Abstract
We previously showed an agarose overlay on keratocytes cultured in media containing pharmacological levels of insulin enhanced collagen processing and collagen fibril formation. In this study, we compared collagen processing by keratocytes cultured in media containing physiological levels of IGF-I, TGF-β, FGF-2, and PDGF in standard and in agarose overlay cultures. Pepsin digestion/SDS PAGE was used to determine the levels of procollagen secreted into the media and the collagen content of the ECM associated with the cell layer. Distribution of collagen type I and fibronectin in the ECM of the agarose cultures was determined by immunoflorescence. Collagen fibril and keratocyte morphology was evaluated by electron microscopy. The agarose overlay significantly enhanced the cell number in the IGF-I, TGF-β and PDGF treated cultures by 2–3 fold. The overlay also significantly enhanced the processing of procollagen to collagen fibrils from 29% in standard cultures to 63–68% in agarose cultures for the IGF-I and PDGF cultures, and from 66% in standard culture to 85% in agarose culture for the TGF-β cultures. Cell accumulation and collagen processing was not enhanced by agarose overlay of the FGF-2 treated cultures. Collagen type I and fibronectin were more uniformly distributed and the collagen fibrils smaller in the ECM of the TGF-β treated cultures. Keratocytes in the FGF-2 treated cultures were in close cell contact with few collagen fibrils while IGF-I, TGF-β, and PDGF cultures had an extensive ECM with abundant collagen fibrils. The results of this study indicate that the agarose overlay enhances collagen fibril assembly and cell accumulation by keratocytes when both collagen synthesis and cell proliferation are stimulated.
Introduction
The avascular cornea functions as a major refractive element of the eye. The stroma is 90% of the thickness of the cornea and it consists of a uniquely transparent extracelllular matrix (ECM) and keratocytes. The stromal ECM is comprised of lamellae containing primarily collagen types I and V that are arranged in uniformly sized and spaced fibrils with keratan sulfate proteoglycans (keratocan and lumican) or chondroitin sulfate proteoglycans (decorin) between the fibrils (Birk et al., 1986; Chakravarti, 2006; Kao et al., 2006; Quantock and Young, 2008). The highly organized ECM is synthesized and secreted by keratocytes, which are dendritic in morphology and are sparsely scattered between the lamellae (Hahnel et al., 2000; Poole et al., 1993). The transparency of the cornea is dependent on to the precise and regular arrangement of the collagen fibrils in the ECM (Maurice, 1970). This arrangement can be disrupted upon wounding and transparency can be compromised (Birk et al., 1990; Hassell et al., 1983; Ljubimov et al., 1998).
Although the keratocytes readily proliferate and are biosynthetically active during corneal development, they exhibit a relatively low level of activity in the adult cornea and are considered “quiescent” (Jester et al., 1994; Muller et al., 1995; Zieske et al., 2001). However, the keratocytes can become active again when a wound occurs to the cornea stroma. The keratocytes damaged during wounding undergo apoptosis (Helena et al., 1998; Zieske et al., 2001). Some of the viable remaining keratocytes are activated to proliferate and migrate to the wound site (Del Pero et al., 1990; Hanna et al., 1989; Zieske et al., 2001) to form regions of hypercellularity; densely packed cells with only a sparse or “provisional” ECM (Lance et al., 1988; Lee et al., 1982; Sundarraj et al., 1998). The keratocytes can go on to stratify by producing an extensive collagenous ECM that is either a normal ECM which restores transparency or an abnormal fibrotic ECM, which is opaque or can remain hypercellular, resulting in a hypercellular scar (Dawson et al., 2005; Hassell et al., 1983; Ljubimov et al., 1998; Maguen et al., 1997). Thus, keratocytes first go through a proliferative, ECM deficient hypercellular phase that is then followed by either a collagenous phase or a fibrocollagenous phase that produces keratocyte stratifcation.
We found that the phases of ECM synthesis seen during wound healing in vivo could be replicated by the action of FGF-2, IGF-I, TGF-β, and PDGF on keratocytes in vitro (Etheredge et al., 2009). FGF-2 stimulated the highest level of proliferation but did not stimulate collagen synthesis. In contrast, IGF-I, TGF-β, and PDGF stimulated proliferation and high levels of collagen synthesis. TGF-β also stimulated hyaluronan, biglycan, and fibronectin synthesis, components of the fibrotic ECM. Therefore, we proposed (Etheredge et al., 2009) that in wound healing; 1) FGF-2 would induce the hypercellular phase, producing the sparse, collagen-deficient, provisional matrix, 2) the action of IGF-I or PDGF would then induce the collagenous phase that results in stratification with a normal stromal ECM that restores transparency or 3) the action of TGF-beta would induce the fibrocollagenous phase that results in stratification with an opaque ECM that causes blindness.
Collagen is first made as triple helical procollagen molecule assembled from three polypeptides composed of N- and C-terminal globular domains separated by a glycine-rich central triple helical region (Canty and Kadler, 2005). In vivo, the N- and C-terminal globular domains are rapidly removed, allowing the triple helical molecules to laterally associate and form collagen fibrils. Fibroblasts in standard cell culture do not readily process procollagen to collagen (Goldberg et al., 1972). As a result, the procollagen accumulates in the culture medium and only a portion is processed into collagen fibrils associated with the cells. We have found, however, that insulin stimulates procollagen synthesis and an agarose overlay on keratocytes cultured in media containing insulin increased collagen processing and the formation of an ECM associated with the keratocytes (Hassell et al., 2008). In that study, however, insulin was used at pharmacological levels. In the current study, we use this agarose overlay cell culture system to compare collagen processing and ECM assembly by keratocytes cultured in physiological levels of each of the of growth factors that induce the different phases of corneal stroma wound repair.
Results
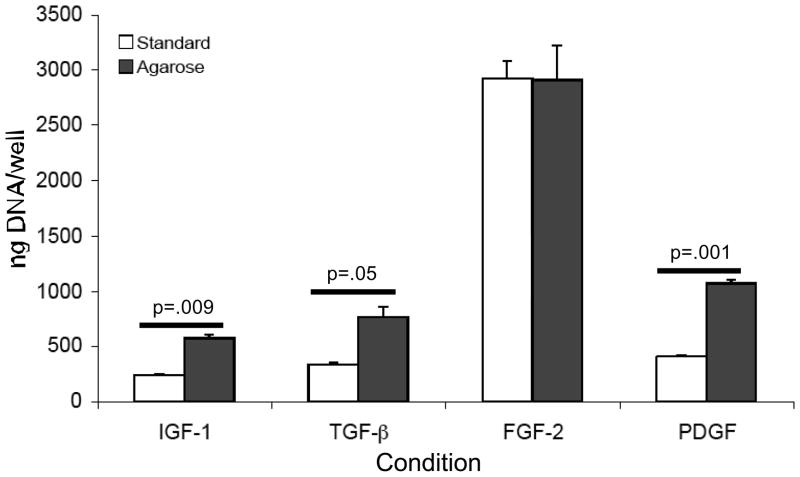
Keratocytes were cultured in media containing IGF-I, TGF-β, FGF-2 or PDGF using standard and agarose overlay conditions in six well plates. The cultures were harvested on day 20 and both the media and the cell layer in the well were digested separately with pepsin. The DNA in the pepsin digest of the cell layer was determined and was expressed per well (Fig. 1). The DNA values for keratocytes cultured in FGF-2 was significantly higher than that of keratocytes cultured in IGF-I, TGF-β and PDGF: ~10-fold higher in standard culture and 3 to 4-fold higher in agarose overlay cultures. The DNA content of keratocyte cultures in media containing IGF-I, TGF-β and PDGF and under agarose, however, was significantly higher, by 2 to 3-fold, than that of keratocytes in standard culture with these growth factors.
Figure 1.
DNA content per well of the cell layers of keratocytes in standard and in agarose overlay cultures. Keratocytes were cultured in media containing IGF-I, TGF-β, FGF-2, or PDGF and harvested for analysis on day 20. Keratocytes cultured in IGF-I, TGF-β and PDGF contained significantly more DNA per well, and therefore more cells, when cultured under agarose than in standard culture. FGF-2 cultures contained significantly (p< 0.02) more DNA per well, and therefore more cells, than the other cultures.
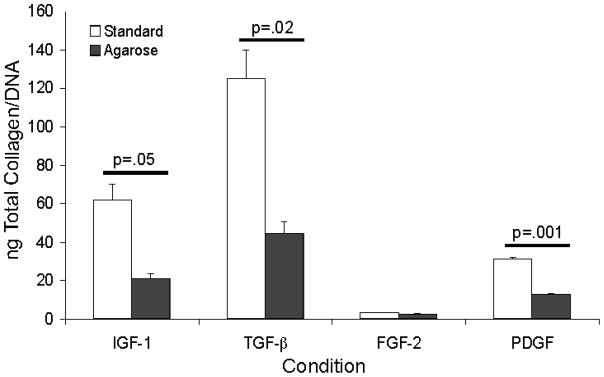
Pepsin digestion converted the insoluble, crosslinked collagen fibrils in the cell layer to soluble collagen and the procollagen in the media to collagen. The amount of collagen in the media and cell layer was determined, the media and cell layer values for each well were combined and expressed per DNA value for that well. Collagen production by keratocytes cultured in media containing FGF-2 was significantly lower than that of keratocytes cultured in IGF-I, TGF-β and PDGF in both standard and agarose cultures. Cultures treated with IGF-I, TGF-β, and PDGF and overlayed with agarose contained significantly lower (63–68% lower) levels of collagen than the standard cultures (Fig. 2).
Figure 2.
Collagen levels associated with the cell layer and media combined of keratocytes in standard and in agarose overlay cultures normalized for DNA content in the cell layer. Keratocytes were cultured in media containing IGF-I, TGF-β, FGF-2, or PDGF and harvested on day 20. IGF-I, TGF-β, and PDGF treated agarose cultures contained significantly less total collagen than standard cultures. FGF-2 cultures contained significantly (p< 0.02) less collagen than the other cultures.
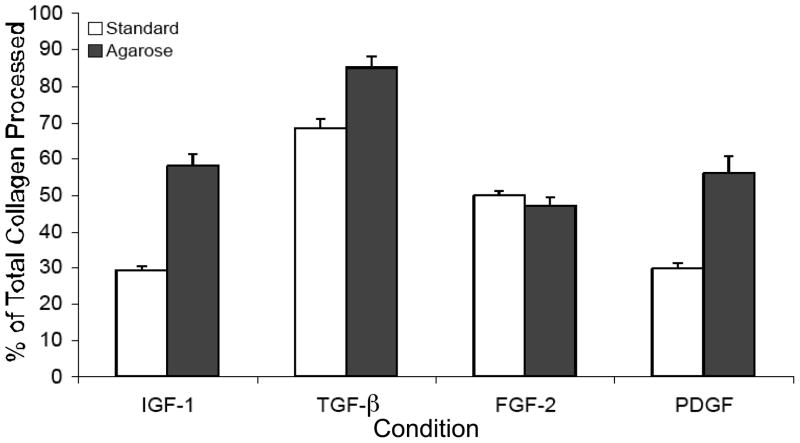
We next calculated the procollagen to collagen conversion rate by expressing the amount of collagen in the cell layer as a percent of the total collagen (cell layer plus media) produced (Fig. 3). The results show that keratocytes cultured in IGF-I and PDGF in standard culture converted ~29% of the total collagen produced to collagen fibrils and agarose overlay significantly increased this conversion rate to 56–58%. Keratocytes cultured in TGF-β in standard culture, however, converted 66% of the total collagen to collagen fibrils, significantly more than IGF-I and PDGF in standard culture and the agarose overlay of TGF-β treated cultures increased conversion to 85%, a value significantly greater than for TGF-β in standard culture. Collagen conversion by FGF-2 treated cultures was not enhanced by agarose overlay.
Figure 3.
Procollagen to collagen conversion rates for keratocytes in standard and agarose cultures. Collagen amounts associated with the cell layer were divided by the amounts in the cell layer and media combined and expressed as a percent. Compared to standard cultures, agarose significantly enhanced procollagen conversion to collagen in cultures treated with IGF-I (p=.008), TGF-β (p=.004), and PDGF (p=.01). Standard cultures treated with TGF-β were significantly more efficient at converting procollagen to collagen than IGF-I (p=.007) and PDGF (p=.007).
The distribution of collagen type I and fibronectin within the cell layer of the agarose cultures was determined by immunohistochemistry using rabbit antibodies to collagen type I and to fibronectin followed by a FITC labeled second goat anti rabbit antibody (Fig. 4). Nuclei were stained with DAPI. The location of the nuclei showed that keratocytes tended to cluster in aggregates when cultured in IGF-I, but were more uniformly distributed when cultured in the other growth factors. The antibody to collagen type I showed that collagen type I in IGF-I treated cultures was deposited in linearly connected clumps that corresponded with the cell aggregates. Collagen type I deposited in the TGF-β treated cultures was distributed more uniformly in large homogenous patches. Reactivity for collagen type I in FGF-2 treated cultures was virtually non-existent. Collagen type I in the PDGF treated cultures was deposited in both intensely staining clumps and in faintly staining patches. The antibody to fibronectin revealed fibronectin was distributed in both thick and thin linear tracks: thick linear tracks in the IGF-I and PDGF treated cultures, much thinner and more homogeneously distributed linear tracks in the TGF-β treated cultures and both thick and thin linear tracks in the FGF-2 treated cultures. Although the reactivity for fibronectin was clearly more extensive than the reactivity for collagen type I in the FGF-2 treated cultures, it was still relatively sparse.
Figure 4.
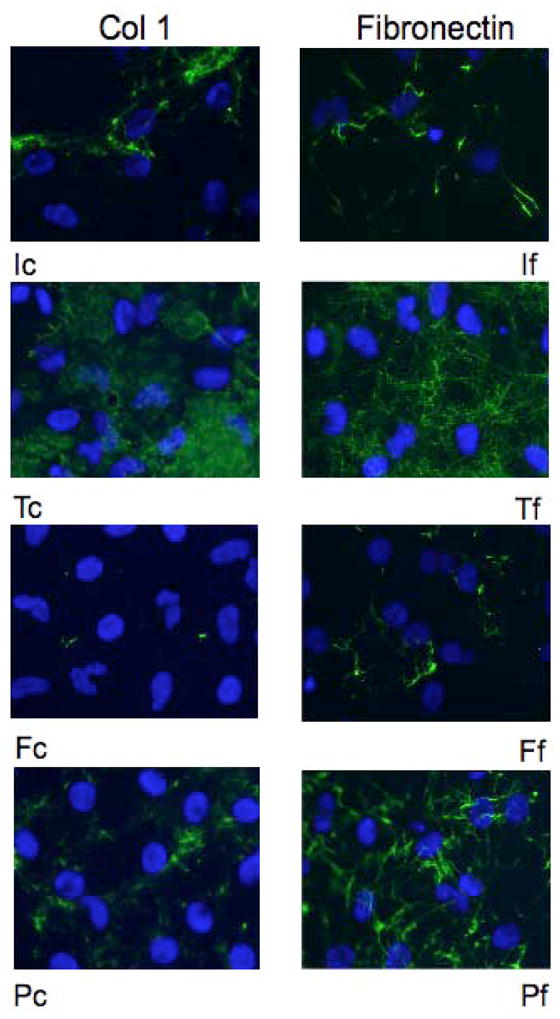
Immunoflouresence reactivity for type I collagen and fibronectin in the cell layers of keratocytes cultured under agarose and in media containing IGF-I (I), TGF-β (T), FGF-2 (F), or PDGF (P). The agarose was removed on day 20 of culture and cell layers reacted with antibodies to either collagen type I (c) or fibronectin (f). Nuclei were visualized using DAPI. Collagen type I was distributed in clumps and in large patches while fibronectin was distributed in thick and thin tracks.
The size and organization of the collagen fibrils in the ECM associated with the cell layer of agarose overlay cultures was analyzed by transmission electron microscopy. There was an extensive ECM containing numerous collagen fibrils between cells in the IGF-I, TGF-β and PDGF treated cultures (Fig. 5). The diameter of fibrils in the TGF-β treated culture were approximately one half the diameter and were more uniformly oriented than that seen in IGF-I treated cultures. The fibrils in the PDGF treated cultures showed the characteristics seen in both the TGF-β and IGF-I cultures. There was little space between the cells for an ECM in the FGF-2 treated cultures. The keratocytes were densely packed and the collagen fibrils in the space between the cells were sparse. The cell surfaces in FGF-2 treated cultures showed extensive regions of cell-cell contact.
Figure 5.
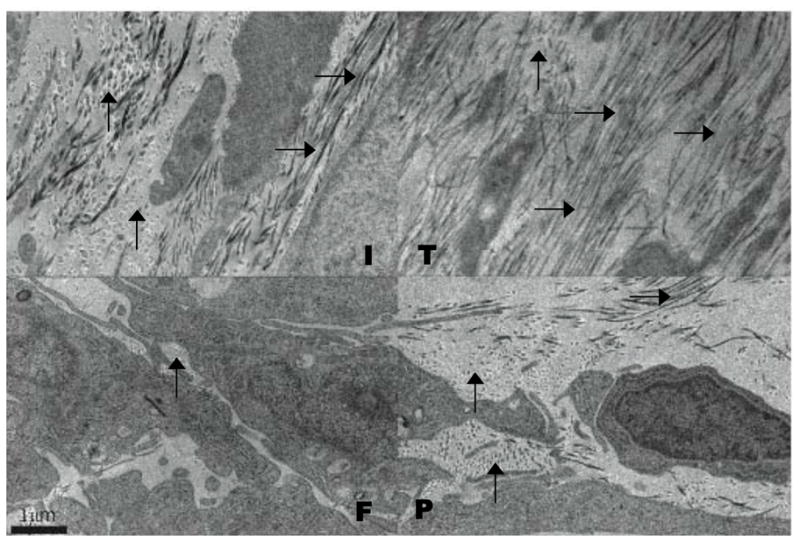
Electron microscopy of the cell layers of keratocytes cultured under agarose. Keratocytes were cultured in media containing IGF-I (I), TGF-β (T), FGF-2 (F), or PDGF (P) and fixed for electron microscopy on day 20. Collagen fibrils were abundant in the ECM of the IGF-I TGF-β, and PDGF treated cultures while FGF-2 treated cultures exhibited little ECM with few collagen fibrils. Vertical arrows point to collagen fibrils in cross section and horizontal arrows point to collagen fibrils in longitudinal section.
Discussion
We previously showed that, in standard culture, TGF-β, IGF-I and PDGF stimulated both cell proliferation and collagen synthesis; but FGF-2 only stimulated cell proliferation (Etheredge et al., 2009). The results of the studies reported here show that compared to standard culture; the agarose overlay enhanced both the cell number and the processing of procollagen to collagen and assembly into fibrils by keratocytes cultured in media containing IGF-I, TGF-β and PDGF, but not in keratocytes cultured in FGF-2. Thus, agarose overlay enhanced cell proliferation only when both collagen synthesis and cell proliferation were stimulated by growth factors. This suggests that the enhanced deposition of collagen fibrils by cultures under agarose may be allowing the keratocytes to proliferate to a higher cell density or stratify.
The results of the current study also show TGF-β treated keratocytes to be more efficient at processing procollagen to collagen in standard culture than IGF-I or PDGF treated keratocytes. TGF-β has previously been shown to enhance the proliferation and cell stratification of human fibroblasts in standard culture and that both the proliferation and stratification could be blocked by antibodies to fibronectin (Clark et al., 1997). We previously found TGF-β stimulated higher levels of fibronectin synthesis by keratocytes than PDGF and IGF-I (Etheredge et al., 2009) and the results of this report showed that the distribution of fibronectin in the ECM of the TGF-β treated cultures, as determined using immunefluorescence microscopy, was in thinner tracks and more homogeneously distributed than that of the IGF-I and PDGF treated cultures. Fibronectin can bind to collagen and has been shown to be necessary for the assembly of collagen types I and III into fibrils (Kadler et al., 2008; McDonald et al., 1982; Velling et al., 2002). The high levels of fibronectin production seen in TGF-β treated cultures and its unique distribution may be responsible for the higher levels of procollagen to collagen conversion seen in the TGF-β treated keratocytes in standard culture.
Although the TGF-β treated keratocytes in standard culture were more efficient at converting procollagen to collagen and subsequent assembly into fibrils than the IGF-I and PDGF treated keratocytes this conversion efficiency could be enhanced even further by agarose overlay. We previously proposed (Hassell et al., 2008) that the agarose overlay may be acting as a molecular sieve barrier to retard the diffusion of procollagen into the media. This would increase the concentration and co-localize procollagen and the procollagen peptidases at the cell surface, facilitating procollagen processing to collagen and fibril assembly. However, since an interaction of fibronectin with collagen also may be required for collagen fibril formation, the agarose overlay may increase the collagen-fibronectin association by the same barrier-to-diffusion type mechanism and enhance collagen fibril formation.
The agarose overlay reduced total collagen production in the IGF-I, TGF-β and PDGF treated cultures by 63–68%. The N- and C-terminal peptides on procollagen that are removed during procollagen processing have been shown to specifically inhibit procollagen synthesis (Paglia et al., 1979; Wiestner et al., 1979). Thus, the increased processing of procollagen by keratocytes cultured under agarose would produce increased levels of N- and C-terminal procollagen peptides that subsequently act on the keratocytes to reduce procollagen production.
The immune-localization of collagen and fibronectin demonstrated that they were deposited with the cell layer in distinctly different patterns in response to each of the different growth factors. The keratocytes cultured in IGF-I formed aggregates and collagen was deposited in clumps while the fibronectin was in thick tracks associated with these cell aggregates. In contrast, the collagen was deposited in large patches and the fibronectin in thin tracks and both were more uniformly distributed in the cell layer of keratocytes cultured in TGF-β. The pattern of collagen distribution in the PDGF treated cultures was intermediate between that seen in the IGF-I and TGF-β treated cultures. Similarly, the pattern of distribution of fibronectin deposited in the PDGF treated cultures also had characteristics of both the IGF-I and TGF-β cultures: it was distributed throughout the culture, but it was in thick bands, like the IGF-cultures. Fibronectin distribution in the FGF-2 treated cultures was considerably more extensive than that of collagen. These observations suggest that the collagen and fibronectin deposited in the ECM co-distributed primarily in the TGF-β treated cultures, the cultures with the highest procollagen to collagen conversion rate.
Electron microscopy revealed differences in the collagen fibrils that were deposited in the ECM by keratocytes cultured in the different growth factors. The fibrils in the IGF-I treated cultures were organized in lamellae while the fibrils in the TGF-β treated cultures were thinner and were more uniformly oriented. Although there was abundant fibril deposition in the IGF-I, TGF-β, and PDGF treated cultures, there were only a few fibrils seen in the FGF-2 treated cultures. The keratocytes were densely packed together with extensive close cell contacts, leaving little space for an ECM. This cell/matrix ratio mediated by culture in FGF-2 is similar to the situation seen for keratocytes in the hypercellular phase of corneal stromal wound healing in vivo (Lance et al., 1988; Lee et al., 1982; Sundarraj et al., 1998).
Experimental Procedures
Materials
All chemicals and growth factors were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. CyQuant kit, DMEM F/12, polyacrylamide gels, as well as pre-cast polyacrylamide gels and reagents that were used for protein separation and transfer were purchased from Invitrogen (Carlsbad, CA) and ECL Western blot analysis system was purchased from GE Healthcare (Piscataway, NJ). Costar cell culture plates and chamber slides were purchased from Fisher Scientific (Suwanee, GA). Rabbit polyclonal antibodies to collagen type I were obtained from Cosmo Bio Co. (Tokyo, Japan) and to fibronectin from Chemicon International (Temecula, CA). FITC conjugated anti rabbit antibodies were obtained from Novus Biologicals (Littleton, CO).
Keratocyte isolation and culture
Bovine eyes harvested from 12-month calves were purchased from Pel Freez Biologicals (Rogers, AR) and keratocytes were isolated as previously described (Berryhill et al., 2001). Briefly, corneas were removed and keratocytes isolated using two sequential collagenase digestions. The culture medium used throughout was serum-free DMEM/F12 containing antibiotics and 1mM 2-phospho-ascorbic acid (DMEM). Cells were plated at ~400,000 cells/well in six well plates; ~200,000 cells/well in gelatin coated, 2-well slides; and ~1,000,000 cells/60 mm dish on day 0. The medium was changed on day 4 with DMEM or to DMEM supplemented with 10ng IGF-I, 2ng TGF-β, 10ng FGF-2, or 10ng PDGF/ml, the concentrations used previously for keratocytes cultured in vitro (Etheredge et al., 2009). That study showed keratocytes cultured in medium without any growth factor do not proliferate and produce very low levels of collagen, levels similar to that of FGF-2 treated keratocytes. Therefore, we omitted cultures without growth factor in this study and compared the cultures with the different growth factors to each other.
Keratocyte cultures were overlayed with 3% agarose in DMEM on day 6 as previously described (Hassell et al., 2008). In brief, 3 grams of agarose in 50 mls of distilled water was autoclaved, cooled to 37°C and mixed with an equal volume of 37°C 2X DMEM. The medium was removed from cultures and the cells were overlayed with a volume of 3% agarose sufficient to produce a layer ~1mm thick on top of the cells. This volume of 3% agarose was 1.0ml/well in 6 well plates, 0.4ml/well in 2 well slides and 2.0ml/60mm dish. The agarose was allowed to solidify at room temperature for 5–10 minutes and DMEM supplemented with 10ng IGF-I, 2ng TGF-β, 10ng FGF-2, or 10ng PDGF/ml was then added to the respective wells. Large volumes of media were added: 8ml/well of six well plates, 2ml/well of two well slides and 16ml/60mm dish. Cultures were harvested on day 20 without intervening media changes.
Collagen measurement
Medium and cell layer of each culture were pepsin digested as previously described to isolate collagen (Etheredge et al., 2009; Hassell et al., 2008). Briefly, the eight mls of culture medium from each well of a six well plate was removed from the well and adjusted to 0.5 M acetic acid by the addition of 230 μl of glacial acetic acid. Four ml of 0.5 M acetic acid was added to the cell layer in each well of the culture plate. The medium and cell layer samples were chilled to 4°C. A pepsin stock (4mg pepsin per ml of 0.5 M a cetic acid) was prepared and 100 μl of pepsin stock was added to the medium and 50μl of pepsin stock to cell layer samples from each well. The samples were digested overnight at 4°C with the culture plates on a rocker. The digests in each well were recovered and an additional four ml of 0.5 M acetic acid containing pepsin was added to each well for a second overnight digestion. The two sequential digests for each well were combined. Both the media and the cell layer digests were neutralized to inactivate the pepsin and then dialyzed against distilled water. Aliquots of 700 μl were removed from the dialyzed cell layer digests and used to determine DNA content. The dialyzed media and cell layer digests were lyophilized and reconstituted in 200 μl of 1X LDS sample buffer (Invitrogen). Samples of 20 μl were reduced and eletrophoresed on 3–8% tris-acetate gels. Gels were soaked in Simply blue safe stain according to the manufacturer’s instructions (Invitrogen) to stain the collagen. The gel lanes were scanned using a Bio-Rad GS-710 calibrated scanning densitometer and the optical density of the α, β and higher molecular weight chains of collagen was measured using Quantity One software.
Collagen also was isolated from bovine corneas by pepsin digestion, and specifically precipitated by extensive dialysis against cold distilled water. The precipitated collagen was solubilized in 0.01N HCl, the amount quantified by measuring its absorbance at 228 nM and used as a standard in SDS/PAGE analysis for collagen content.
DNA measurement
The DNA extracted from the cell layers of each well of harvested cultures by pepsin digestion was measured using a Cyquant Assay Kit and following the instructions provided with the kit. Calf thymus DNA was used as a standard.
Fixation and immunohistochemistry
Cells on glass slides were fixed in 4% paraformaldehyde in PBS for 1 hr and rinsed with 1% glycine. The agarose overlay was punctured with a pipette tip at the edge of the culture dish and removed. Nonspecific antigens were blocked with 2.5% BSA in PBS at room temperature. Slides were then incubated with a rabbit anti-bovine collagen type I antibody at 1:500 or rabbit anti-bovine fibronectin antibody at 1:1000 in dilution buffer (1% BSA, 1% Tween 20 in 1x PBS) for 2 hrs. Unbound antibody was washed off with two 5-minute washes in PBS/Tween 20. The primary antibodies were detected by FITC conjugated anti-rabbit antibody at 1:500 in dilution buffer incubated for 1 hour in the dark. Slides were then washed twice with PBS for 5-minutes each wash in the dark. Cover slips were mounted with DAPI mounting medium, which stain the nuclei of all cells. Slides incubated with the secondary antibody alone served as the negative control. Positive controls were sections of fresh bovine corneal tissue.
Electron microscopy
Transmission electron microscopy was used to evaluate the organization of ECM deposited by keratocytes cultured under agarose. Keratocytes cultured in 60mm plates were fixed in 2.5% glutaraldehyde, 4% paraformaldehyde, 0.1M sodium cacodylate, and 8.0 mM calcium chloride. The agarose overlay was removed as described above. Cultures were post-fixed with 1% osmium tetroxide. The samples were dehydrated using a graded ethanol series. Cell layers in 100% ethanol were removed from the dish as a sheet using a cell scraper, transferred to a 1.5ml tube containing propylene oxide and then infiltrated and embedded in a mixture of Embed 812, nadic methyl anhydride, dodecenylsuccinic anhydride and DMP-30 (Electron Microscopy Sciences, PA). Sections of 90nm thickness were examined using a JEOL 1400 transmission electron microscope equipped with a Gatan Orius camera.
Statistics
All DNA and collagen values were the mean of 3 determinations. Statview Version 5 (SAS Institute, Cary, NC) was used for statistical comparisons. Samples were analyzed using a paired t test and standard deviation was used when n=3.
Acknowledgments
Supported by Grant EY08104 (JRH), EY008104 (LE) and EY05129 (DEB) from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berryhill BL, et al. Production of prostaglandin D synthase as a keratan sulfate proteoglycan by cultured bovine keratocytes. Invest Ophthalmol Vis Sci. 2001;42:1201–7. [PubMed] [Google Scholar]
- Birk DE, et al. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95(Pt 4):649–57. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- Birk DE, et al. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27:1470–7. [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–53. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Chakravarti S. Focus on molecules: keratocan (KERA) Exp Eye Res. 2006;82:183–4. doi: 10.1016/j.exer.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Clark RA, et al. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170:69–80. doi: 10.1002/(SICI)1097-4652(199701)170:1<69::AID-JCP8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Dawson DG, et al. Histologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Arch Ophthalmol. 2005;123:741–56. doi: 10.1001/archopht.123.6.741. [DOI] [PubMed] [Google Scholar]
- Del Pero RA, et al. A refractive and histopathologic study of excimer laser keratectomy in primates. Am J Ophthalmol. 1990;109:419–29. doi: 10.1016/s0002-9394(14)74608-2. [DOI] [PubMed] [Google Scholar]
- Etheredge L, et al. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest Ophthalmol Vis Sci. 2009;50:3128–36. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- Goldberg B, et al. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972;69:3655–9. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnel C, et al. The keratocyte network of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2000;19:185–93. doi: 10.1097/00003226-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Hanna KD, et al. Corneal stromal wound healing in rabbits after 193-nm excimer laser surface ablation. Arch Ophthalmol. 1989;107:895–901. doi: 10.1001/archopht.1989.01070010917041. [DOI] [PubMed] [Google Scholar]
- Hassell JR, et al. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys. 1983;222:362–9. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- Hassell JR, et al. Increased stromal extracellular matrix synthesis and assembly by insulin activated bovine keratocytes cultured under agarose. Exp Eye Res. 2008;87:604–11. doi: 10.1016/j.exer.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helena MC, et al. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–83. [PubMed] [Google Scholar]
- Jester JV, et al. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–43. [PubMed] [Google Scholar]
- Kadler KE, et al. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WW, et al. Focus on molecules: lumican. Exp Eye Res. 2006;82:3–4. doi: 10.1016/j.exer.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance SE, et al. Diamond burring and surgical keratectomy. Morphologic comparison in the rabbit. Arch Ophthalmol. 1988;106:830–4. doi: 10.1001/archopht.1988.01060130900049. [DOI] [PubMed] [Google Scholar]
- Lee RE, et al. The healing of linear nonperforating wounds in rabbit corneas of different ages. Invest Ophthalmol Vis Sci. 1982;23:660–5. [PubMed] [Google Scholar]
- Ljubimov AV, et al. Extracellular matrix changes in human corneas after radial keratotomy. Exp Eye Res. 1998;67:265–72. doi: 10.1006/exer.1998.0511. [DOI] [PubMed] [Google Scholar]
- Maguen E, et al. Alterations of corneal extracellular matrix after multiple refractive procedures: a clinical and immunohistochemical study. Cornea. 1997;16:675–82. [PubMed] [Google Scholar]
- Maurice DM. The transparency of the corneal stroma. Vision Res. 1970;10:107–8. doi: 10.1016/0042-6989(70)90068-4. [DOI] [PubMed] [Google Scholar]
- McDonald JA, et al. Role of fibronectin in collagen deposition: Fab′ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982;92:485–92. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller LJ, et al. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557–67. [PubMed] [Google Scholar]
- Paglia L, et al. Inhibition of procollagen cell-free synthesis by amino-terminal extension peptides. Biochemistry. 1979;18:5030–4. doi: 10.1021/bi00589a034. [DOI] [PubMed] [Google Scholar]
- Poole CA, et al. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci. 1993;106(Pt 2):685–91. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- Quantock AJ, Young RD. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn. 2008 doi: 10.1002/dvdy.21579. [DOI] [PubMed] [Google Scholar]
- Sundarraj N, et al. Proteoglycan distribution during healing of corneal stromal wounds in chick. Exp Eye Res. 1998;67:433–42. doi: 10.1006/exer.1998.0540. [DOI] [PubMed] [Google Scholar]
- Velling T, et al. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–81. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- Wiestner M, et al. Inhibiting effect of procollagen peptides on collagen biosynthesis in fibroblast cultures. J Biol Chem. 1979;254:7016–23. [PubMed] [Google Scholar]
- Zieske JD, et al. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72:33–9. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]