Abstract
N-methyl-D-aspartate receptor (NMDAR) mediated excitotoxicity is a probable proximate mechanism of neurodegeneration in Huntington disease (HD). Striatal neurons express the NR2B-NMDAR subunit at high levels, and this subunit is thought to be instrumental in causing excitotoxic striatal neuron injury. We evaluated the efficacy of 3 NR2B-selective antagonists in the R6/2 transgenic fragment model of HD. We evaluated ifenprodil (10 mg/kg; 100 mg/kg), RO25,6981 (10 mg/kg), and CP101,606 (30 mg/kg). Doses were chosen on the basis of pilot acute maximally tolerated dose studies. Mice were treated with twice daily subcutaneous injections. Outcomes included survival, motor performance declines assessed with the rotarod, balance beam task, and activity measurements, and post-mortem striatal volumes. No outcome measure demonstrated any benefit of treatments. Lack of efficacy of NR2B antagonists in the R6/2 model has several possible explanations including blockade of beneficial NMDAR mediated effects, inadequacy of the R6/2 model, and the existence of multiple proximate mechanisms of neurodegeneration in HD.
Keywords: Huntington disease, striatum, excitoxicity, glutamate
Huntington disease (HD) is an incurable, autosomal dominant neurodegenerative disorder characterized by involuntary movements, psychiatric problems, and dementia (Warby et al., 2007). The median onset of HD is around age 40 with inexorable progression to death over a period of approximately 15–20 years. HD is uncommon with an approximate prevalence of 5–10/100000 among populations of European descent but its onset in midlife and prolonged course causes costs disproportionate to prevalence. The pathologic hallmark of HD is striatal degeneration though recent neuroimaging data indicates early neocortical atrophy as well (Vonsattel and DiFiglia, 1998; Rosas et al., 2008).
HD and 7 other neurodegenerative disorders are caused by increased CAG repeats within coding portions of their respective genes. Neurodegeneration results primarily from “gain of function” neurotoxicity of expanded polyglutamine (polyQ) repeats. While expanded polyQ domains are the primary agents of neurotoxicity, the surrounding protein sequences are thought to modulate expanded polyQ domain interactions with other cellular constituents, suggesting that the precise neurotoxic effects of different expanded polyQ proteins will vary from disease to disease (Imarisio et al., 2007; Orr and Zoghbi, 2007; Lim et al., 2008).
Excitotoxicity is the oldest creditable proposed proximate mechanism of neurodegeneration for HD (Coyle and Schwarcz, 1976; McGeer and McGeer, 1976). The excitotoxic hypothesis of neurodegeneration in HD is supported by several strong lines of evidence. Histopathologic features of lesions produced by acute intrastriatal administration of N-methyl-D-aspartate receptor (NMDAR) agonists resemble the striatal pathology of HD (Bazzett and Albin, 2001; Fan and Raymond, 2007). Increased NMDAR mediated currents and enhanced susceptibility to exogenous NMDAR agonists is described in murine genetic HD models (Levine et al., 1999; Chen et al., 1999; Cepeda et al., 2001; Zeron et al., 2001; 2002; 2004; Li et al., 2003; 2004; Shehadeh et al., 2006; Fan and Raymond, 2007; Milnerwood et al., 2010). The open channel NMDAR antagonist remacemide ameliorates the phenotype of the R6/2 transgenic fragment model of HD and other interventions that reduce excitotoxicity have beneficial effects in the R6/2 model (Ferrante et al., 2002, Stack et al., 2007).
NMDARs are heterotetrameric structures consisting of 2 NR1 subunits and varying combinations of NR2A-D or NR3A-C subunits (Kew and Kemp, 2005; Waxman and Lynch, 2005). NR2 subunit composition modulates the functional properties of NMDARs. NMDAR activation has both excitotoxic and neuronal survival promoting effects (Hardingham, 2009). Both NR2 subunit composition and receptor location are suggested to influence the neurotoxic versus survival promoting effects of NMDAR activation. Considerable literature suggests that activation of extrasynaptic NMDARs promotes neuronal death with activation of intrasynaptic NMDARs promoting neuronal survival (Hardingham et al., 2002; Papadia et al., 2008; Leveille et al., 2008; 2010; Martel et al., 2009). Other recent literature suggests that NR2B containing NMDARs are excitotoxic mediators while NR2A containing NMDARs promote neuronal survival (Liu et al., 2007). Striatal neurons express both NR2A and NR2B containing NMDARs (Standaert et al., 1999; Kuppenbender et al., 2000). Work by Raymond and colleagues indicates selective potentiation of striatal NR2B containing NMDAR effects in murine genetic HD models with an early increase in extrasynaptic NMDAR signaling in a transgenic HD mouse model (Zeron et al., 2002; 2004; Li et al., 2004; Milnerwood et al., 2010). We showed recently that enhancing NR2B containing NMDAR neurotransmission in vivo exacerbates selective striatal neuron degeneration in a knockin murine genetic model of HD (Heng et al., 2009). Targeting NR2B containing NMDARs is a rational approach to neuroprotection in HD. Selective antagonists exist for NR2B containing NMDARs, and at least one of these compounds, ifenprodil, is marketed for use in humans (Loftis and Janowsky, 2003; Kew and Kemp, 2005). We evaluated the efficacy of three selective NR2B antagonists – ifenprodil, RO25,6981 and CP101,606 - in the R6/2 fragment transgenic model of HD.
Methods
Animals
All experiments were performed with the R6/2 murine model of HD maintained on a CBA × C57BL/6 genetic background. Founder mice were purchased from Jackson Labs (Bar Harbor, ME). We maintained a breeding colony by crossing R6/2 males to F1 CBA × C57BL/6 females. Every mouse was genotyped and assayed for repeat length of the CAG expansion (Laragen, Los Angeles, CA). Repeat length was maintained between 110–120 (mean = 115 ± 2.1). For all experiments, both male and female R6/2 mice were used. Animals were housed in Specific Pathogen Free (SPF) conditions with a 12-h light/dark cycle maintained at 23°C. Mice were grouped by gender in large cages enriched with an igloo and a horizontal running wheel, no more than 7 mice per cage, and provided with food and water ad libitum. Supplemental wet chow mixed with organic peanut butter was provided daily. All procedures were conducted in strict compliance with the Guide for the Care and Use of Laboratory Animals as adopted by the NIH and approved by the Committee on Use and Care of Animals (UCUCA), University of Michigan, and the Veterinary Medical Unit (VMU) at the Ann Arbor Veterans Affairs Medical Center.
Drug Injections
Three NR2B selective antagonists were tested; ifenprodil (10 mg/kg and 100 mg/kg in10% hydroxypropyl-β-cyclodextrin [HPβCD]), RO25,6981 (10 mg/kg in1% N-methyl pyrrolidone [NMP] in 50 mM citrate, pH 4.5), and CP101,606 (30 mg/kg in 10% HPβCD/3% DMSO). Perzinfotel (EAA-090; 10mg/kg in 10% PEG400 in 50mM Phosphate, pH 8), a selective NR2A antagonist, was evaluated as well. Drug doses and routes of administration for ifenprodil and RO25,6981were chosen on the basis of maximally tolerated (MTD) dose studies sponsored by the High Q Foundation (New York, NY) and performed by Psychogenics (Tarrytown, NY). Formulation and pharmacokinetic studies were performed by Pharmatek (San Diego, CA) and Melior Discovery (Malvern, PA), respectively. For ifenprodil, the elimination T1/2 was 4.4 hours with a Tmax of 0.5 hours. Bioavailability was 47% and brain penetrance was 420%. For RO25,6981, the elimination T1/2 was 5.1 hours with a Tmax of 0.5 hours. Bioavailability was 137% and brain penetrance was 53%. Doses selected were consistent with prior published literature studying in vivo effects of these compounds (Boyce et al., 1999; Mennitti et al., 2000; Murray et al., 2000). For CP101,606, doses were chosen on the basis of prior published reports (Boyce et al., 1999; Mennitti et al., 2000; Murray et al., 2000). . In preliminary testing, perzinfotel had undetectable blood levels with all routes of administration but was evaluated because of prior reports suggesting efficacy in models of ischemia and pain (Kinney et al., 1998; Sun et al., 2004; Brandt et al., 2005). Dose was chosen on the basis of prior literature. Ten to 22 R6/2 mice (of either sex) were allocated to each treatment or control group. Control groups were run with each treatment group for a total of 47 control animals. Drug or vehicle control animals were given subcutaneous injections twice daily at rotating sites from 6 weeks of age until death. Animals were treated between 6 am and 8 am for the first injection and between 5 pm and 7 pm for the second injection.
Behavioral Tests
Motor function was assessed by balance beam and rotarod performance, and activity monitoring. Animals were filmed crossing 41 cm suspended balance beams (20, 11 and 5 mm diameter) and scored for time to traverse beams and number of hindlimb slips (Heng et al., 2009). Films were analyzed blinded to treatment conditions with time to traverse beam and foot slips measured from films. Animals failing to cross the beam in 30 seconds or halting on the beam were scored at 30 seconds. Hindlimb slips in animals halting on the beam were scored at 30 hindlimb slips. The rotarod (Model 7650; Ugo Basile) task was accelerated rotation from 4–40 rpm over 4 min, measuring latency to fall. Activity cages (Advanced Concepts, Ann Arbor, MI) with photosensors recorded the number of small movements (beam breaks) and cage traversals (crosses) animals made over a 2 hr period. Baseline behavioral evaluation was performed at 5 weeks of age. Baseline balance beam and rotarod tasks were done daily for five days, then twice a week from 6 weeks of age until death. Activity cage measurements and weights were taken weekly from 5 weeks until death. Behavioral evaluations were performed between 10 am and Noon.
Pathologic Examination
Brains were harvested from mice after natural death and immersion fixed in a 4% paraformaldehyde solution at 4C for 24 hours, then cryoprotected in 20% sucrose. Consecutive 40µm sections were cut on a sliding microtome in the parasagittal plane. A 1 in 5 series of sections was selected, mounted, and stained with cresyl violet. Total striatal area was determined using NIS-Elements Software (Nikon Instruments, Inc.). Striatal volumes were calculated with the Cavalieri principle. A second set of sections was used to determine the accumulation of neuronal intranuclear inclusions (NIIs). Tissue was incubated with an antibody directed at the htt N-terminus (Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:250) and immunostaining was visualized with Vector ABC Elite kits (Vector Laboratories, Burlingame, CA) using DAB as the chromagen.
Statistical Analysis
Twice weekly behavioral data were averaged for each animal to produce weekly values. Statistical analysis of mortality and behavioral data was done using a Linear Mixed Model within SPSS. Gender effects were evaluated within the Linear Mixed Model and no effect of gender on any outcome variable, other than weight, or interaction of gender with outcome was found. With the exception of weight data, both genders were combined for final analysis and presentation. Preliminary analysis showed that all vehicle controls were essentially identical and all vehicle animals were aggregated for comparison with treatments. Striatal volumes were compared with t-tests and Bonferroni corrected for multiple comparisons. Data variation is represented as standard error of the mean in all figures.
Results
Mortality and Weight
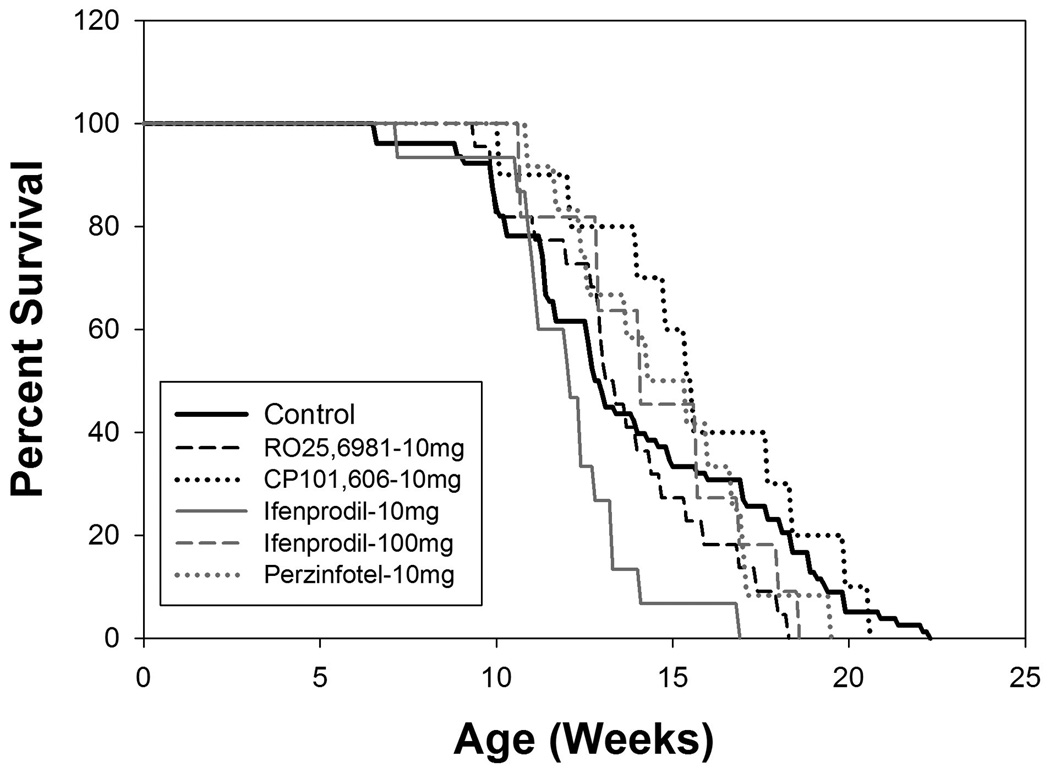
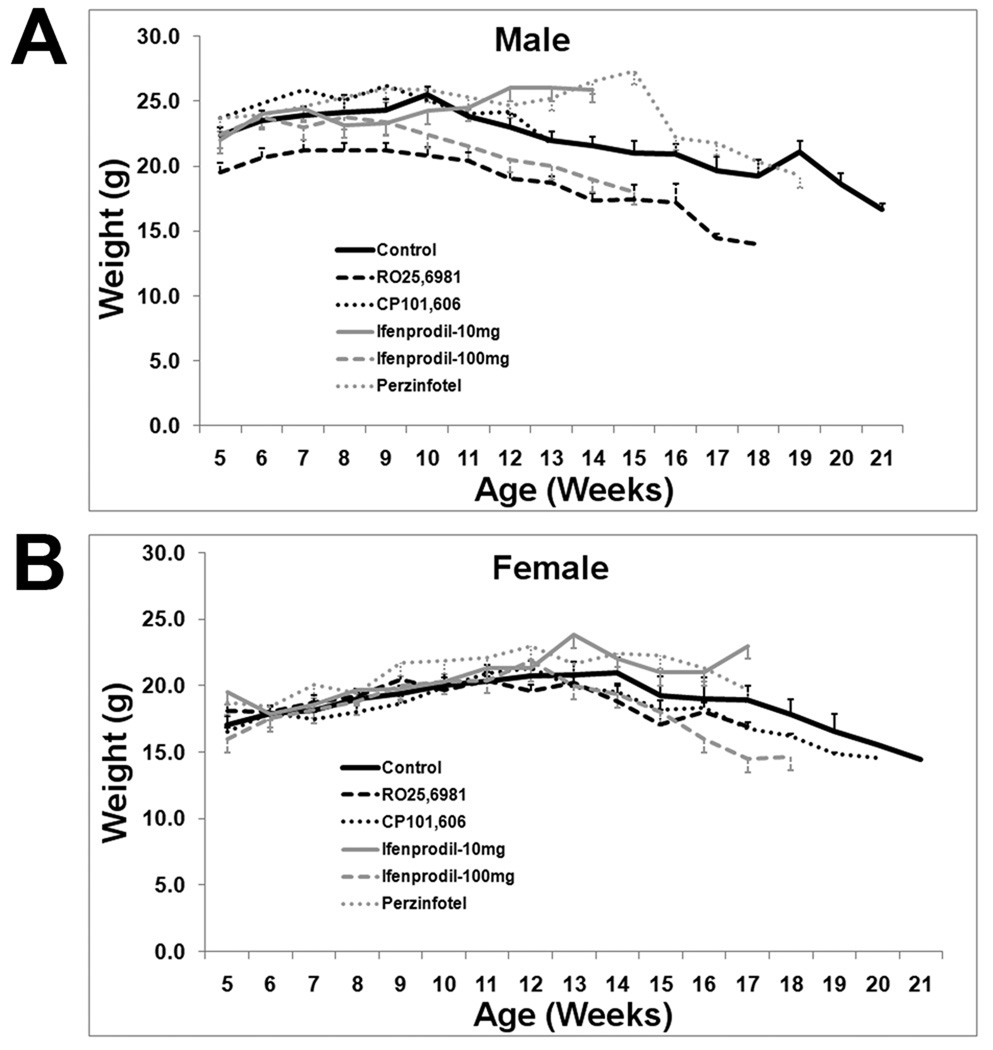
In the aggregate control group, there was 50% mortality by approximately 13 weeks of age and all control mice were deceased by 23 weeks of age (Figure 1). These results are similar to the prior work of Stack et al. (2005) and Menalled et al. (2009) with this model of HD. There was no effect of any treatment on mortality (Figure 1). Control male weights peaked at approximately 10 weeks and then gradually declined. Female weights peaked somewhat later, around 13 weeks, and then declined. Similar to mortality data, there was no effect of any treatment on weight trajectories (Figure 2).
Figure 1.
Kaplan- Meier survival curves for treatment groups (N = 12 – 15 per group) and aggregate control group (N = 45). There is no difference between any treatment group and control group.
Figure 2.
Weights for all treatment and aggregate control groups. There is no difference between any treatment group and control group.
Motor Behavior
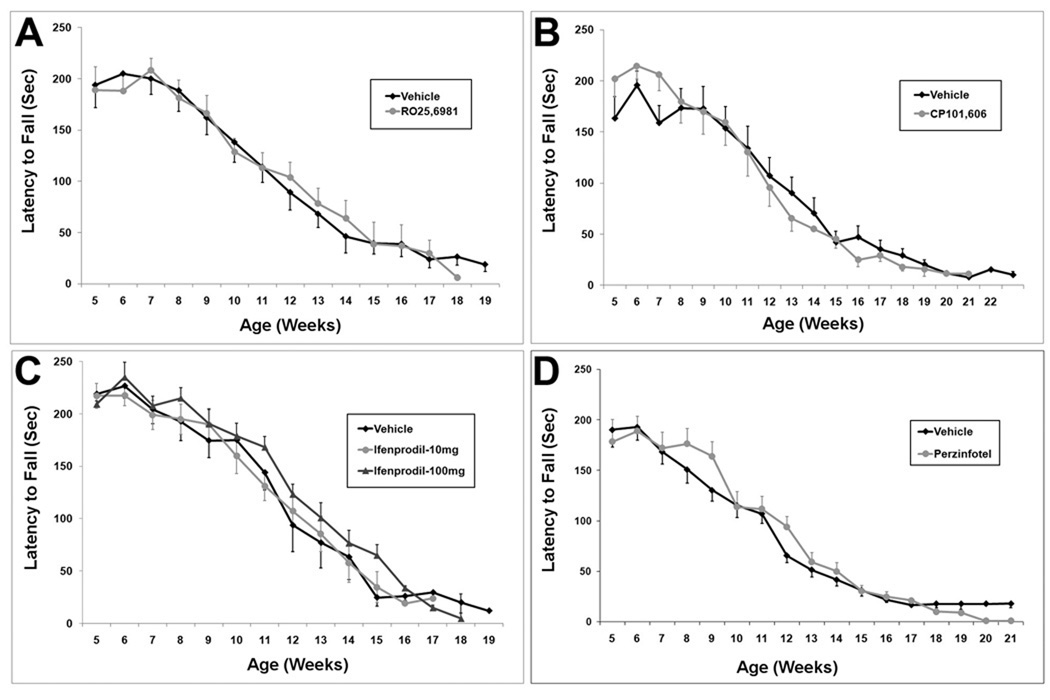
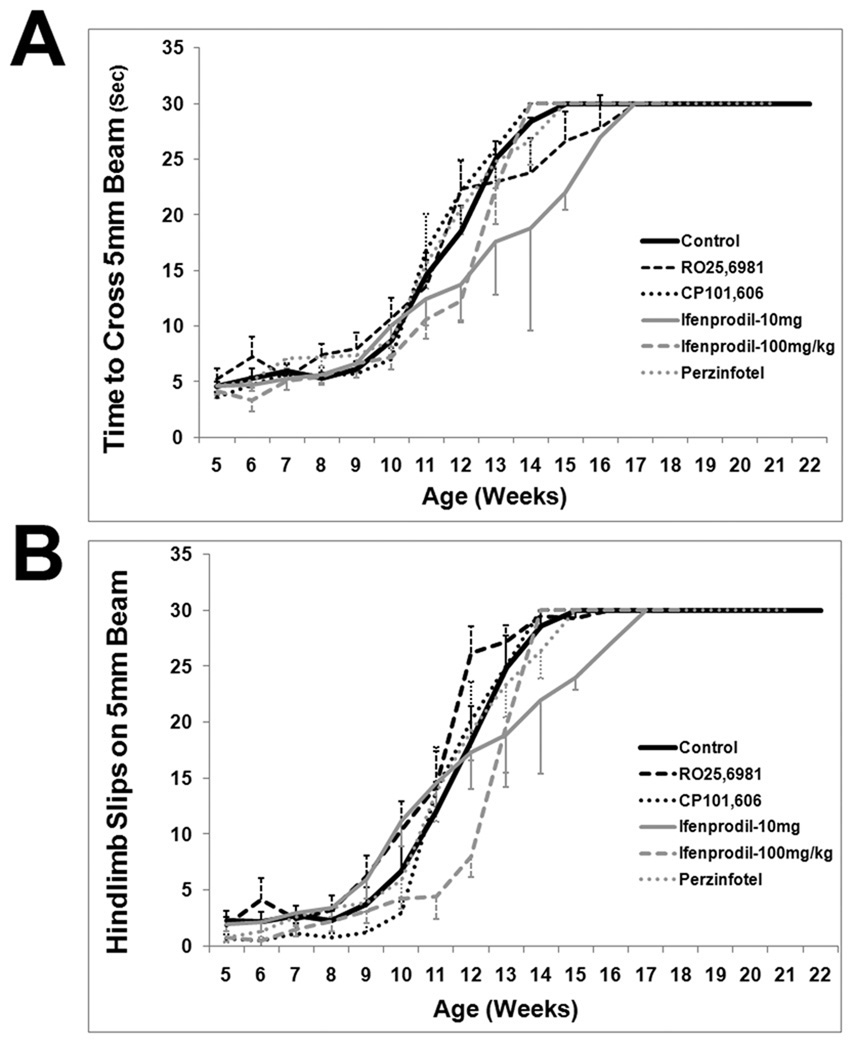
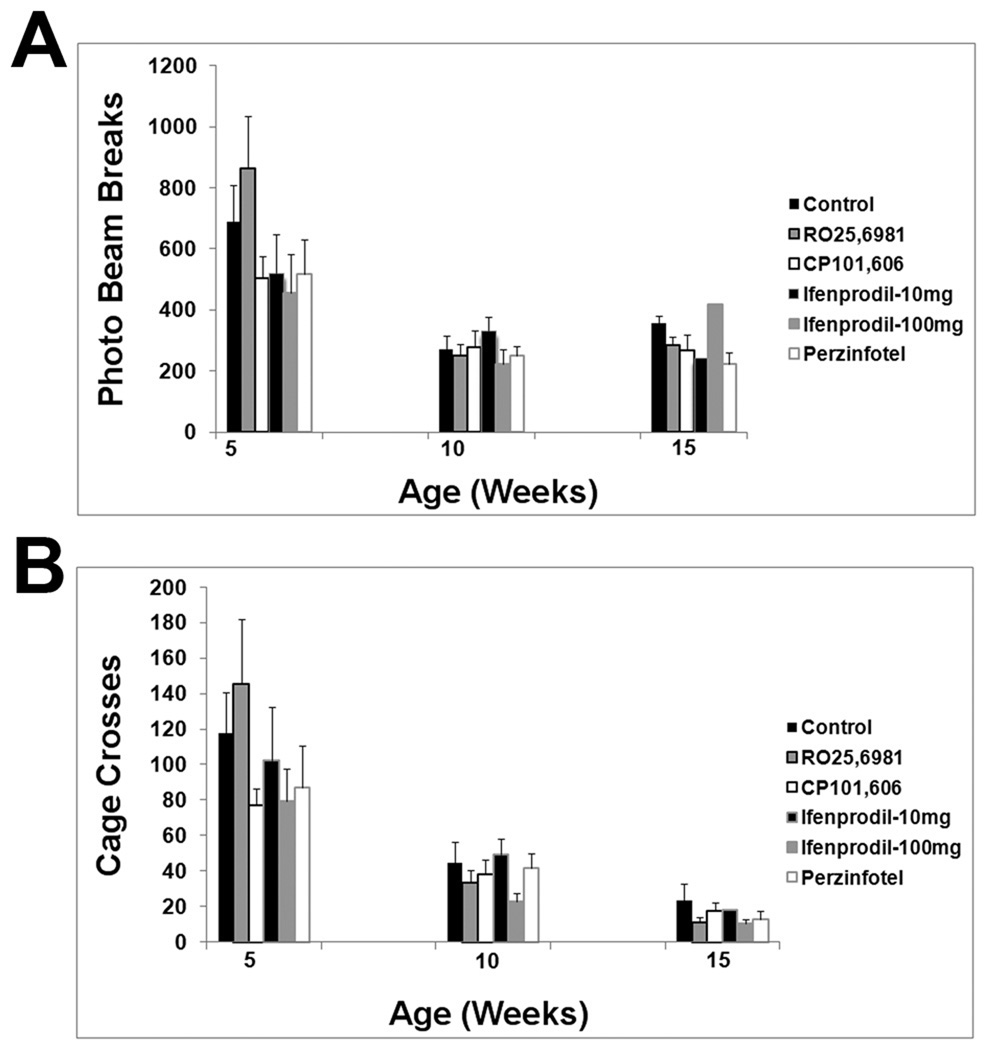
Motor performance declined steadily from baseline in all treatment groups and the control group. From approximately 7 weeks of age onward, rotarod performance declined linearly (Figure 3). Control animal rotarod performance data is similar to the prior results of Stack et al. (2005) and Menalled et al. (2009). There was no effect of any treatment on the rate or magnitude of decline in rotarod performance (Figure 3). Similar results were found with the balance beam task. After about 7 weeks of age, balance beam performance, as measured either by time to cross the beam or by hindlimb slips, deteriorated progressively. By approximately 14–15 weeks, mice were severely impaired on the balance beam task. Our control balance beam data is similar to that of Carter et al. (1999). There was no effect of any treatment on declining performance in thebalance beam task (Figure 4; Supplemental Figures 1 and 2). Similar results were obtained with activity cage measurements, a measure of spontaneous activity. Total beam breaks, a measure of all movements, declined from 5 weeks onward and appeared to plateau by 10 weeks (Figure 5). Cage traversals (crosses) declined from 5 weeks onwards and continued to decline up to 15 weeks (Figure 5). Again, there was no effect of any treatment on activity measurements (Figure 5).
Figure 3.
Rotarod performance in all treatment groups. Each treatment group compared with aggregate control group. (A) RO25,6981. (B) CP101,606. (C) Ifenprodil. (D) Perzinfotel. There is no difference between any treatment group and control group.
Figure 4.
Balance beam performance on a 5 mm wide beam. (A) Time to cross beam. (B) Number of hindlimb slips. There is no difference between any treatment group and control group. See Supplemental Data for performance on 11 mm and 20 mm beams, which also show no difference between any treatment group and control group.
Figure 5.
Activity cage measures at 5 weeks, 10 week, and 15 weeks of age. (A) Total number of photo beam breaks. (B) Number of cage crossing events. There is no difference between any treatment group and control group at any time point.
Striatal Pathology
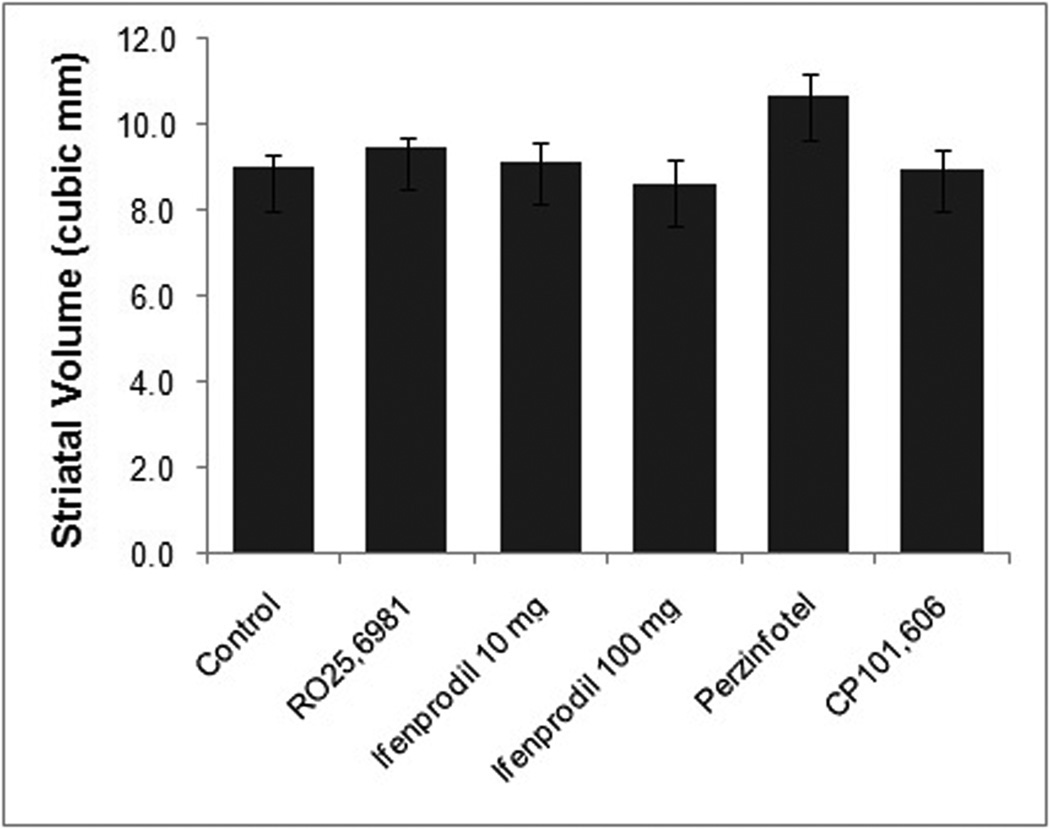
There was no effect of any treatment on post-mortem striatal volume (Figure 6). Neither was there a qualitative effect of any treatment on striatal NII deposition. Specifically, there was no qualitative effect of NR2B antagonist treatment on NII areal density or size (Supplemental Figure 3).
Figure 6.
Striatal volumes measured post-mortem. There is no difference between any treatment group and the control group.
Discussion
We found little evidence for efficacy of these selective NR2B antagonists in the R6/2 model of HD. There were no effects on motor behavior measures, mortality, or striatal volumes. Our results are discordant with a large body of data indicating that NMDAR mediated excitotoxicity is a proximate mechanism of neurodegeneration in HD. NMDAR mediated excitotoxicity is one of the best supported proposed proximate mechanisms for neurodegeneration in HD. Recent work with murine genetic models of HD, including transgenic fragment models, full length transgenic models, and knockin models supports a role for NMDAR mediated excitotoxicity. Some of this data implicates NR2B containing NMDARs specifically as mediators of excitotoxic injury in HD murine genetic models (Zeron et al., 2002; 2004; Li et al., 2004; Heng et al., 2009; Milnerwood et al., 2010).
NMDAR activation produces both excitotoxic and pro-survival effects in neurons. Most data suggests that activation of extrasynaptic NMDARs is neurotoxic while activation of synaptic NMDARs promotes neuronal survival (Hardingham et al., 2002; Leveille et al., 2008; 2010; Papadia et al., 2008; Martel et al., 2009; Hardingham, 2009). Some data also suggests that NR2 subunit composition is salient (Liu et al., 2007). Some data suggests that mature neuronal synaptic NMDARs preferentially express NR2A subunits while NR2B containing NMDARs tend to be extrasynaptic (Tovar and Westbrook, 1999). Other results, however, suggest that neurons express NR2A and NR2B subunit containing NMDARs in both synaptic and extrasynaptic locations (Stanika et al., 2009). Mature medium spiny striatal neurons express both NR2A and NR2B subunits and it is likely that medium spiny extrasynaptic NMDARs preferentially contain NR2B subunits (Standaert et al., 1999; Kuppenbender et al., 2000; Milnerwood et al., 2010).
Okamoto et al. (2009) demonstrated preferential activation of extrasynaptic NMDARs and consequent neurotoxicity in neurons transfected with mhtt constructs. Similar data were presented by Milnerwood et al. (2010) in studies of striatal slice preparations from the YAC128 full length murine transgenic model of HD. Milnerwood et al. (2010) and Okamoto et al. (2009) document beneficial effects of relatively low doses of the open channel NMDAR antagonist memantine in YAC128 mice in vivo. These doses of memantine selectively antagonize pathologically activated extrasynaptic NMDARs in vitro. At higher doses, memantine treatment exacerbated striatal degeneration in YAC128 mice. Higher doses probably inhibit both extra- and intrasynaptic NMDARs, obviating the benefits of inhibiting extrasynaptic NMDARs and possibly exacerbating mutant huntingtin toxicity by inhibiting the neuroprotective effects of synaptic NMDAR activation. We used high doses of NR2B antagonists in an effort to obtain maximal effects. It is plausible that this approach resulted in blockade of pro-survival signaling effects of synaptic NMDARs, counteracting any beneficial effects of reducing the excitotoxic effects of extrasynaptic NMDAR activation.
There are other possible explanations for our results. One possibility is that the agents we employed are insufficiently selective. The available data, however, indicates that ifenprodil, RO25,6981, and CP101,606 are selective NR2B NMDAR antagonists and may exhibit uncompetitive NMDAR antagonism (Mott et al., 1998; Loftis and Janowsky, 2003; Kew and Kemp, 2005). The precise stoichiometry of striatal NMDARs is unknown but both heterodimers (NR1 plus either NR2A or NRB) and heterotrimers (NR1 plus NR2A and NR2B) may be present. CP101,606 is probably an antagonist of heterodimeric NR2B-NMDARs only, and its failure could reflect excitotoxicity mediated by heterotrimeric (NR1 plus NR2A and NR2B) NMDARs (Chazot et al, 2002). Ifenprodil and RO25,6981, however, are probable antagonists of heterodimeric and heterotrimeric NR2B containing receptors (Chazot et al, 2002).
Another possibility would be inadequacies of the R6/2 model. R6/2 is a transgenic fragment model with an exon 1 containing construct with the expanded CAG repeat domain and adjacent promoter sequence. This line has the advantage of an aggressive phenotype secondary to an expanded polyQ protein, the core pathogenetic mechanism of this disease family. This transgenic fragment model does not reproduce all the genetic regulatory sequences or protein context of mutant huntingtin. The early onset, early mortality, extra-CNS features, and diffuse expression of NII pathology in R6/2 mice is different from the phenotype of knockin or full length transgenic mutant genetic murine models that may have better construct and face validity (Heng et al., 2008; Morton et al., 2009; Menalled et al., 2009). Nonetheless, the R6/2 line does reproduce crucial features of HD and the aggressive phenotype provides excellent endpoints for evaluating interventions (Heng et al., 2008). The predictive validity of the R6/2 line and all other HD murine models is presently unknown because of a paucity of human clinical trial data.
Hansson et al. (1999, 2001) described resistance to acute intrastriatal administration of NMDAR agonists in R6/2 mice and the related R6/1 line. This is an age-related phenomenon, with NMDAR agonist resistance developing as mice matured. We began treatments at 6 weeks, an age at which R6/2 mice exhibit considerable resistance to acute intrastriatal NMDAR agonist administration. This raises the possibility that initiating treatment at an earlier point would produce different results. Stack et al. (2007), however, found that 2 interventions known to reduce excitotoxic striatal injury, decortication and 6-hydroxydopamine lesions, reduced striatal pathology in R6/2 mice when performed at 6 weeks of age.
The accumulated datafavor a significant role for extrasynaptic-NR2B subunit containing NMDAR mediated excitotoxicity in striatal neurodegeneration in HD. Clinically available antagonists such as memantine or ifenprodil may be candidates for clinical trials in HD. Selective NR2B antagonists have been studied in pilot clinical trials in depression and L-dopa induced dyskinesias with some positive effects (Nutt et al., 2008; Preskorn et al., 2008; Skolnick et al., 2009). Our data suggest that targeting extrasynaptic NMDARs specifically will be difficult. The neuroprotective effects of activating synaptic NMDARs indicates the presence of a dome- or U- shaped dose-response curve for neuroprotection via NMDAR blockade. Determining appropriate doses of memantine or a selective NR2B antagonist for neuroprotective trials in HD subjects will be crucial. Dose selection may require some type of human pharmacodynamic biomarker of neuroprotective NMDAR blockade – a formidable obstacle. Further evaluation of memantine and selective NR2B antagonists is required prior to trials.
Supplementary Material
Balance beam performance on an 11 mm wide beam. (A) Time to cross beam. (B) Number of hindlimb slips. There is no difference between any treatment group and control group.
Balance beam performance on a 20 mm wide beam. (A) Time to cross beam. (B) Number of hindlimb slips. There is no difference between any treatment group and control group.
Neuronal intranuclear inclusions (NIIs) in the striatum of 13 week R6/2 mice. No qualitative effect of treatments on NII areal density or size. Treatment groups shown are (A) Vehicle Control (B) RO25,6981 (C) 10 mg/kg Ifenprodil (D) 100 mg/kg Ifenprodil (E) CP101,606 and (F) Perzinfotel. Arrows point to examples of NIIs which are visible in all panels. Scale bar = 20µm.
Acknowledgements
Supported by a VA Merit Review grant, R03 NS054810, R21 NS059537, and the High Q Foundation. We thank the anonymous reviewers for helpful criticism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazzett T, Albin RL. Huntington Disease: A Model Excitotoxic Chronic Neurodegeneration. In: Palomo T, Beninger RJ, Archer T, editors. Neurodegenerative Brain Disorders. 2001. pp. 259–288. [Google Scholar]
- Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38(5):611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Brandt MR, Cummons TA, Potestio L, Sukoff SJ, Rosenzweig-Lipson S. Effects of the N-methyl-D-aspartate receptor antagonist perzinfotel [EAA-090; [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)-ethyl]phosphonic acid] on chemically induced thermal hypersensitivity. J. Pharmacol. Exp. Ther. 2005;313(3):1379–1386. doi: 10.1124/jpet.105.084467. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J.Neurosci. 1999;19(8):3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Ariano MA, Calvert CR, Flores-Herna’ndez J, Chandler SH, Leavitt BR, Hayden MR, Levine MS. NMDA receptor function in mouse models of Huntington disease. J. Neurosci. Res. 2001;66:525–539. doi: 10.1002/jnr.1244. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Lawrence S, Thompson CL. Studies on the subtype selectivity of CP-101,606: evidence for two classes of NR2B-selective NMDA receptor antagonists. Neuropharmacology. 2002;42(3):319–324. doi: 10.1016/s0028-3908(01)00191-5. [DOI] [PubMed] [Google Scholar]
- Chen N, Luo T, Wellington C, Metzler M, McCutcheon K, Hayden MR, Raymond LA. Subtype-specific enhancement of NMDA receptor currents by mutant huntingtin. J. Neurochem. 1999;72:1890–1898. doi: 10.1046/j.1471-4159.1999.0721890.x. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature. 1976;263(5574):244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- Fan MMY, Raymond LA. N-Methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog. Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J. Neurosci. 2002;22(5):1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannson O, Petersen A, Leist L, Nicotera P, Castilho RF, Brundin P. Transgenic mice expressing a Huntington's disease mutation are resistant to quinolinate acid-induced striatal excitotoxicity. Proc. Nat. Acad. Sci. USA. 1999;96:8727–8732. doi: 10.1073/pnas.96.15.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Guatteo E, Mercuri NB, Bernardi G, Li X-J, Castilho R, Brundin P. Resistance to NMDA toxicity correlates with appearance of nuclear inclusions, behavioral deficits and changes in calcium homeostasis in mice transgenic for exon 1 of the huntington gene. Eur. J. Neurosci. 2001;14:1492–1504. doi: 10.1046/j.0953-816x.2001.01767.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem. Soc. Trans. 2009;37(Pt 6):1147–1160. doi: 10.1042/BST0371147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Heng MY, Detloff PJ, Albin RL. Rodent genetic models of Huntington disease. Neurobiol. Dis. 2008;32(1):1–9. doi: 10.1016/j.nbd.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Heng MY, Detloff PJ, Wang PL, Tsien JZ, Albin RL. In vivo evidence for NMDA receptor- mediated excitotoxicity in a murine genetic model of Huntington disease. J. Neurosci. 2009;29:3200–3205. doi: 10.1523/JNEUROSCI.5599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179(1):4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kinney WA, Abou-Gharbia M, Garrison DT, Schmid J, Kowal DM, Bramlett DR, Miller TL, Tasse RP, Zaleska MM, Moyer JA. Design and synthesis of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)-ethyl]phosphonic acid (EAA-090), a potent N-methyl-D-aspartate antagonist, via the use of 3-cyclobutene-1,2-dione as an achiral alpha-amino acid bioisostere. J. Med. Chem. 1998;41(2):236–246. doi: 10.1021/jm970504g. [DOI] [PubMed] [Google Scholar]
- Küppenbender KD, Standaert DG, Feuerstein TJ, Penney JB, Jr, Young AB, Landwehrmeyer GB. Expression of NMDA receptor subunit mRNAs in neurochemically identified projection and interneurons in the human striatum. J. Comp. Neurol. 2000;419(4):407–421. doi: 10.1002/(sici)1096-9861(20000417)419:4<407::aid-cne1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Léveillé F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C, Tolkovsky AM, Hardingham GE. Suppression of the intrinsic apoptosis pathway by synaptic activity. J. Neurosci. 2010;30(7):2623–2635. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Klapstein GJ, Koppel A, Gruen E, Cepeda C, Vargas ME, Jokel ES, Carpenter EM, Zanjani H, Hurst RS, Efstratiadis A, Zeitlin S, Chesselet MF. Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington’s disease. J. Neurosci. Res. 1999;58:515–532. [PubMed] [Google Scholar]
- Li L, Fan M, Icton CD, Chen N, Leavitt BR, Hayden MR, Murphy TH, Raymond LA. Role of NR2B-type NMDA receptors in selective neurodegeneration in Huntington disease. Neurobiol. Aging. 2003;24:1113–1121. doi: 10.1016/j.neurobiolaging.2003.04.003. [DOI] [PubMed] [Google Scholar]
- Li L, Murphy TH, Hayden MR, Raymond LA. Enhanced striatal NR2B-containing N-methyl-D-aspartate receptor-mediated synaptic currents in a mouse model of Huntington’s disease. J. Neurophysiol. 2004;92:2738–2746. doi: 10.1152/jn.00308.2004. [DOI] [PubMed] [Google Scholar]
- Lim J, Crespo-Barreto J, Jafar-Nejad P, Bowman AB, Richman R, Hill DE, Orr HT, Zoghbi HY. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature. 2008;452(7188):713–718. doi: 10.1038/nature06731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007;27(11):2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol. Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Martel MA, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, NR2B-containing N-methyl-d-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158:334–343. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Duplication of biochemical changes of Huntington’s chorea by intrastriatal injections of glutamic and kainic acids. Nature. 1976;263:517–519. doi: 10.1038/263517a0. [DOI] [PubMed] [Google Scholar]
- Menalled L, El-Khodor BF, Patry M, Suárez-Fariñas M, Orenstein SJ, Zahasky B, Leahy C, Wheeler V, Yang XW, MacDonald M, Morton AJ, Bates G, Leeds J, Park L, Howland D, Signer E, Tobin A, Brunner D. Systematic behavioral evaluation of Huntington's disease transgenic and knock-in mouse models. Neurobiol. Dis. 2009;35(3):319–336. doi: 10.1016/j.nbd.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Pagnozzi MJ, Butler P, Chenard BL, Jaw-Tsai SS, Frost White W. CP-101,606, an NR2B subunit selective NMDA receptor antagonist, inhibits NMDA and injury induced c-fos expression and cortical spreading depression in rodents. Neuropharmacology. 2000;39(7):1147–1155. doi: 10.1016/s0028-3908(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice. Neuron. 2010;65(2):178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Glynn D, Leavens W, Zheng Z, Faull RL, Skepper JN, Wight JM. Paradoxical delay in the onset of disease caused by super-long CAG repeat expansions in R6/2 mice. Neurobiol. Dis. 2009;33(3):331–341. doi: 10.1016/j.nbd.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat. Neurosci. 1998;1(8):659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- Murray F, Kennedy J, Hutson PH, Elliot J, Huscroft I, Mohnen K, Russell MG, Grimwood S. Modulation of [3H]MK-801 binding to NMDA receptors in vivo and in vitro. Eur. J. Pharmacol. 2000;397(2–3):263–270. doi: 10.1016/s0014-2999(00)00263-6. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS, Landen JW. Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov. Disord. 2008;23(13):1860–1866. doi: 10.1002/mds.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med. 2009;15(12):1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008;11(4):476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain. 2008;131(Pt 4):1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehadeh J, Fernandes HB, Zeron Mullins MM, Graham RK, Leavitt BR, Hayden MR, Raymond LA. Striatal neuronal apoptosis is preferentially enhanced by NMDA receptor activation in YAC transgenic mouse model of Huntington disease. Neurobiol.Dis. 2006;21:392–403. doi: 10.1016/j.nbd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009;30(11):563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Stack EC, Kubilus JK, Smith K, Cormier K, Del Signore SJ, Guelin E, Ryu H, Hersch SM, Ferrante RJ. Chronology of behavioral symptoms and neuropathological sequela in R6/2 Huntington's disease transgenic mice. J. Comp. Neurol. 2005;490(4):354–370. doi: 10.1002/cne.20680. [DOI] [PubMed] [Google Scholar]
- Stack EC, Dedeoglu A, Smith KM, Cormier K, Kubilus JK, Bogdanov M, Matson WR, Yang L, Jenkins BG, Luthi-Carter R, Kowall NW, Hersch SM, Beal MF, Ferrante RJ. Neuroprotective effects of synaptic modulation in Huntington's disease R6/2 mice. J. Neurosci. 2007;27(47):12908–12915. doi: 10.1523/JNEUROSCI.4318-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG, Friberg IK, Landwehrmeyer GB, Young AB, Penney JB., Jr Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Brain Res. Mol. Brain Res. 1999;64(1):11–23. doi: 10.1016/s0169-328x(98)00293-9. [DOI] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc. Natl. Acad. Sci. USA. 2009;106(24):9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Chiu D, Kowal D, Simon R, Smeyne M, Zukin RS, Olney J, Baudy R, Lin S. Characterization of two novel N-methyl-D-aspartate antagonists: EAA-090 (2-[8,9-dioxo-2,6-diazabicyclo [5.2.0]non-1(7)-en2-yl]ethylphosphonic acid) and EAB-318 (R-alpha-amino-5-chloro-1-(phosphonomethyl)-1H-benzimidazole-2-propanoic acid hydrochloride) J. Pharmacol. Exp. Ther. 2004;310(2):563–570. doi: 10.1124/jpet.104.066092. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19(10):4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57(5):369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Warby SC, Graham RK, Hayden MR. Huntington disease. 2007 http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=huntington.
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Chen N, Moshaver A, Lee AT, Wellington CL, Hayden MR, Raymond LA. Mutant huntingtin enhances excitotoxic cell death. Mol. Cell. Neurosci. 2001;17:41–53. doi: 10.1006/mcne.2000.0909. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Fernandes HB, Krebs C, Shehadeh J, Wellington CL, Leavitt BR, Baimbridge KG, Hayden MR, Raymond LA. Potentiation of NMDA receptor-mediated excitotoxicity linked with intrinsic apoptotic pathway in YAC transgenic mouse model of Huntington’s disease. Mol. Cell. Neurosci. 2004;25:469–479. doi: 10.1016/j.mcn.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-Daspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Balance beam performance on an 11 mm wide beam. (A) Time to cross beam. (B) Number of hindlimb slips. There is no difference between any treatment group and control group.
Balance beam performance on a 20 mm wide beam. (A) Time to cross beam. (B) Number of hindlimb slips. There is no difference between any treatment group and control group.
Neuronal intranuclear inclusions (NIIs) in the striatum of 13 week R6/2 mice. No qualitative effect of treatments on NII areal density or size. Treatment groups shown are (A) Vehicle Control (B) RO25,6981 (C) 10 mg/kg Ifenprodil (D) 100 mg/kg Ifenprodil (E) CP101,606 and (F) Perzinfotel. Arrows point to examples of NIIs which are visible in all panels. Scale bar = 20µm.