Abstract
The purpose of this study was to determine whether a brief (6–8 sessions) cognitive-behavioral treatment for temporomandibular dysfunction-related pain could be efficacious in reducing pain, pain-related interference with lifestyle and depressive symptoms. The patients were 101 men and women with pain in the area of the temporomandibular joint of at least 3 months duration, randomly assigned to either Standard Treatment (STD; n=49) or to Standard Treatment + Cognitive-Behavioral skills training (STD+CBT; n=52). Patients were assessed at posttreatment (6 weeks), 12 weeks, 24 weeks, 36 weeks, and 52 weeks. Linear mixed model analyses of reported pain indicated that both treatments yielded significant decreases in pain, with the STD+CBT condition resulting in steeper decreases in pain over time compared to the STD condition. Somatization, self-efficacy and readiness for treatment emerged as significant moderators of outcome, such that those low in somatization, or higher in self-efficacy or readiness, and treated with STD+CBT reported lower pain over time. Somatization was also a significant moderator of treatment effects on pain-related interference with functioning, with those low on somatization reporting less pain interference over time when treated in the STD+CBT condition. It was concluded that brief treatments can yield significant reductions in pain, life interference and depressive symptoms in TMD sufferers, and that the addition of cognitive-behavioral coping skills will add to efficacy, especially for those low in somatization, or high in readiness or self-efficacy.
Keywords: Temporomandibular dysfunction, cognitive-behavioral treatment, moderators of treatment, brief treatment, pain, pain-related interference
Cognitive-behavioral therapies (CBT) have demonstrated some success in the treatment of pain related to temporomandibular joint dysfunction (TMD) [6; 8; 33], but few of these studies included systematic, active comparison conditions. Thus there is relatively little evidence regarding the long-term effects of CBT compared to active alternatives, or whether CBT works equally well for all patients.
Turk and colleagues [29] hypothesized that patients who report emotional and physical difficulties would benefit more from a treatment that included cognitive therapy than from a similar comparison treatment that did not. Only those patients who received the treatment that included cognitive therapy demonstrated continued improvements out to 6 months. The authors concluded that cognitive therapy was effective for highly distressed TMD patients. It is not clear, however, whether the addition of cognitive treatment would not also have been useful for more adaptive patients.
In an effort to systematically evaluate moderators of CBT for TMD pain, Turner, et al. [32] examined results from 115 patients who had been given “treatment as usual” from their dentist, and assigned to either an education/attention control condition or to a CBT condition. An array of potential moderators were examined. For the most part the authors were not able to identify baseline patient characteristics that interacted with treatment condition to predict 12-month outcomes. The authors concluded that CBT works equally well for all patients.
A number of dispositional variables have been proposed as potential moderators of CBT. Motivation, or readiness for self-care treatment, may be a moderator of treatment. Those who are not sufficiently motivated to commit to the CBT process may actually fare more poorly in CBT than in less demanding treatments [11].
Catastrophizing, or the tendency to exaggerate a negative mental set, may also moderate treatment. CBT, with its emphasis on developing adaptive cognitions, should produce better results with patients who catastrophize than other active treatments that do not seek to manage catastrophizing.
Somatization is a disposition or trait that manifests as the “tendency to experience and communicate somatic distress in response to psychosocial stress” [14]. CBT, which encourages the patient to directly attend to, and manage, the pain problem, may actually work less well for those high in somatization than a treatment that entails less focus on symptoms.
Self-efficacy, or the confidence to manage pain [1], may also moderate treatment. Those with greater confidence in their ability to cope may more readily adopt, and persist in, the coping skills developed in CBT [16]. CBT may also be more effective than a more passive control treatment for those who demonstrate a “monitoring” coping style, i.e. the tendency to attend to threatening stimuli [15; 21].
The purpose of the present study was to evaluate the marginal long-term efficacy of adding CBT components to an active conservative treatment for TMD. In addition we sought to determine if some patients might fare better, or worse, with CBT than they would with a conservative treatment that put few demands on patients' skills or expectations.
Method
Overview
To explore the questions discussed above, patients with TMD pain were recruited from the community and randomly assigned to one of two treatment conditions: a Standard Treatment (STD) condition entailing the placement of a flat plane disoccluding splint, the prescription of non-steroidal anti-inflammatory drugs, and instruction for a soft diet; or a Standard Treatment plus CBT condition (STD+CBT) in which patients received all elements of STD, but also received cognitive-behavioral coping skills training. Each treatment was 6 weeks long.
Patients (N = 101) were administered a number of instruments designed to measure the potential moderating variables discussed above, and were assessed for pain, pain-related interference, and depressive symptoms every three months for one year. It was hypothesized that patients with higher levels of readiness for treatment, self-efficacy, and monitoring coping style would fare better over the long-term with the addition of CBT (i.e., in the STD+CBT condition), because these patients would be better able to use the coping skills approach. Those higher in catastrophization were also expected to benefit from STD+CBT because of the emphasis placed on combating catastrophization in that condition. It was also hypothesized that those higher in somatization and blunting coping style would fare better with the STD treatment alone.
Participants
Participants were 85 women and 16 men seeking treatment for a complaint of either bilateral or unilateral pain in the area of the temporomandibular joint that had persisted and was noticeable on a daily basis for a period of at least 3 months. This number of participants was sufficient to, at a minimum, detect significant between-group differences at posttreatment on each of the major dependent variables, with a power of .8 and alpha set at .05.
Patients were recruited between October 2003 and July 2007 from the dental clinics in our university-based school of dental medicine (10%), from other dental referrers (< 5%), and from the greater Hartford metropolitan area via newspaper and web-based advertisements offering free short-term treatment. None were referred from specialized facial pain clinics. To be eligible patients needed to have a positive Axis I diagnosis on the Research Diagnostic Criteria [RDC; 4] for temporomandibular disorders (positive on at least one symptom-based Group), and could have no contraindications to TMD treatment (as determined by the consulting oral surgeon, e.g., oral cancer that would require immediate treatment). Patients were excluded for any of the following: lack of fluency in English (as determined by inability to read and understand a statement of informed consent); previous surgery for treatment of TMD pain; history of rheumatoid disease; extensive anatomical destruction or deterioration of the TM joint; diagnosed as having pain of neuropathic or odontogenic origin; carrying a diagnosis of psychosis; current use of antidepressants or anxiolytics; taking opioid pain medication; or pregnancy (due to possible adverse effects in pregnancy with the prescription of non-steroidal anti-inflammatory drugs).
The mean age of the sample was 39.4 years (SD = 12.1). The majority of participants were white (79%), with 9% black, 9% of Hispanic origin, and 3% self-described as Other. Forty-one percent were married or cohabiting. The average years of education was 14.7 (SD = 2.5). The participants reported having chronic TMD pain for 6.7 years on average (SD = 6.6), with a mean pain intensity rating of 3.5 on a scale to 6 (SD = 1.3). Of 196 persons screened, 121 were deemed eligible for the study, and 101 were assigned to treatment. At posttreatment 88% of patients provided data, and 73% provided data at 52 weeks. Losses to follow-up were equivalent across treatment conditions. The final follow-ups were conducted in June of 2008.
Measures and Instruments
General outcome variables: Pain, depression, and interference
Ratings of pain experience at each of assessment points were collected using the Multidimensional Pain Inventory [MPI; 13]. Characteristic pain intensity was calculated on a scale from 0 – 6 by averaging ratings of current pain, average pain, and worst pain in the past week [35].
Depression Symptoms at each assessment point were measured using the 20-item Center for Epidemiological Studies Depression scale [CES-D; 24]. The CES-D is well suited for use in a population with medical problems such as chronic pain in that it relies less on physical symptoms of depression than do other measures. In the current sample the CES-D had an internal reliability of α = .94.
Interference with activities was measured using the interference scale from the MPI. The interference subscale consists of 13 items scored from 0 to 6 on Likert-type scales that ask the respondent the degree to which his/her pain problem has interfered or changed work life, family life, and social life. The Interference score was calculated by averaging the scores of the 13 items. In our sample the scale had a reliability of α = .91.
Potential moderators of treatment effects
Readiness to engage in self-management treatment for chronic pain was assessed using the Pain Stages of Change Questionnaire [PSOCQ; 11; 12]. The PSOCQ assesses readiness using 30 Likert-scaled items scored from 1 (strongly disagree) to 5 (strongly agree), and comprising four scales: Precontemplation (e.g., `All of this talk about how to cope better is a waste of my time'), Contemplation (e.g., `I have been thinking that the way I cope with my pain could improve'), Action (e.g., `I am developing new ways to cope with my pain') and Maintenance (e.g., `I use what I have learned to help keep my pain under control'). The internal reliabilities of these scales ranged from α = .78 for precontemplation to α = .94 for maintenance. In the current study a single “Readiness” variable was calculated (i.e., Contemplation + Action + Maintenance − Precontemplation), based on a formula first used effectively for assessing readiness for alcoholism treatment in Project MATCH [23]. The internal reliability of the composite scale was α = .68.
Both coping and catastrophizing were measured using the 18-item Pain-Related Self-Statements Scale [PRSS; 7]. Patients were asked to indicate on a scale from 0 (Almost Never) to 5 (Almost Always) how often each self-statement comes to mind. This scale was chosen because of its demonstrated validity with TMD patients, and because it is relatively short. In the present sample the reliability of the coping subscale was α = .78, and the reliability of the catastrophizing subscale was α = .86.
Somatization was measured with the somatization subscale of the Symptom Checklist 90 - Revised [SCL-90-R; 3]. The SCL-90 R somatization scale consists of 12 items assessing the degree to which the person has experienced a number of physical sensations or symptoms, with a high score indicating a preoccupation with physical problems. The SCL-90R somatization subscale had an internal reliability of α = .83 in our TMD sample.
Pain management self-efficacy was assessed using the Chronic Pain Self-Efficacy Scale [CPSS; 1]. The CPSS is a 22-item questionnaire designed to measure chronic pain patients' perceived self-efficacy to cope with the consequences of chronic pain. The CPSS is constructed of three subscales: self-efficacy for pain management, self-efficacy for coping, and self-efficacy for coping with symptoms, as well as a total score, which was the score used in the present study (internal reliability α = .96 in this sample).
To measure coping style, patients were administered the Miller Behavioral Style Scale [MBSS; 20]. This inventory presents four hypothetical problem vignettes and asks the subject to choose from a set of solutions that vary in the degree of information or distraction provided. Two subscales are computed: Monitoring (internal reliability α = .70) and Blunting (internal reliability α = .57). Because of the low reliability of the Blunting subscale, only the Monitoring subscale was used here.
Treatment
Treatment in both conditions consisted of six sessions conducted over six weeks (though patients could take up to nine weeks to complete treatment). Sessions were spaced at least one week apart. Treatment was delivered by four Master's level therapists with at least 2 years experience in cognitive-behavioral therapy with medical patients. The same therapists provided both of the study treatments in order to minimize therapist effects. Both treatments were manual-driven. A detailed outline of each session gave the therapists specific guidelines as to what material to cover, what points to emphasize, and the specific kinds of homework to be assigned. The precise content of therapy sessions depended on the individual patient's circumstances and experiences. All treatment sessions in both conditions were audiotaped and reviewed for adherence to the treatment protocol by the first author. Supervision of therapists was conducted biweekly throughout the course of the study.
Standard Treatment (STD)
Standard Treatment consisted of splint therapy plus soft diet and oral anti-inflammatory agents [as per Stack & Stack, 27]. Patients were told that the treatment was intended to change oral habits with respect to clenching and bruxing, and to provide a sufficient respite from pain to allow more adaptive oral habits to emerge. Subjects in this group were fitted with a flat plane occlusal splint during the first treatment visit, one to two weeks after the baseline visit, with instructions to keep it in place continuously (except for eating) for the succeeding 4 weeks.
After 4 weeks it was recommended to patients that they start to taper the splint (e.g., use only as a night guard) in preparation for discontinuing the splint altogether. The purpose of the early discontinuation was to prevent the patient from adapting to the splint or clenching or bruxing on the splint itself. However, patients were allowed to retain the splint, and continue its use, if they preferred (as was the case in about 50% of patients).
In addition to the splint, subjects were also given a 5-week course of non-steroidal anti-inflammatory medication (NSAIDs; naproxen sodium 550 mg PO BID). (Extra strength acetaminophen was substituted for naproxen for those patients who reported having difficulty with NSAIDs or who had gastric ulcer disease. This was the case for 8 patients.) A soft diet was also prescribed, with special attention paid to avoiding foods that require extreme jaw opening (e.g., large sandwiches) or foods that had caused pain in the past (e.g., steak). Patients were asked to continue the NSAIDs and the soft diet until the end of the 6-week treatment period, after which they were informed that they could alter the treatment as they saw fit, but with a recommendation that the soft diet be continued.
All patients were seen once a week during treatment. However, whereas STD+CBT patients received weekly CBT, STD patients received weekly “progress checks” in which a therapist inquired as to the patient's status and monitored the patient's adherence to the basic treatment recommendations, i.e., medication use, splint use, and soft diet. These progress checks served to control for the amount of time and attention received by participants in the STD+CBT condition. As “homework,” patients were asked to keep records of their medication and splint use, and their diets each week. The therapist took care to not deliver any kind of cognitive-behavioral treatment to these patients. Complaints, if any, were met by expressions of sympathy, and encouragement to adhere to the Standard Treatment recommendations.
Standard Treatment + Cognitive-Behavioral Treatment (STD+CBT)
The STD+CBT condition included all aspects of the STD treatment described above. In addition, patients received a brief cognitive-behavioral program that focused on relaxation training, stress management, and cognitive restructuring. Treatment was intended to promote self-efficacy, reduce catastrophization, and increase the use of adaptive coping responses and habit modification. The program addressed the three most significant cognitive factors in TMD pain identified by Turner et al. [31]; beliefs or appraisals, coping and catastrophizing, and a significant behavioral factor, orofacial relaxation [e.g., 2]. The cognitive-behavioral program was based on brief programs developed by Turk, Zaki and Rudy [30] and Mishra, Gatchel and Gardea [22], who reported that a brief (6-session) CB treatment that employed masseter EMG biofeedback was more effective than standard care or CB or biofeedback alone.
The cognitive behavioral program was intended to teach skills to keep the patient from returning to old habits, and to clenching, bruxing, and catastrophizing cognitions that contribute to TMD pain and distress. Sessions covered an introduction and rationale for treatment, relaxation training and self-efficacy enhancement, masseter EMG biofeedback-assisted relaxation with an emphasis on relaxing the masseter muscles, habit modification (especially clenching and bruxing), combating negative thoughts and catastrophization, and stress management. Homework each week took the form of practicing skills discussed in the treatment sessions. Relaxation practice was assigned as homework every week.
Adherence to treatment
Eighty-seven percent of patients attended six treatment sessions, with no significant differences in attendance by treatment condition. Percent adherence to treatment prescriptions common to both treatments, i.e., diet, medication and splint use, was scored by the patient's therapist as Yes or No for each day of the week. Adherence to medication was scored as “Yes” if any of the prescribed medication was taken that day, and any reported splint use was considered adherent. Rates of adherence were as follows: Soft diet = 92%; Medication = 86%; Splint use = 73%. Patients averaged 7 hours of splint use per day, using it most often as a night guard. There were no between-treatment differences in these measures.
Procedure
Intake session
Persons meeting initial eligibility criteria were seen for an intake assessment session in the Dental Clinical Research Center (DCRC) of the University of Connecticut Health Center. Potential subjects were examined by an oral surgeon to rule out neuropathic or odontogenic pain and to classify the person according to the RDC for TMD. Individuals meeting all inclusion/exclusion criteria at this point were told of all procedures involved and administered a consent form. Baseline measures of the major dependent variables were then administered, and impressions were taken for an acrylic, flat-plane disoccluding splint. Patients were given $40.00 for completion of the baseline measures.
Assignment to treatment
Those who agreed to participate were randomized to either the Standard Treatment group (STD; n=49) or to the Standard Treatment + Cognitive-Behavioral Treatment group (STD+CBT; n=52) using a computerized urn randomization procedure [28]. The two conditions were balanced on gender, age, ethnic background, pain level recorded at baseline, and RDC axis I diagnoses. The Project Coordinator entered the urn data during the intake session and informed the participants of their treatment assignments. The first treatment appointment was then scheduled for one to two weeks later, coinciding with the delivery of the splint.
Data collection procedures
A trained M.A.-level research associate, who was not blinded to treatment condition, conducted the pretreatment and follow-up research assessments. Follow-up interviews were conducted in person and were scheduled at 6 weeks (posttreatment), and at weeks 12, 24, 36 and 52. Participants were compensated $25 for each in-person follow-up assessment.
Data Analysis
Analysis of main effects of treatment on each of the three major dependent variables was conducted using a mixed model regression procedure [Proc MIXED; SAS Institute, 25], and an intent-to-treat approach. The mixed model regression procedure was used because it takes advantage of all available data by using a maximum likelihood estimation procedure to estimate the parameters of the multivariate normal regression model [17]. The basic model examined for each dependent variable included a dummy variable representing the treatment effect (STD=0 v. STD+CBT=1), a variable representing time in weeks and scaled continuously (0 weeks to 52 weeks), and the interaction of treatment condition × time. In these analyses both the intercept and the time variable were included as random effects.
Moderation effects were also analyzed for each dependent variable using a mixed model regression procedure. For each analysis the dependent variable was evaluated as a function of treatment condition (dummy variable), time, a grand-mean centered moderator variable (e.g., somatization), condition × moderator, and condition × moderator × time. A Bonferroni-corrected alpha level of .007 was adopted to adjust for the multiple tests on each dependent variable. For both the fixed and random effects an unstructured covariance structure was adopted, based upon accepted fit criteria (−2RLL, AIC) [10].
Results
Treatment Main Effects
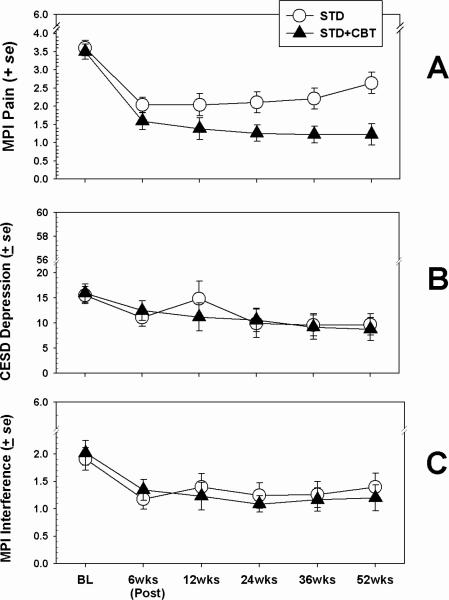
Means (and standard errors) by treatment condition for the outcome variables Characteristic Pain, Depressive Symptoms, and Pain-related Interference are shown in Figure 1. Results of mixed model analyses indicated that for Pain, a significant effect for time emerged (F(1,401) = 28.45; p < .0001), such that reports of pain declined from pretreatment to posttreatment for both conditions. No main effect was observed for treatment condition, but a significant condition × time interaction did emerge ((F(1,401) = 6.57; p < .01); effect size for the difference between slopes d = 0.25), with the STD+CBT condition resulting in greater decreases in pain reports over the 52 weeks than the STD condition.
Figure 1.
Effects of treatment on major outcome variables. BL = `Baseline.'
For both CES-D Depressive Symptom scores and MPI Interference scores the mixed model analyses yielded no main effects for treatment condition, and no interaction effects. Significant effects for time did emerge for both depression score (F(1,401) = 3.54; p < .05) and Interference (F(1,401) = 3.61; p < .05), indicating that scores on these variables declined over time, but that these declines were not differentiated by treatment condition.
Tests of Moderators of Treatment
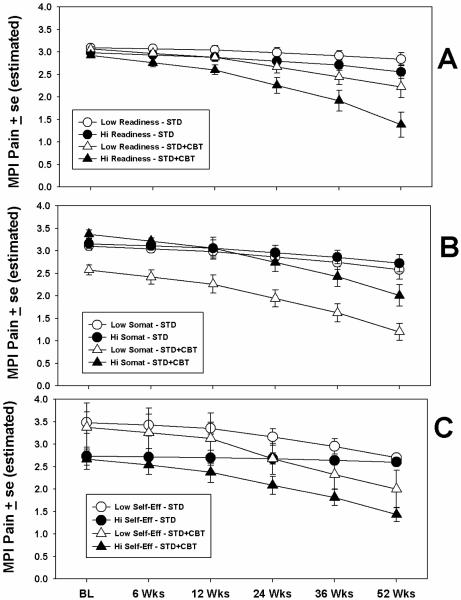
Table 1 shows the results from the tests of moderators of treatment condition on MPI Characteristic Pain over the 52 weeks. As seen in the table, three variables emerged as significant moderators of treatment condition by virtue of significant condition × moderator × time interaction effects: readiness, somatization, and self-efficacy. In order to assess the effects of these interactions on pain levels over time, the estimated means on MPI Pain derived from the mixed model analyses were plotted by condition and time for high and low levels of the moderators. The high and low levels of the moderators were determined by median split for illustrative purposes. The results for the significant interactions are shown in Figure 2.
Table 1.
Results of Mixed Model Analyses of Moderators of Treatment Effects. Dependent Variable is MPI Characteristic Pain Though 52 Weeks.
| Moderating Variable | Term | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderatora | Moderator X Conditiona | Moderator X Condition X Timeb | |||||||
| β | se | F | β | se | F | β c | se | F | |
| Readiness | −0.07 | 0.07 | 2.67 | −.05 | .09 | 0.08 | −0.002 | 0.0011 | 6.06* |
| Coping | −0.55 | 0.17 | 24.59* | −0.12 | 0.25 | 0.05 | −0.004 | 0.0032 | 1.14 |
| Catastrophizing | 0.64 | 0.12 | 32.07* | 0.15 | 0.15 | 5.19 | 0.006 | 0.0044 | 2.41 |
| Somatization | 0.08 | 0.02 | 13.97* | 0.01 | 0.02 | 1.35 | 0.001 | 0.0001 | 9.62* |
| Self-Efficacy | −0.03 | 0.01 | 27.63* | −0.01 | 0.01 | 0.79 | −0.0002 | 0.0001 | 8.63* |
| Optimism | −0.07 | 0.03 | 8.44* | −0.01 | 0.04 | 0.35 | −0.001 | 0.0003 | 1.06 |
| MBSS Monitoring | −0.01 | 0.05 | 0.03 | 0.01 | 0.06 | 0.04 | 0.001 | 0.0011 | 1.15 |
df=1/400
df=2/400
Estimate for interaction term when Condition = 1 (STD+CBT)
Note:
p < .007
β =beta, standardized mixed regression coefficient; se = standard error of beta
Figure 2.
Moderating effects of three patient variables on treatment. Dependent variable is characteristic pain, measured using the MPI. High and low levels on the moderator variables were determined by median split. Values plotted are derived from estimates from mixed model regression analyses. BL = `Baseline.'
As seen in the figure, those higher in readiness to engage in self-management who were assigned to the STD+CBT condition tended to report less pain over time than those assigned to STD, and less pain than those assigned to STD+CBT but who were low in readiness (Panel A). Similarly, the STD+CBT condition appeared to work best for those scoring low on somatization. Those high in somatization tended to have similar results regardless of treatment condition (Panel B).
The results for pain management self-efficacy are shown in Panel C. As the figure illustrates, the main effect for self-efficacy is evident by lower pain scores at baseline for those in the High Self-Efficacy categories, regardless of treatment. However, whereas those high in self-efficacy in the STD+CBT condition apparently benefited from treatment over time, high-self-efficacy patients in the STD condition did not. That is, self-efficacy for pain management appeared to alter treatment response, but only for those in the STD+CBT condition; regardless of self-efficacy level at baseline, those high and low in self-efficacy in the STD condition had similar outcomes at the end of the 52 weeks.
Table 2 shows summarizes the results of tests of moderators of treatment condition on CES-D Depression scores over the follow-up period. None of the variables tested interacted significantly with treatment condition to affect depression scores over time, although all the variables except readiness were significant overall predictors of depression scores.
Table 2.
Results of Mixed Model Analyses of Moderators of Treatment Effects. Dependent Variable is CES-D Depression Score Through 52 Weeks.
| Moderating Variable | Term | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderatora | Moderator X Conditiona | Moderator X Condition X Timeb | |||||||
| β | se | F | β | se | F | β c | se | F | |
| Readiness | −0.62 | 0.53 | 3.05 | −0.63 | 0.65 | 0.54 | 0.02 | 0.01 | 1.90 |
| Coping | −2.19 | 1.36 | 7.08* | −1.66 | 1.56 | 0.08 | 0.04 | 0.04 | 1.09 |
| Catastrophizing | 4.47 | 1.12 | 30.29* | 0.21 | 1.66 | 0.02 | 0.01 | 0.05 | 3.18 |
| Somatization | 0.90 | 0.13 | 62.86* | −0.17 | 0.20 | 0.67 | 0.001 | 0.006 | 0.06 |
| Self-Efficacy | −0.31 | 0.07 | 38.77* | −0.08 | 0.09 | 0.72 | 0.000 | 0.002 | 0.01 |
| Optimism | −1.44 | 0.35 | 29.58* | −0.31 | 0.47 | 0.42 | −0.009 | 0.014 | 0.41 |
| MBSS Monitoring | 1.16 | 0.39 | 11.24* | 0.50 | 0.54 | 0.86 | 0.007 | 0.016 | 0.20 |
df=1/400
df=2/400
Estimate for interaction term when Condition = 1 (STD+CBT)
Note:
p < .007
β =beta, standardized mixed regression coefficient; se = standard error of beta
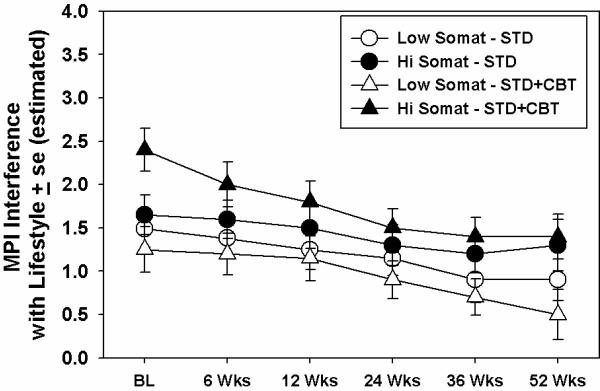
Results of moderation analyses on MPI Interference with lifestyle scores are shown in Table 3. As was the case with the analysis of depression scores, almost all of these variables were predictive of interference overall. Only somatization moderated the effect of treatment on interference however. The effect is seen in Figure 3. As the figure depicts, those low in somatization tended to fare better overall, regardless of treatment condition. Those who fared best were those low in somatization treated in the STD+CBT condition.
Table 3.
Results of Mixed Model Analyses of Moderators of Treatment Effects. Dependent Variable is MPI Interference with Lifestyle Through 52 Weeks.
| Moderating Variable | Term | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderatora | Moderator X Conditiona | Moderator X Condition X Timeb | |||||||
| β | se | F | β | se | F | β c | se | F | |
| Readiness | −0.14 | 0.07 | 3.27 | 0.01 | 0.10 | 0.94 | 0.003 | 0.001 | 2.58 |
| Coping | −0.56 | 0.13 | 29.61 | −0.06 | 0.15 | 0.13 | 0.005 | 0.004 | 0.75 |
| Catastrophizing | 0.62 | 0.14 | 25.72* | −0.08 | 0.17 | 0.21 | −0.003 | 0.004 | 0.89 |
| Somatization | 0.07 | 0.02 | 20.27* | −0.02 | 0.03 | 0.85 | −0.000 | 0.000 | 6.96* |
| Self-Efficacy | −0.05 | 0.01 | 39.72* | -0.02 | 0.01 | 4.05 | −0.000 | 0.000 | 4.23 |
| Optimism | −0.15 | 0.05 | 14.93* | −0.04 | 0.07 | 0.53 | 0.000 | 0.001 | 1.55 |
| MBSS Monitoring | −0.01 | 0.01 | 1.08 | −0.02 | 0.07 | 0.07 | 0.001 | 0.001 | 0.22 |
df=1/400
df=2/400
Estimate for interaction term when Condition = 1 (STD+CBT)
Note:
p < .007
β =beta, standardized mixed regression coefficient; se = standard error of beta
Figure 3.
Moderating effects of somatization on treatment. Dependent variable is pain-related interference with lifestyle, measured using the MPI. High and low levels on the somatization variable were determined by median split. Values plotted are derived from estimates from a mixed model regression analysis. BL = `Baseline.'
Discussion
A finding that is common to this field is that patients with TMD-related pain generally tend to improve to some extent, regardless of treatment [e.g., 34]. This appeared to be true in the present study. Patients in both conditions, STD and STD+CBT, improved significantly, particularly from pre- to posttreatment. On average, patients reported a 40% decrease in pain. Similar improvements were seen for patients in both treatment conditions in CES-D depression scores and in scores on the MPI Interference scale. In general, then, a closely monitored, conservative treatment may be sufficient to help most people with these disorders. The addition of CBT appeared to have to have some advantages, however. The trend over time, at least for recording of pain, tended to return to baseline levels for those in the STD condition whereas those in the STD+CBT condition tended to report continuing declines in pain for a year after treatment.
A somewhat more complex picture emerges from the analyses of potential moderators of treatment effects. For the most part, the addition of CBT to standard treatment conferred the most advantage to the most adaptive patients. The present results did not, for example, support the hypothesis of Turk et al. [29] that CBT would be most useful for those with more dysfunction, such as depressive symptoms, at least in this sample. In the current study CES-D depression scores declined in both conditions equally; the addition of CBT did not result in additional decreases in dysphoria.
The addition of CBT to standard treatment did contribute significantly to decreases in pain and pain-related interference for those who scored low on the somatization scale at baseline, but not for those who scored high. The results in this study are thus similar to those of Dworkin et al. [6] in that those classified as high in somatization did not benefit from CBT. It is not a surprise that for each of the dependent variables analyzed somatizing emerged as a significant predictor. Macfarlane et al. [18] have suggested that orofacial pain may be a manifestation of somatization. Patients with chronic pain complaints who score high on somatization tend to report pain that is more diffuse, more severe and more difficult to localize and treat [9; 26]. In patients with TMD somatization is related to more widely dispersed pain [5]. It may be the case that the overconcern with bodily symptoms noted by McCreary et al. [19] interferes with the patient's ability to do the mental work (e.g., reframing, problem solving, etc.) required by CBT.
The results for readiness and for self-efficacy, on the other hand, were very much in the directions expected. Both readiness and self-efficacy emerged as main effects, such that higher levels of each were predictive of lower pain scores. The most interesting findings were 1) that those patients who were more ready, or motivated for treatment, and 2) those who were more confident about their ability to manage their pain, benefited significantly more from the CBT elements than did those lower in readiness or self-efficacy. Patients who entered treatment high in motivation and confidence not only recorded lower pain, but also were better able to use treatment. These findings were not the result of a high correlation between readiness and self-efficacy; the correlation was r=.35. The constructs are related (and perhaps complementary) but not identical.
The results of the present study were somewhat different from those obtained by Turner et al. [32], the study that is closest to this one in terms of populations and treatments. Our ability to detect treatment × moderator effects may have been the result of the somewhat more sensitive analyses used in the present study. Another difference from the Turner et al. study, however, was our inability to detect main effects for treatment condition on depression scores and pain-related interference scores over time. These discrepancies may have been attributable to the somewhat less sensitive measures used in the present study, and may represent a limitation.
Another limitation was our inability to blind the research associate to patient's treatment conditions. The failure to detect between-condition differences on two of the three dependent variables, however, would suggest that experimenter bias was not operating to influence reporting of outcomes. An additional problem was the relatively low severity of the patient sample studied here. The fact that mean pain ratings tended to be moderate may have hindered our ability to detect between-condition effects, and may limit the generalizability of the study somewhat.
In summary, the present results suggest that standard conservative dental treatment, even brief ones, may be sufficient for most patients who present with TMD-related orofacial pain. The addition of cognitive-behavioral coping skills will add to efficacy, however, especially for those low in somatization and high in readiness and self-efficacy. In the present study, it appeared that CBT worked best for those who were best prepared to use it. It therefore may be clinically useful to assess key constructs such as somatization, readiness, and self-efficacy to manage chronic pain, and to add intervention components that will serve to increase readiness and boost self-efficacy for managing TMD pain.
Acknowledgements
Support for this project was provided by grants R01-DE14607 from the National Institute on Dental and Craniofacial Research, and by General Clinical Research Center grant M01-RR06192 from the National Institutes of Health. None of the authors have any financial or other relationships that might lead to a conflict of interest. The authors would like to acknowledge Zeena Tawfik-Yonkers, Carlos Ibanez, Sharon Cooper, Kimberly Corey, Jennifer Scagliotti, Howard Steinberg, Kevin Vowles, Lisa Burgio, and Megyn Clement for their work in the conduct of this study.
Footnotes
Portions of this work were presented at the May 2008 meeting of the International Association for Dental Research, in Toronto, Ontario, Canada.
References
- [1].Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- [2].Crider AB, Glaros AG. A meta-analysis of EMG biofeedback treatment of temporomandibular disorders. J Orofac Pain. 1999;13(1):29–37. [PubMed] [Google Scholar]
- [3].Derogatis LR. SCL-90-R: Administration scoring and procedures manual. National Computer Systems; Minneapolis, MN: 1994. [Google Scholar]
- [4].Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6(4):301–355. [PubMed] [Google Scholar]
- [5].Dworkin SF, Sherman J, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain. 2002;16(3):207–220. [PubMed] [Google Scholar]
- [6].Dworkin SF, Turner JA, Wilson L, Massoth D, Whitney C, Huggins KH, Burgess J, Sommers E, Truelove E. Brief group cognitive-behavioral intervention for temporomandibular disorders. Pain. 1994;59(2):175–187. doi: 10.1016/0304-3959(94)90070-1. [DOI] [PubMed] [Google Scholar]
- [7].Flor H, Behle DJ, Birbaumer N. Assessment of pain-related cognitions in chronic pain patients. Behav Res Ther. 1993;31(1):63–73. doi: 10.1016/0005-7967(93)90044-u. [DOI] [PubMed] [Google Scholar]
- [8].Gardea MA, Gatchel RJ, Mishra KD. Long-term efficacy of biobehavioral treatment of temporomandibular disorders. J Behav Med. 2001;24(4):341–359. doi: 10.1023/a:1010682818427. [DOI] [PubMed] [Google Scholar]
- [9].Gil KM, Phillips G, Abrams MR, Williams DA. Pain drawings and sickle cell disease pain. Clin J Pain. 1990;6(2):105–109. doi: 10.1097/00002508-199006000-00005. [DOI] [PubMed] [Google Scholar]
- [10].Judge GG, Griffiths WE, Hill RC, Lutkepohl H, Lee T-C. The theory and practice of econometrics. Wiley; New York: 1985. [Google Scholar]
- [11].Kerns RD, Rosenberg R. Predicting responses to self-management treatments for chronic pain: application of the pain stages of change model. Pain. 2000;84(1):49–55. doi: 10.1016/S0304-3959(99)00184-0. [DOI] [PubMed] [Google Scholar]
- [12].Kerns RD, Rosenberg R, Jamison RN, Caudill MA, Haythornthwaite J. Readiness to adopt a self-management approach to chronic pain: the Pain Stages of Change Questionnaire (PSOCQ) Pain. 1997;72(1–2):227–234. doi: 10.1016/s0304-3959(97)00038-9. [DOI] [PubMed] [Google Scholar]
- [13].Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- [14].Lipowski ZJ. Somatization: the concept and its clinical application. Am J Psychiatry. 1988;145(11):1358–1368. doi: 10.1176/ajp.145.11.1358. [DOI] [PubMed] [Google Scholar]
- [15].Litt MD, Nye C, Shafer D. Preparation for oral surgery: evaluating elements of coping. J Behav Med. 1995;18(5):435–459. doi: 10.1007/BF01904773. [DOI] [PubMed] [Google Scholar]
- [16].Litt MD, Shafer DM, Ibanez CR, Kreutzer DL, Tawfik-Yonkers Momentary pain and coping in temporomandibular disorder pain: Exploring mechanisms of cognitive behavioral treatment for chronic pain. Pain. 2009;145:160–168. doi: 10.1016/j.pain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; New York: 1987. [Google Scholar]
- [18].Macfarlane TV, Blinkhorn AS, Davies RM, Ryan P, Worthington HV, Macfarlane GJ. Orofacial pain: just another chronic pain? Results from a population-based survey. Pain. 2002;99(3):453–458. doi: 10.1016/S0304-3959(02)00181-1. [DOI] [PubMed] [Google Scholar]
- [19].McCreary CP, Clark GT, Oakley ME, Flack V. Predicting response to treatment for temporomandibular disorders. J Craniomandib Disord. 1992;6(3):161–169. [PubMed] [Google Scholar]
- [20].Miller SM. Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. J Pers Soc Psychol. 1987;52(2):345–353. doi: 10.1037//0022-3514.52.2.345. [DOI] [PubMed] [Google Scholar]
- [21].Miller SM, Brody DS, Summerton J. Styles of coping with threat: implications for health. J Pers Soc Psychol. 1988;54(1):142–148. doi: 10.1037//0022-3514.54.1.142. [DOI] [PubMed] [Google Scholar]
- [22].Mishra KD, Gatchel RJ, Gardea MA. The relative efficacy of three cognitive-behavioral treatment approaches to temporomandibular disorders. J Behav Med. 2000;23(3):293–309. doi: 10.1023/a:1005562126071. [DOI] [PubMed] [Google Scholar]
- [23].Project MATCH Research Group Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- [24].Radloff LS. The CES-D scale: A self-report depression scale for the general population. Applied Psychosocial Measurement. 1977;1:385–401. [Google Scholar]
- [25].SAS . Book SAS/STAT software: changes and enhancements through V7 and V8. SAS Institute; City: 1999. SAS/STAT software: changes and enhancements through V7 and V8. [Google Scholar]
- [26].Sherman JJ, LeResche L, Huggins KH, Mancl LA, Sage JC, Dworkin SF. The relationship of somatization and depression to experimental pain response in women with temporomandibular disorders. Psychosom Med. 2004;66(6):852–860. doi: 10.1097/01.psy.0000140006.48316.80. [DOI] [PubMed] [Google Scholar]
- [27].Stack BC, Jr., Stack BC., Sr. Temporomandibular joint disorder. Am Fam Physician. 1992;46:143–150. [PubMed] [Google Scholar]
- [28].Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. In: Donovan DM, Mattson ME, editors. Alcoholism treatment matching research: Methodological and clinical approaches. 1994. pp. 70–75. [DOI] [PubMed] [Google Scholar]
- [29].Turk DC, Rudy TE, Kubinski JA, Zaki HS, Greco CM. Dysfunctional patients with temporomandibular disorders: evaluating the efficacy of a tailored treatment protocol. J Consult Clin Psychol. 1996;64(1):139–146. doi: 10.1037//0022-006x.64.1.139. [DOI] [PubMed] [Google Scholar]
- [30].Turk DC, Zaki HS, Rudy TE. Effects of intraoral appliance and biofeedback/stress management alone and in combination in treating pain and depression in patients with temporomandibular disorders. J Prosthet Dent. 1993;70(2):158–164. doi: 10.1016/0022-3913(93)90012-d. [DOI] [PubMed] [Google Scholar]
- [31].Turner JA, Dworkin SF, Mancl L, Huggins KH, Truelove EL. The roles of beliefs, catastrophizing, and coping in the functioning of patients with temporomandibular disorders. Pain. 2001;92(1–2):41–51. doi: 10.1016/s0304-3959(00)00469-3. [DOI] [PubMed] [Google Scholar]
- [32].Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127(3):276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [33].Turner JA, Mancl L, Aaron LA. Brief cognitive-behavioral therapy for temporomandibular disorder pain: effects on daily electronic outcome and process measures. Pain. 2005;117(3):377–387. doi: 10.1016/j.pain.2005.06.025. [DOI] [PubMed] [Google Scholar]
- [34].Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain. 2006;121(3):181–194. doi: 10.1016/j.pain.2005.11.017. [DOI] [PubMed] [Google Scholar]
- [35].Von Korff M. Epidemiological and survey methods: assessment of chronic pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. The Guilford Press; New York: 2001. pp. 603–618. [Google Scholar]