | 23.4 | |
| B2 | 48.8 | 35.2 | |
| C | 22.6 | 34.9 | |
| Reference Vessel Size, mean, median | 3.2, 3 | 3.1, 3 | <0.001 |
| Lesion Length, mean, median | 13.3, 12 | 18.8, 15 | <0.001 |
| Diameter % Stenosis, mean, median | 86.0, 90 | 85.2, 90 | 0.004 |
| Total Occlusion, % | 10.6 | 9.3 | 0.41 |
| Rotational Atherectomy, % | 10.9 | 4.0 | <0.001 |
| TIMI Grade 0/1 Post-Procedure, % | 0.1 | 0.2 | 0.34 |
| Lesion Deemed Successful by Operator, % |
99.3 | 99.2 | 0.84 |
| Angiographic Success, % | 99.4 | 99.7 | 0.45 |
| Procedural Success, % | 98.0 | 99.7 | 0.003 |
| Final % Stenosis, mean, median | 3.0, 0 | 1.6, 0 | <0.001 |
| Major Dissection (During procedure), % | 8.8 | 2.9 | <0.001 |
| Major Dissection (Persisted after procedure), % |
0.6 | 0.0 | 0.05 |
| Perforation, % | 0.1 | 0.2 | 0.83 |
| Persistent Flow Reduction, % | 1.0 | 0.9 | 0.84 |
| Abrupt closure in lab, % | 0.8 | 0.0 | 0.02 |
Denotes significance across groups. ACC = American College of Cardiology; AHA = American Heart Association; BA = balloon angioplasty; BMS = bare metal stent; DES = drug eluting stent; TIMI = Thrombolysis In Myocardial Infarction.
Table 2.
Presentation and Angiographic Characteristics
| Parameter | BMS (N=884) |
DES (N=653) |
P-Value |
|---|---|---|---|
| Stable Angina, % | 28.0 | 23.0 | 0.03 |
| Unstable Angina, % | 39.8 | 32.0 | 0.002 |
| Acute Myocardial Infarction, % | 23.2 | 25.4 | 0.32 |
| Cardiogenic shock, % | 2.0 | 1.5 | 0.46 |
| Elective Procedure | 63.3 | 61.4 | 0.65 |
| Medications <24hrs Prior to or During Procedure |
|||
| Aspirin, % | 93.7 | 91.4 | 0.09 |
| Clopidogrel and/or Ticlopidine, % | 68.7 | 86.1 | <0.001 |
| GP IIb/IIIa Receptor Antagonist, % | 31.7 | 36.6 | 0.04 |
| Abnormal Left Ventricular Function, % | 27.2 | 28.3 | 0.68 |
| Ejection Fraction, mean, median | 54.0, 55 | 52.9, 55 | 0.41 |
| Vessel Disease (≥50% stenosis), % | <0.001 | ||
| 0 | 0.1 | 0.2 | |
| 1 | 42.7 | 29.7 | |
| 2 | 32.5 | 32.6 | |
| 3 | 24.7 | 37.5 | |
| Technically Amenable to Complete Revascularization with CABG, % |
87.1 | 77.0 | <0.001 |
| Technically Amenable to Complete Revascularization with PCI, % |
80.1 | 84.8 | 0.02 |
Denotes significance across groups. BA = balloon angioplasty; BMS = bare metal stent; CABG = coronary artery bypass graft surgery; DES = drug eluting stent; PCI = percutaneous coronary intervention.
Procedural Complications
Overall, there was a high angiographic success rate (99.7% vs. 99.4%; p = 0.45), with a higher procedural success rate (99.7% vs. 98.0%; p = 0.003) in the DES group when compared to BMS (Table 3). Major dissections occurred in 6.3% lesions during stent implantation and the incidence was significantly higher among lesions treated with BMS as compared to lesions treated with DES (8.8% vs. 2.9%) (Table 3). A small number of dissections persisted after stent placement (0.6% and all treated with BMS). Abrupt vessel closure occurred in 0.8% of patients (all BMS), all of which was transient.
30-day outcomes
The follow-up rate for 30 day outcomes was 96.5%. The incidence of the primary safety outcome of death or myocardial infarction was similar by stent group (4.4% vs. 2.9%; P = 0.14) (Table 4). In a propensity score adjusted analyses, DES usage was associated with a reduction in 30-day death/myocardial infarction (HR = 0.53, 95% CI = 0.28-1.01; p = 0.05), compared to the BMS group.
Table 4.
30-day and 1 year Efficacy and Safety Outcomes
| Parameter | BMS (N=884) |
DES (N=653) |
P-Value |
|---|---|---|---|
| 30-Day Outcomes | |||
| Primary Safety Outcome (Death/ Myocardial Infarction ) |
4.4 | 2.9 | 0.14 |
| Primary Efficacy Outcome (CABG/repeat PCI) | 2.5 | 1.4 | 0.13 |
| 1-year Outcomes | |||
| Primary Safety Outcome (Death/ Myocardial Infarction ) |
10.5 | 9.3 | 0.45 |
| Death | 4.9 | 3.9 | 0.35 |
| Myocardial Infarction | 5.8 | 5.6 | 0.90 |
| Primary Efficacy Outcome (CABG/repeat PCI) | 15.3 | 10.0 | 0.003 |
BA= balloon angioplasty; BMS = bare metal stent; CABG = coronary artery bypass graft surgery; DES = drug eluting stent; PCI = percutaneous coronary intervention.
Similarly, in the unadjusted analyses, the incidence of the primary efficacy outcome of repeat revascularization was no different between the two stent groups (Table 4). In the propensity score adjusted analyses, there was no difference in the risk of 30-day repeat revascularization (HR = 0.55, 95% CI = 0.22-1.39; p = 0.20).
1-year Outcomes
The follow-up rate for 1-year outcomes was 96.7%. The cumulative one-year incidence of the primary safety outcome was no different between the BMS or the DES patient groups (10.5% vs. 9.3%; p = 0.45) (Figure 2), with no difference in the incidence of both death or myocardial infarction (Table 4). Similarly, in a propensity score adjusted analyses, DES usage was as safe as compared to BMS (HR = 0.78, 95% CI = 0.53-1.15; p = 0.20), with no difference in the risk of death (HR = 0.73, 95% CI = 0.40-1.32; p = 0.30) or myocardial infarction (HR = 0.81, 95% CI = 0.49-1.36; p = 0.43) compared to the BMS group.
Figure 2.
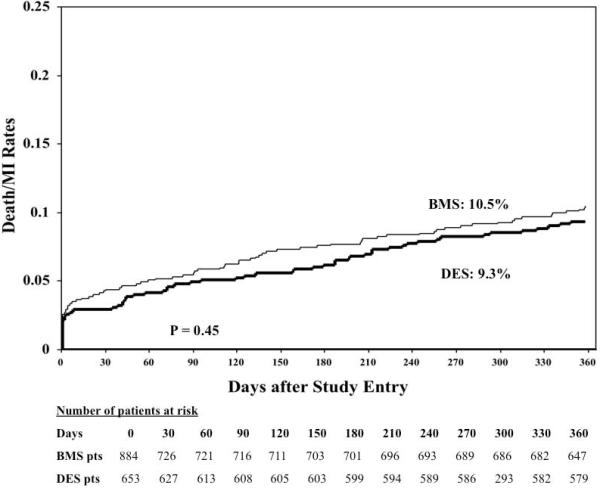
1-year primary safety outcome (death/myocardial infarction) between patients treated with a drug eluting stent (DES) when compared to those treated with a bare metal stent (BMS).
Moreover, in the unadjusted analyses, the incidence of the primary efficacy outcome of repeat revascularization was significantly lower with DES compared to BMS (Figure 3). In the propensity score adjusted analyses, there was a 43% reduction in the risk of repeat revascularization (HR = 0.57, 95% CI = 0.40-0.82; p = 0.002) in favor of DES group when compared to the BMS group. We also assessed the predictors of repeat revascularization in a multivariable model. DES usage was associated with a 40% reduction (HR = 0.60; 95% CI 0.43-0.85; p = 0.003) in the risk of repeat revascularization when compared with BMS in a model controlling for age, gender, diabetes status, hypertension, triple vessel disease, unstable angina at presentation, attempted ulcerated lesion, attempted tortuous lesion, ostial lesion, long acting nitrates and clopidogrel at discharge.
Figure 3.
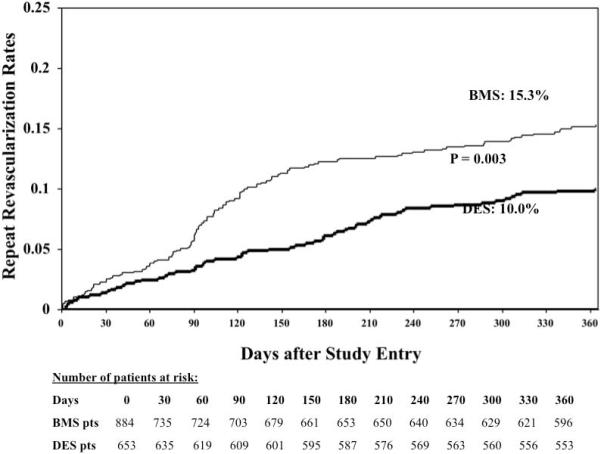
1-year primary efficacy outcome (repeat revascularization) between patients treated with a drug eluting stent (DES) when compared to those treated with a bare metal stent (BMS).
Rotational Atherectomy and Stenting
Among the 1537 stented patients, 116 (7.5%) underwent rotational atherectomy prior to stenting. There was no difference in primary efficacy and safety outcomes regardless of the use of rotational atherectomy (data not shown).
Stent Thrombosis
The data on stent thrombosis was collected only from waves 4 and 5. In patients with a calcified lesion treated with DES, the rate of stent thrombosis was 0.64% which was no different from the stent thrombosis rate in patients with non-calcified lesions (0.62%; p = 0.97). However, we do not have data on stent thrombosis in waves 1-3 and hence no direct comparison could be made between the DES and BMS groups.
DISCUSSION
The results of the present study showed that PCI of moderate to severe calcified lesion with DES is safe and is associated with a significant reduction in the risk for repeat revascularization when compared to PCI with BMS. The results remained even after adjusting for a propensity score that incorporated important variables.
Calcified Coronary Lesions
Calcified coronary lesions represents an advanced stage in the atherosclerotic process whereby a soft plaque is converted to a fibrocalcific plaque. Treatment of calcific lesions is challenging with higher rates of procedural failure, stent under-deployment, lower post-procedural minimal luminal diameter and acute gain and elevated risk of restenosis (1,2). In the percutaneous transluminal angioplasty era, presence of lesion calcification was a significant predictor of major complications (9).
Contemporary stent trials have traditionally excluded patients with calcified lesions. The resistant calcific lesion may prevent complete stent expansion. Moreover, neointimal hyperplasia is mainly a function of smooth muscle cells migrating from the media into intima, resulting in restenosis within stents. Drug eluting stents have largely reduced the risk of restenosis by reducing neointimal hyperplasia due to smooth muscle cell proliferation. In calcific lesions however, the cellular component (including smooth muscle cells) are vastly reduced and hence the neointimal hyperplasia component of restenosis might be small. The efficacy of DES in this setting is therefore questionable.
Bare Metal Stents for Calcified Lesions
Most of the data on the efficacy and safety of BMS for calcified lesions is derived from small non-randomized studies. The largest series of 540 patients by Mosseri et al. showed a high rate of target lesion revascularization-TLR (19.6%) and major adverse cardiac events (MACE- death, myocardial infarction, TLR, any revascularization) (26.2%) (10). Similarly, the Taxus IV substudy of 126 BMS patients with moderate to severe calcification on fluoroscopy found an incidence of TLR of 11.9% with MACE of 16.8% (11). Finally, in a small series of 41 patients with superficial calcification >60 degrees on IVUS, Seo et al. showed a similar high rate of TLR (19.5%) and MACE (24.4%) (12). Our results, obtained from 884 patients with BMS, a sample size greater than all the above three studies combined, showed a similar high rate of repeat revascularization and of death/MI/repeat revascularization (22.4%).
Drug Eluting Stents for Calcified Lesions
The data on DES for calcified lesions are even more sparse. Li et al. evaluated 135 patients with calcified lesions (defined as any calcification on angiography) treated with DES (sirolimus-eluting stent) and reported an incidence of TLR of 6.9% at 8 months follow-up, which was not different from the non-calcified control group (13). Kawaguchi et al. evaluated 152 patients with moderate or severe calcification on fluoroscopy treated with a sirolimus-eluting stent and showed a low rate of TLR (7.3%) and MACE (13.8%) at 12 months (14). Similarly, in the Taxus IV substudy of 121 pateints receiving paclitaxel-eluting stents, low rates of TLR (5.1%) and MACE (11.8%) were reported (11). Our study of 653 DES patients, a sample size more than all the above studies combined together, showed a similar low incidence of repeat revascularization (10%) and death/MI/repeat revascularization rates (16.4%). Similarly, the theoretical concern of risk of stent thrombosis was not seen, with an incidence of stent thrombosis in the DES patients of 0.64%, which was comparable to DES stent thrombosis rates for non-calcified lesions, though there was no direct comparison between the DES and BMS arms.
DES vs. BMS for Calcified Lesions
To our knowledge only two studies have compared the outcomes of DES and BMS in patients with calcified lesions. Seo et al. compared 41 BMS patients with 38 DES patients and showed a lower rate of TLR (19.5% vs. 7.9%) and MACE (24.4% vs. 7.9%) with DES at 9 months follow-up. Similarly, in the Taxus IV substudy with 126 BMS patients against 121 DES patients, reported a lower rate of TLR (11.9% vs. 5.1%) and MACE (16.8% vs. 11.8%) with DES at 12 months follow-up. In our study, the largest series thus far, the DES patients, despite being a significantly higher risk group than other published studies, showed similar safety as the BMS group but at the same time was more efficacious at preventing repeat revascularization. This was true even in a propensity score adjusted analysis.
In our study, in a subgroup analyses of stenting with or without rotational atherectomy, there was no beneficial effect of rotational atherectomy for either efficacy or safety outcomes. However, prior studies have shown better outcomes with rotational atherectomy and stenting compared to stenting alone or rotational atherectomy and balloon angioplasty alone (15). Rotational atherectomy and stenting, however, was performed in only 7.5% of our patients and thus our analysis was limited by a small sample size. Moreover, it is possible that the decision to use rotational atherectomy was based on the degree of calcification, which was not assessed in this study.
Study Limitations
This study was not a randomized trial and therfore the findings could be influenced by unrecognized confounders. We tried to adjust for this by using a propensity adjusted analysis but we can not exclude an impact of unmeasured variables. However, it is still the largest series to-date comparing real-world patients from multiple centers across America. Given that the Dynamic Registry enrolls unselected patients, it is often difficult to track TLR because patients could have undergone multiple procedures prior to as well as after their index PCI. Rather, the lesion location data were collected on a segment basis. In addition, patients were recuited over time and changes in practice (including medical management) could have influenced the outcome. The identification of lesion calcification did not mandate intravascular ultrasound examination and hence we do not have data on the location (superficial vs. deep) or the extent of calcification. Calcification on fluoroscopy is specific but less sensitive and hence patients with calcification not identified by fluoroscopy might have been excluded. In addition, the dynamic registry does not have a angiographic core laboratory analysis of angiograms and no routine follow-up angiography were performed.
Conclusions
In this largest series to date, DES implanted in patients with moderate to severe calcified lesion was safe and was associated with a significant reduction in the risk of repeat revascularization compared to patients receiving BMS. Further randomized trials are needed to fully explore the role of DES in patients with calcified coronary lesions.
Acknowledgments
This study was supported in part by grant HL 22392 from the National Heart, Lung, and Blood Institute, Bethesda, MD
Footnotes
Author Disclosures
Sripal Bangalore: None
Helen A. Vlachos: None
Faith Selzer: None
Robert L. Wilensky: None
Kevin E Kip: None
David O. Williams: None
David P. Faxon: None
References
- 1.Vavuranakis M, Toutouzas K, Stefanadis C, Chrisohou C, Markou D, Toutouzas P. Stent deployment in calcified lesions: can we overcome calcific restraint with high-pressure balloon inflations? Catheter Cardiovasc Interv. 2001;52(2):164–72. doi: 10.1002/1522-726x(200102)52:2<164::aid-ccd1041>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Farb A, Burke AP. Coronary angioplasty from the perspective of atherosclerotic plaque: morphologic predictors of immediate success and restenosis. Am Heart J. 1994;127(1):163–79. doi: 10.1016/0002-8703(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 3.Pocock SJ, Lansky AJ, Mehran R, Popma JJ, Fahy MP, Na Y, Dangas G, Moses JW, Pucelikova T, Kandzari DE. Angiographic surrogate end points in drug-eluting stent trials: a systematic evaluation based on individual patient data from 11 randomized, controlled trials. J Am Coll Cardiol. 2008;51(1):23–32. doi: 10.1016/j.jacc.2007.07.084. and others. [DOI] [PubMed] [Google Scholar]
- 4.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–23. doi: 10.1056/NEJMoa035071. and others. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221–31. doi: 10.1056/NEJMoa032441. and others. [DOI] [PubMed] [Google Scholar]
- 6.Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584–91. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Seeger JD, Kurth T, Walker AM. Use of propensity score technique to account for exposure-related covariates: an example and lesson. Med Care. 2007;45(10 Supl 2):S143–8. doi: 10.1097/MLR.0b013e318074ce79. [DOI] [PubMed] [Google Scholar]
- 8.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, Robins JM. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–70. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 9.Bredlau CE, Roubin GS, Leimgruber PP, Douglas JS, Jr., King SB, 3rd, Gruentzig AR. In-hospital morbidity and mortality in patients undergoing elective coronary angioplasty. Circulation. 1985;72(5):1044–52. doi: 10.1161/01.cir.72.5.1044. [DOI] [PubMed] [Google Scholar]
- 10.Mosseri M, Satler LF, Pichard AD, Waksman R. Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc Revasc Med. 2005;6(4):147–53. doi: 10.1016/j.carrev.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Moussa I, Ellis SG, Jones M, Kereiakes DJ, McMartin D, Rutherford B, Mehran R, Collins M, Leon MB, Popma JJ. Impact of coronary culprit lesion calcium in patients undergoing paclitaxel-eluting stent implantation (a TAXUS-IV sub study) Am J Cardiol. 2005;96(9):1242–7. doi: 10.1016/j.amjcard.2005.06.064. and others. [DOI] [PubMed] [Google Scholar]
- 12.Seo A, Fujii T, Inoue T, Onoda S, Koga A, Tanaka Y, Chin K, Kurusu T, Takikawa K, Shibata T. Initial and long-term outcomes of sirolimus-eluting stents for calcified lesions compared with bare-metal stents. Int Heart J. 2007;48(2):137–47. doi: 10.1536/ihj.48.137. and others. [DOI] [PubMed] [Google Scholar]
- 13.Li JJ, Xu B, Yang YJ, Chen JL, Qiao SB, Ma WH, Qin XW, Yao M, Liu HB, Wu YJ. Effects of sirolimus-eluting stent on calcified coronary lesions. Chin Med J (Engl) 2008;121(1):6–11. and others. [PubMed] [Google Scholar]
- 14.Kawaguchi R, Tsurugaya H, Hoshizaki H, Toyama T, Oshima S, Taniguchi K. Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients. Cardiovasc Revasc Med. 2008;9(1):2–8. doi: 10.1016/j.carrev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann R, Mintz GS, Kent KM, Pichard AD, Satler LF, Popma JJ, Hong MK, Laird JR, Leon MB. Comparative early and nine-month results of rotational atherectomy, stents, and the combination of both for calcified lesions in large coronary arteries. Am J Cardiol. 1998;81(5):552–7. doi: 10.1016/s0002-9149(97)00983-1. [DOI] [PubMed] [Google Scholar]


