Abstract
Cerebellar damage typically results in ataxia and can be caused by stroke, tumor or one of many forms of degenerative disease. Since few pharmacological options are available, most treatments rely heavily on rehabilitation therapy. Little data exist on methods for tracking the progression of ataxia, which is critical for assessing the efficacy of current and newly developing treatments. Here, we tracked the severity of ataxia, with a particular emphasis on gait and balance dysfunction, in a group of individuals with cerebellar damage using the International Cooperative Ataxia Rating Scale (ICARS) and several instrumented laboratory measures of gait and balance impairments over one year. We found that the ICARS was able to distinguish between subjects with static lesions and those with degenerative disorders, was sensitive to increases in ataxia severity occurring over one year, and correlated well with specific instrumented measures of gait in persons with cerebellar degeneration. These results suggest the ICARS is a valuable tool for clinicians and investigators to document and track long-term changes in gait and balance performance in individuals with cerebellar degenerative disorders.
Keywords: ICARS, cerebellum, walking, ataxia, clinical assessment, sensitivity
INTRODUCTION
Gait ataxia is one of the most common and debilitating signs of cerebellar damage1 and is characterized by unsteadiness, variable foot placement, widened stance, a veering path of movement, and abnormal inter-joint coordination2–5. The severity of gait ataxia is correlated with the degree of impaired balance4,6,7. Substantial recovery of gait may be possible following cerebellar damage of a single, focal etiology; e.g., several studies have now indicated that motor recovery from ischemic cerebellar stroke is generally excellent, with minimal to no residual deficits in up to 83% of patients8–10. In contrast, progressive functional declines are common among individuals with cerebellar degenerative diseases such as spinocerebellar ataxia. The rates of decline vary widely depending on many factors, including the specific diagnosis, age at disease onset, gender, and the length of trinucleotide repeats in the case of some autosomal dominant ataxias11. Currently, no treatments exist to reverse or substantially reduce motor disability caused by cerebellar degeneration. Nevertheless continual improvements in pharmacological agents12–15 and targeted rehabilitation interventions16–19 may eventually lead to amelioration or at least some slowing of the progression of gait ataxia.
For effectiveness of any of these new avenues to be determined in randomized trials, clinical assessment tools that appropriately measure and track changes in the severity of ataxia must be available. The most widely used and accepted clinical ataxia severity scale is the International Cooperative Ataxia Rating Scale20 (ICARS). Yet to date, little attention has been paid to the utility of the ICARS in clinical trials. Ideally, a meaningful clinical rating scale for ataxia must have established reliability and validity, be sensitive to small changes over time, discriminate between static and progressive cerebellar lesions, and relate to function in some way. The ICARS has been shown to be reliable in cerebellar patients21–24 and to have criterion-related and external validity, though internal validity is unclear22,23. To date, however, no study has been able to demonstrate sensitivity of the ICARS to change in persons with chronic cerebellar disease23. In addition, the relationship between ICARS scores and instrumented movement tests has not previously been examined.
We sought to identify behavioral measures sensitive to the progression of gait ataxia in persons with cerebellar degenerative disorders. Specifically, we wanted to determine the sensitivity of the ICARS and a series of common laboratory gait and balance measures to cerebellar disease progression, and whether the ICARS and instrumented measures are related to one another. We measured motor performance in a group of adults with either static or degenerative cerebellar damage at three time intervals over one year. We predicted we would be able to dissociate persons with progressive degeneration from those with static lesions by the presence or absence, respectively, of motor declines over time and that ICARS scores would correlate with instrumented measures of walking.
METHODS
Subjects
Eighteen subjects with cerebellar damage, either due to a progressive, degenerative disease (“degenerative” group) or to a non-degenerative single injury to the cerebellum (“static” group), participated in the study. Inclusion criteria were: cerebellar damage confirmed by MRI or CT scan, clinical evidence of at least mild ataxia (ICARS score ≥ 5), ≥ two months since time of onset, ability to stand unsupported for > five seconds, and ability to walk unsupported and without any assistive devices for > 10 meters. Exclusion criteria were: radiological or clinical evidence of involvement of brain structures outside the cerebellum, significant orthopaedic or other medical conditions that could affect motor performance, or current participation in any therapies for ataxia. Cerebellar subjects participated in three testing sessions: baseline, a six-month follow-up, and a 12-month follow-up. These timeframes are typical for clinical trials and longitudinal reports in this patient population24–26. Data were also collected from two groups of 10 age-matched controls during a single session to provide reference values for typical healthy adult gait and balance measures. See Table 1 for detailed subject characteristics. All subjects gave their informed consent prior to participating and the institutional human studies committee approved the study.
Table 1.
Cerebellar subject characteristics
| Subj | Age (y) | Sex | Diagnosis | Time Since Onset (y) | Total ICARS |
|---|---|---|---|---|---|
| Static group | |||||
| S1 | 62 | M | L PICA stroke | 0.2 | 12 |
| S2 | 16 | M | R cerebellar astrocytoma resection | 0.4 | 12 |
| S3 | 68 | M | R PICA and SCA strokes | 2.3 | 16 |
| S4 | 20 | M | Cerebellar tumor resection | 7.0 | 21 |
| S5 | 52 | M | L AICA stroke | 4.7 | 22 |
| S6 | 29 | F | Medulloblastoma resection | 13.4 | 30 |
| S7 | 45 | F | Static childhood cerebellar lesion of unknown origin | 30.3 | 46 |
| mean (SEM) | 41.7 (7.7) | 2 F, 5 M | 8.3 (4.0) | 22.7 (4.6) | |
| Degenerative group | |||||
| D1 | 68 | M | SAOA | 12 | |
| D2 | 39 | F | SCA 2 | 13 | |
| D3 | 52 | M | FAOA | 14 | |
| D4 | 31 | M | SCA 8 | 30 | |
| D5 | 51 | F | SAOA | 31 | |
| D6 | 34 | F | SCA 6 | 35 | |
| D7 | 44 | M | SAOA | 35 | |
| D8 | 56 | M | SAOA | 36 | |
| D9 | 48 | M | SCA 6 and SCA 8 | 40 | |
| D10 | 47 | M | SAOA | 40 | |
| D11 | 69 | F | SCA 6 | 65 | |
| mean (SEM) | 49.0 (3.7) | 4 F, 7 M | 31.9 (4.6) | ||
| tot mean (SEM) | 46.2 (3.7) | 28.3 (3.4) | |||
Values for Age, Time Since Onset and Total ICARS are from the baseline testing session. Abbreviations: Subj=Subject number; ICARS=International cooperative ataxia rating scale; M=male; F=female; L=left; R=right; PICA=posterior inferior cerebellar artery; AICA=anterior inferior cerebellar artery; SCA=superior cerebellar artery; SAOA=sporadic adult onset ataxia; FAOA= familial adult onset ataxia; SCA 2,6,8=spinocerebellar ataxia type 2, type 6, type 8; y=years. Data were also collected from two groups of ten age-matched controls to provide reference values for the gait (3 females; mean age ± SEM, 46.3±5.8 years) and balance (4 females; 46.0±5.1 years) measures.
Paradigm
The ICARS20 was used to clinically quantify the severity of ataxia in cerebellar subjects. The ICARS is a 100-point ordinal scale that quantifies ataxia in four categories of movement: posture and gait, limb kinetics, speech, and eye movements. Higher scores indicate greater ataxia.
Balance was assessed in three tasks with subjects standing on a force plate (Kistler 9281, Kistler Instrument Corp., Switzerland) with eyes open and arms folded: quiet standing with feet together, weight shifting laterally, and weight shifting forward and backward, both as far as possible with feet shoulder-width apart. Three 20-second trials of each condition were collected.
Gait testing consisted of walking as fast as possible across a level 8-meter walkway without assistive devices or orthotics. Five walking trials were collected. Rest breaks were provided as needed to minimize fatigue.
Data collection
The ICARS was scored by the same experienced examiner. Force plate center of pressure (COP) coordinates (sampling rate, 1000 Hz) were used to quantify balance. Foot positions were recorded via infrared-emitting diodes placed on the fifth metatarsal heads, lateral malleoli and lateral knee joint spaces to represent the feet, ankles and knees. Positions were recorded in 3D (sampling rate, 100 Hz) using two Optotrak 3020 sensors (Optotrak System; Northern Digital Inc., Waterloo ON).
Data analysis
Position data were low-pass filtered at 10 Hz. Custom software from Matlab (The MathWorks, Natick MA) were used for all other analyses. During static standing, balance deficits were quantified using mean sway amplitude and sway variance. Mean sway amplitude measured the average deviation of the COP from its center using the equation: , where n represents the number of data samples in each static balance trial and x and y represent the COP locations in the medio-lateral and antero-posterior dimensions, respectively, for each data sample27. Sway variance was calculated as the sway amplitude coefficient of variation for each trial: . During weight shifting, balance deficits were quantified by weight shift distances, or the average peak-to-peak distances traveled by the COP in the desired direction (medio-laterally for the lateral weight shift task; antero-posteriorly for the fore-aft task) and normalized to foot spread (for lateral weight shifts) or foot length (fore-aft shifts).
Walking was quantified by a number of variables selected to address known features of cerebellar gait ataxia: stride length, stride width, cadence, stance time, double support time, and walking speed. Stride was defined as the period from initial contact on one foot to the next initial contact on the same foot. Stride length was measured as the forward distance traveled by the ankle marker during one stride. Stride width was measured as the lateral distance between the two ankle markers at the time of initial contact. Cadence was recorded as the average number of steps per minute. Stance time and double support time, the time when both feet are in contact with the ground, were normalized to stride time.
Statistical analyses were completed using Statistica software (StatSoft, Inc., Tulsa OK). At baseline, comparisons among control, static cerebellar and degenerative cerebellar groups were completed using a one-way analysis of variance (ANOVA). To compare differences between cerebellar groups across visits, we used a two-factor (group × visit) ANOVA with repeated measures on one factor. When any ANOVA was significant, post hoc analysis was done using Tukey’s honest significant different test. For the ICARS measures only, we used a Mann Whitney U test to compare static and degenerative groups and Friedman ANOVA by ranks to compare across visits. Post hoc comparisons were made using the Wilcoxon matched pairs test. In the cerebellar degeneration group, we assessed the relationship between ICARS scores and walking performance with Spearman rank order correlations. The level for statistical significance was set to p<0.05.
RESULTS
All 18 cerebellar subjects completed all three visits. The six and 12-month follow-up visits occurred, on average, 6.78±0.37 and 13.33±0.40 months after the baseline visit. There were no differences in these values between static and degenerative groups (p=0.87 and p=0.53, respectively).
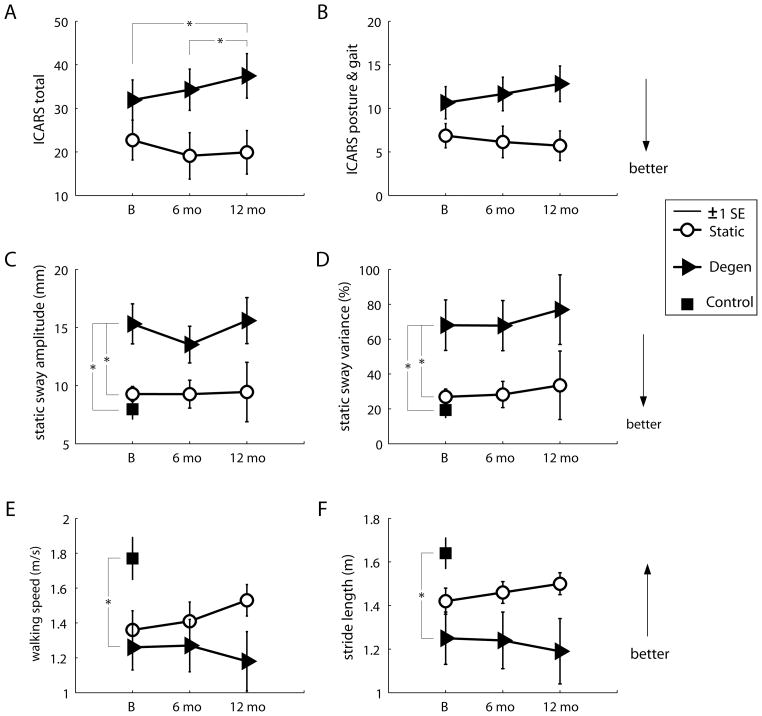
Figures 1A, B show total ICARS scores and posture and gait ICARS subscores for both cerebellar groups. Total ICARS scores did not differ between cerebellar groups at baseline (p=0.17). For the degenerative but not the static group, there was a significant worsening of ICARS scores over the three visits (p=0.012); scores increased over the year (baseline vs. 12-month visit, p=0.029) and over the second six-month period (six-month vs. 12-month visit, p=0.028). In contrast, scores tended to remain stable, and in some cases improved, in the static group (p=0.163). Similar results were noted for posture and gait ICARS subscores (Fig. 1B). Specifically, there was no significant difference in posture and gait subscores between groups at baseline (p=0.09), however the degenerative group showed a trend towards worsening of posture and gait subscores over the three visits (p=0.067). The static group showed no such difference across visits (p=0.86). For the other ICARS subscores, there were neither any significant between-groups differences at baseline (limb kinetics, p=0.24; speech, p=0.20; oculomotor, p=0.82), nor any differences between visits. However there was a trend towards significant worsening of limb kinetics subscores within the degenerative group (p=0.058). See Table 2 for details of the other ICARS subscores at each visit.
Figure 1. Selected results from the comparison of control, cerebellar static and cerebellar degenerative groups over time.
ICARS scores: average total scores (A) and posture and gait subscores (B). Static balance measures: average mean sway amplitudes (C) and sway variances (D). Gait measures: average walking speeds (E) and stride lengths (F). Asterisks indicate significant differences (p<0.05). Abbreviations: ICARS=International cooperative ataxia rating scale; Degen=cerebellar degenerative group; SE=standard error; B=baseline; mo=months.
Table 2.
Additional results for the cerebellar groups
| Measure | Group | Baseline | 6 Months | 12 Months | Significance (p values) | |
|---|---|---|---|---|---|---|
| B | x Visits | |||||
| ICARS limb kinetics | Static Degen |
11.3 ± 2.6 16.4 ± 2.7 |
10.0 ± 3.0 17.4 ± 2.6 |
10.3 ± 2.6 18.5 ± 2.8 |
0.246 | 0.341 (S), 0.058 (D) |
| ICARS speech | Static Degen |
1.1 ± 0.6 2.2 ± 0.6 |
0.6 ± 0.4 2.4 ± 0.6 |
1.3 ± 0.7 2.3 ± 0.5 |
0.246 | 0.061 (S), 0.905 (D) |
| ICARS oculomotor | Static Degen |
3.0 ± 0.6 3.0 ± 0.5 |
2.9 ± 0.5 3.4 ± 0.6 |
2.7 ± 0.6 3.3 ± 0.5 |
0.860 | 0.472 (S), 0.296 (D) |
| Lateral weight shift (%) | Static Degen Cont |
92.5 ± 4.4 83.2 ± 7.7 95.0 ± 5.6 |
104.2 ± 6.8 82.0 ± 8.3 |
100.2 ± 8.0 86.6 ± 6.6 |
0.376 | 0.225 (G), 0.197 (V), 0.241 (GxV) |
| Fore-aft weight shift (%) | Static Degen Cont |
48.4 ± 4.8 44.4 ± 3.2 49.1 ± 2.7 |
52.1 ± 3.8 43.2 ± 4.2 |
46.8 ± 3.1 44.5 ± 3.0 |
0.562 | 0.541 (G), 0.436 (V), 0.795 (GxV) |
| Stride width (mm) | Static Degen Cont |
288.1 ± 27.7 297.7 ± 31.5 207.5 ± 18.0 |
241.1 ± 45.7 321.6 ± 26.7 |
263.5 ± 36.9 267.9 ± 32.4 |
0.044* | 0.599 (G), 0.180 (V), 0.095 (GxV) |
| Cadence (steps/min) | Static Degen Cont |
113.7 ± 5.8 118.5 ± 5.2 128.0 ± 5.2 |
116.1 ± 6.6 119.3 ± 5.2 |
119.8 ± 4.4 112.7 ± 6.7 |
0.215 | 0.861 (G), 0.552 (V), 0.222 (GxV) |
| Stance time (%) | Static Degen Cont |
62.0 ± 0.7 62.6 ± 2.1 54.7 ± 0.4 |
62.0 ± 0.7 63.8 ± 2.1 |
62.1 ± 0.6 67.0 ± 3.3 |
0.001* | 0.438 (G), 0.081 (V), 0.100 (GxV) |
| Double support time (%) | Static Degen Cont |
11.7 ± 0.6 11.6 ± 2.0 10.0 ± 0.7 |
12.0 ± 1.0 13.8 ± 2.1 |
12.6 ± 0.9 13.8 ± 1.8 |
0.628 | 0.699 (G), 0.020* (V), 0.213 (GxV) |
For dynamic balance measures, ‘%’ represents weight shift distances as a percent of foot spread/length; for walking measures, ‘%’ represents time, as a percentage of the total stride duration. Abbreviations: ICARS=International cooperative ataxia rating scale; Static=static cerebellar group; Degen=degenerative cerebellar group; Cont=healthy control group; S=effect of visit within static group; D=effect of visit within degenerative group; G=main effect of group; V=main effect of visit; GxV=group-by-visit interaction effect. Values represent means ± 1 SEM. P values represent the level of statistical significance (values p<0.05 are indicated in bold and with asterisks). In the column labeled ‘B’, values are generated from the one-way ANOVA (for balance and gait measures) or the Mann Whitney U (for ICARS scores) comparing baseline performance across groups. In the column labeled ‘x Visits’, values are from the factorial ANOVA (balance and gait) or Friedman ANOVA by ranks (ICARS) comparing groups across all visits.
Static balance testing results are depicted in Figures 1C, D. At baseline, mean sway amplitudes differed among all three groups (p<0.001), with the degenerative group showing worse postural sway compared to either the control group (post hoc, p<0.001) or the static group (post hoc, p=0.008), but no difference between control and static groups (post hoc, p=0.74). Similarly, the factorial analysis showed an overall increase in postural sway in the degenerative group compared to the static group (group effect, p=0.04), but no effects of visit or any group × visit interaction, indicating that neither cerebellar group appeared to demonstrate much change in static postural sway over the course of the year (Fig. 1C). Sway variance also differed across the groups at baseline (p=0.002). Again, differences were attributable to impaired postural control in the degenerative (degenerative vs. control, p=0.002; degenerative vs. static, p=0.019) but not the static group (static vs. control, p=0.85; Fig. 1D). There were no changes in sway variance over the three visits, nor any interaction effects. Dynamic weight shifting performance did not differ across groups at baseline (lateral, p=0.38; fore-aft, p=0.56) or across visits (see Table 2).
At baseline, we found significant differences across groups for many of the walking parameters: cerebellar subjects generally took shorter strides (p=0.02), wider steps (p=0.04), spent more time in stance (p=0.001) and walked slower (p=0.02) than controls. However there were no differences between degenerative and static groups on any of these measures (all p>0.49). There were no significant differences between the three groups in cadence (p=0.22) or percent double support time (p=0.63; see Table 2).
Over time, the static cerebellar group tended to walk faster and take longer strides, whereas the degenerative cerebellar group tended to walk slower and take shorter strides (group × visit interactions, p=0.007 and p=0.042, respectively; Fig. 1E, F). There were no significant post hoc differences between visits for walking speed or stride length. There was a significant effect of visit for percent double support time (p=0.02). The cerebellar group as a whole increased double support times at the six-month follow up (post hoc, p=0.02) and at the 12-month follow up compared to baseline (post hoc, p=0.01). We found no significant changes across groups or visits for stride width, cadence or percent stance time (see Table 2).
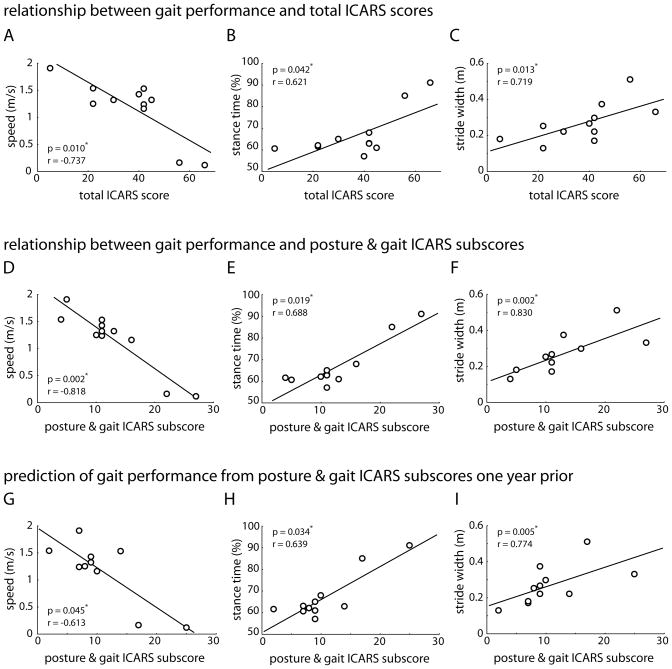
Figures 2A–F show the correlations between ICARS scores and gait performance for the individuals in the cerebellar degenerative group at the 12 month visit. We selected gait performance measures of walking speed, stance time and stride width for these analyses because these parameters have been specifically reported to be impaired in individuals with cerebellar disorders and shown to be strongly related to balance function4. Correlations between total ICARS scores and walking performance were strong and significant (Fig. 2A–C; speed, r=−0.737; stance time, r=0.621; stride width, r=0.719). Correlations between posture and gait ICARS subscores and walking were also significant (Fig. 2D–F; speed, r=−0.818; stance time, r=0.688; stride width, r=0.830). Notably, each of these produced a higher correlation coefficient and greater statistical significance than the corresponding correlation using total ICARS scores.
Figure 2. Scatterplots showing the correlation between specific walking parameters and ICARS scores for all subjects in the cerebellar degenerative group.
Correlation between total ICARS scores and walking speed (A), stance time (B) and stride width (C) at the 12-month visit. Correlation between posture and gait ICARS subscores and walking speed (D), stance time (E) and stride width (F) at the 12-month visit. Correlation between posture and gait ICARS subscores at baseline and walking speed (G), stance time (H) and stride width (I) at the 12-month visit. Spearman correlation coefficients and significance levels are provided in each of the panels; asterisks indicate significant differences (p<0.05). Abbreviations: ICARS=International cooperative ataxia rating scale.
To determine whether the ICARS could predict later gait performance, we calculated correlation coefficients between ICARS scores at baseline and walking performance at the 12 month follow-up. Overall, total ICARS scores were not a significant predictor of gait measures one year later. The correlations between total ICARS score and walking speed, stance time and stride width were r=−0.439 (p=0.176), r=0.438 (p=0.177) and r=0.551 (p=0.079), respectively. However, posture and gait ICARS subscores did predict all three walking measures (see Fig. 2G–I; speed, r=−0.613; stance time, r=0.639; stride width, r=0.774).
DISCUSSION
Using both clinical ICARS scores and a battery of instrumented laboratory measures, we tracked gait and balance over one year in individuals with cerebellar dysfunction due either to a static lesion or degenerative disease.
Baseline performance
All the individuals with cerebellar damage showed evidence of significant ataxia, as measured by ICARS scores. The differences between cerebellar groups and healthy controls on the balance and walking tests provide further evidence that the cerebellar subjects in our sample were significantly ataxic2,3,5,28,29. Interestingly, although both had ataxia, balance and walking appeared to be somewhat less affected in the static group than the degenerative cerebellar group (see Fig. 1C, D). This could be due to slightly more severe ataxia in the degenerative group (though baseline ICARS scores were statistically not different between groups), or it may be related to differences in lesion location or volume between groups and/or the possibility of better natural recovery in the static group, in which subjects had only a single injury.
Longitudinal changes
In subjects with cerebellar degeneration, total ICARS scores were sensitive to declines in motor performance over a one-year period and even over the second six-month period (Fig. 1A). Posture and gait ICARS subscores showed borderline sensitivity, with the degenerative group trending towards a significant increase over the year (p=0.067). Two principal measures of walking performance, gait speed and stride length, showed similar trends longitudinally (compare Figs. 1A, B with 1E, F), but we were unable to detect statistically significant worsening in the degenerative group using any instrumented walking measure. Therefore total ICARS scores were the only measure we made that showed clear sensitivity to change. The advantage of the ICARS over our instrumented laboratory measures for this type of comparison is probably that the ICARS assesses all forms of ataxia broadly, whereas our instrumented tests focused exclusively on balance and gait impairments. Thus the ICARS may have been able to detect more subtle declines occurring over multiple motor domains.
Two individuals in the static cerebellar group had injuries less than one year old. Conceivably, they may have experienced natural recovery over the year-long study period, which could have falsely exaggerated differences between static and degenerative groups over time. To test for this, we reanalyzed the data with these two individuals removed from the group and found that both of the previously significant group × visit interaction effects remained (walking speed, p=0.02; stride length, p=0.04). This verifies that the differences over time are attributable to a decline in the degenerative group and not to any improvement in the static group.
Our longitudinal results differ from another study that reported total ICARS scores were not sensitive to change over one year23. Although baseline ICARS scores were similar across subjects in these two studies, the variability across sessions appears to be somewhat lower in the current report. This could be due to the fact that our subjects were tested by the same examiner at each visit. The rate of disease progression may have also differed across subjects in the two study samples.
Relationship between ICARS and instrumented walking measures
Despite our small sample (11 individuals with cerebellar degeneration), we found strong correlations between total ICARS scores and walking speed, percent stance time, and stride width. As might be expected there were even stronger correlations between posture and gait ICARS subscores and the same walking parameters. Based on our data, posture and gait ICARS subscores are the better predictor of gait ataxia, explaining 47–69% of the variance in gait deficits in persons with cerebellar degeneration, compared to 38–54% for total ICARS scores. Also, posture and gait subscores, but not total ICARS scores, correlated with walking function one year later. Thus, not only is the ICARS sensitive to change, the posture and gait component is a significant predictor of gait performance one year later.
New clinical assessment tools have become available for measurement of ataxia severity, such as the Scale for the Assessment and Rating of Ataxia (SARA30), the Ataxia Functional Composite Scale31, and the Brief Ataxia Rating Scale32. To date, the ICARS has been more widely utilized33–35 and recently, it was shown to be correlated with cerebellar volumetry in persons with pure cerebellar degeneration36. However newer scales have unique features that may improve evaluation of ataxia, e.g., the SARA can be completed faster and may have better construct validity than the ICARS30. Further studies should directly compare these measures’ psychometric properties and relationships to recovery or progression of cerebellar ataxia in patients with different types of cerebellar lesions.
Conclusions
We showed that the ICARS and certain instrumented gait measures can distinguish subjects with static versus degenerative cerebellar lesions over time. Importantly, the ICARS appears to be sensitive to even mild increases in the severity of ataxia occurring over one year. Total ICARS scores and posture and gait ICARS subscores both are highly correlated with gait performance, though posture and gait subscores are more strongly related, and can predict gait performance one year later. These data suggest the ICARS may serve as a gross substitute for some instrumented measures of gait in persons with degenerative cerebellar disease. Longer longitudinal studies with larger sample sizes are needed to validate this and to determine how these results differ across disease types. Further, it should be examined whether the ICARS is sensitive to improvements of ataxia in individuals with static lesions.
Acknowledgments
The authors would like to thank Rebecca Bunoski, Natalie Zamora and Ari Bezman for their assistance with data collection, and Shih-Chiao Tseng for helpful review of this manuscript. This work was supported by NIH (NICHD/NCMRR) grants K01 HD050369, K01 HD049476, K12 HD055931 and R01 HD040289, and the Foundation for Physical Therapy.
Footnotes
Financial disclosures/Conflict of interest: The following provides full financial disclosures from all authors relative to the research covered in the submitted manuscript, regardless of date. Morton: NIH K01 HD050369 (NICHD/NCMRR), the Foundation for Physical Therapy. Tseng: NIH K12 HD055931 (NICHD/NCMRR). Zackowski: NIH K01 HD049476 (NICHD/NCMRR). Daline: N/A. Bastian: NIH R01 HD040289 (NICHD/NCMRR).
AUTHOR ROLES
Morton: 1C, 2B, 2C, 3A, 3B. Tseng: 2B, 2C, 3A, 3B. Zackowski: 1C, 2C, 3A, 3B. Daline: 1C, 2B, 3B. Bastian: 1A, 1B, 2A, 2B, 2C, 3B.
FINANCIAL DISCLOSURES
The following provides full financial disclosures from all authors of all other support unrelated to this research and covering the past year (from the date of submission).
Morton: University of Iowa, Foundation for Physical Therapy Research Grant. Tseng: Temple University. Zackowski: Kennedy Krieger Institute. Daline: University of Minnesota Medical Center, Fairview. Bastian: Kennedy Krieger Institute, Johns Hopkins Brain Sciences Institute, NIH (NICHD/NCMRR) R01 HD048741, NIH (NINDS) R21 NS061189, NIH (NICHD/NCMRR) R21 HD060169.
References
- 1.Thach WT, Bastian AJ. Role of the cerebellum in the control and adaptation of gait in health and disease. Prog Brain Res. 2004;143:353–366. doi: 10.1016/s0079-6123(03)43034-3. [DOI] [PubMed] [Google Scholar]
- 2.Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- 3.Hallet M, Massaquoi SG. Physiologic studies of dysmetria in patients with cerebellar deficits. Can J Neurol Sci. 1993;20(Suppl 3):S83–S92. [PubMed] [Google Scholar]
- 4.Morton SM, Bastian AJ. Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol. 2003;89(4):1844–1856. doi: 10.1152/jn.00787.2002. [DOI] [PubMed] [Google Scholar]
- 5.Ilg W, Golla H, Thier P, Giese MA. Specific influences of cerebellar dysfunctions on gait. Brain. 2007;130(Pt 3):786–798. doi: 10.1093/brain/awl376. [DOI] [PubMed] [Google Scholar]
- 6.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 7.Dichgans J, Diener HC. Clinical evidence for functional compartmentalization of the cerebellum. In: Bloedel JR, Dichgans J, Precht W, editors. Cerebellar Functions. Berlin: Springer-Verlag; 1985. pp. 126–147. [Google Scholar]
- 8.Tohgi H, Takahashi S, Chiba K, Hirata Y. Cerebellar infarction: clinical and neuroimaging analysis in 293 patients. Stroke. 1993;24:1697–1701. doi: 10.1161/01.str.24.11.1697. [DOI] [PubMed] [Google Scholar]
- 9.Jauss M, Krieger D, Hornig C, Schramm J, Busse O. Surgical and medical management of patients with massive cerebellar infarctions: results of the German-Austrian Cerebellar Infarction Study. J Neurol. 1999;246:257–264. doi: 10.1007/s004150050344. [DOI] [PubMed] [Google Scholar]
- 10.Kelly PJ, Stein J, Shafqat S, et al. Functional recovery after rehabilitation for cerebellar stroke. Stroke. 2001;32(2):530–534. doi: 10.1161/01.str.32.2.530. [DOI] [PubMed] [Google Scholar]
- 11.Klockgether T, Lüdtke R, Kramer B, et al. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain. 1998;121(Pt 4):589–600. doi: 10.1093/brain/121.4.589. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Yoshida K, Miyazaki D, Morita H, Ikeda S. Spinocerebellar ataxia type 6 (SCA6): clinical pilot trial with gabapentin. J Neurol Sci. 2009;278(1–2):107–111. doi: 10.1016/j.jns.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Tanabe M, Nakano T, Honda M, Ono H. Glycine transporter blockade ameliorates motor ataxia in a mouse model of spinocerebellar atrophy. J Pharmacol Sci. 2009;109(3):444–448. doi: 10.1254/jphs.08329fp. [DOI] [PubMed] [Google Scholar]
- 14.Marmolino D, Acquaviva F. Friedreich’s Ataxia: From the (GAA)(n) Repeat Mediated Silencing to New Promising Molecules for Therapy. Cerebellum. 2009;8(3):245–259. doi: 10.1007/s12311-008-0084-2. [DOI] [PubMed] [Google Scholar]
- 15.Torashima T, Koyama C, Iizuka A, et al. Lentivector-mediated rescue from cerebellar ataxia in a mouse model of spinocerebellar ataxia. EMBO Rep. 2008;9(4):393–399. doi: 10.1038/embor.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sliwa JA, Thatcher S, Jet J. Paraneoplastic subacute cerebellar degeneration: functional improvement and the role of rehabilitation. Arch Phys Med Rehabil. 1994;75(3):355–357. doi: 10.1016/0003-9993(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 17.Gill-Body KM, Popat RA, Parker SW, Krebs DE. Rehabilitation of balance in two patients with cerebellar dysfunction. Phys Ther. 1997;77(5):534–552. doi: 10.1093/ptj/77.5.534. [DOI] [PubMed] [Google Scholar]
- 18.Perlmutter E, Gregory PC. Rehabilitation treatment options for a patient with paraneoplastic cerebellar degeneration. Am J Phys Med Rehabil. 2003;82(2):158–162. doi: 10.1097/00002060-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Harris-Love MO, Siegel KL, Paul SM, Benson K. Rehabilitation management of Friedreich ataxia: lower extremity force-control variability and gait performance. Neurorehabil Neural Repair. 2004;18(2):117–124. doi: 10.1177/0888439004267241. [DOI] [PubMed] [Google Scholar]
- 20.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 21.Storey E, Tuck K, Hester R, Hughes A, Churchyard A. Inter-rater reliability of the International Cooperative Ataxia Rating Acale (ICARS) Mov Disord. 2004;19(2):190–192. doi: 10.1002/mds.10657. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz-Hubsch T, Tezenas du Montcel S, Baliko L, et al. Reliability and validity of the International Cooperative Ataxia Rating Scale: a study in 156 spinocerebellar ataxia patients. Mov Disord. 2006;21(5):699–704. doi: 10.1002/mds.20781. [DOI] [PubMed] [Google Scholar]
- 23.Schoch B, Regel JP, Frings M, et al. Reliability and validity of ICARS in focal cerebellar lesions. Mov Disord. 2007;22(15):2162–2169. doi: 10.1002/mds.21543. [DOI] [PubMed] [Google Scholar]
- 24.Timmann D, Gerwig M, Frings M, Maschke M, Kolb FP. Eyeblink conditioning in patients with hereditary ataxia: a one-year follow-up study. Exp Brain Res. 2005;162(3):332–345. doi: 10.1007/s00221-004-2181-x. [DOI] [PubMed] [Google Scholar]
- 25.Sorbi S, Forleo P, Fani C, Piacentini S. Double-blind, crossover, placebo-controlled clinical trial with L-acetylcarnitine in patients with degenerative cerebellar ataxia. Clin Neuropharmacol. 2000;23:114–118. doi: 10.1097/00002826-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Trujillo-Martín MM, Serrano-Aguilar P, Monton-Alvarez F, Carrillo-Fumero R. Effectiveness and safety of treatments for degenerative ataxias: a systematic review. Mov Disord. 2009;24(8):1111–1124. doi: 10.1002/mds.22564. [DOI] [PubMed] [Google Scholar]
- 27.Mauritz KH, Dichgans J, Hufschmidt A. Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain. 1979;102(3):461–482. doi: 10.1093/brain/102.3.461. [DOI] [PubMed] [Google Scholar]
- 28.Diener HC, Dichgans J, Bacher M, Gompf B. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol. 1984;57(2):134–142. doi: 10.1016/0013-4694(84)90172-x. [DOI] [PubMed] [Google Scholar]
- 29.Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10(3):247–259. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz-Hubsch T, Tezenas du Montcel S, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurol. 2006;66:1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 31.Assadi M, Leone P, Veloski JJ, Schwartzman RJ, Janson CG, Campellone JV. Validating an Ataxia Functional Composite Scale in spinocerebellar ataxia. J Neurol Sci. 2008;268(1–2):136–139. doi: 10.1016/j.jns.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Schmahmann JD, Gardner R, MacMore J, Vangel MG. Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord. 2009;24(12):1820–1828. doi: 10.1002/mds.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich’s ataxia: a randomised, placebo-controlled trial. Lancet Neurol. 2007;6(10):878–886. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- 34.Gabsi S, Gouider-Khouja N, Belal S, et al. Effect of vitamin E supplementation in patients with ataxia with vitamin E deficiency. Eur J Neurol. 2001;8(5):477–481. doi: 10.1046/j.1468-1331.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- 35.Yabe I, Sasaki H, Yamashita I, Takei A, Tashiro K. Clinical trial of acetazolamide in SCA6, with assessment using the Ataxia Rating Scale and body stabilometry. Acta Neurol Scand. 2001;104(1):44–47. doi: 10.1034/j.1600-0404.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 36.Richter S, Dimitrova A, Maschke M, et al. Degree of cerebellar ataxia correlates with three-dimensional mri-based cerebellar volume in pure cerebellar degeneration. Eur Neurol. 2005;54(1):23–27. doi: 10.1159/000087241. [DOI] [PubMed] [Google Scholar]