Abstract
Introduction
Some studies have found that antidepressants increase serum brain-derived neurotrophic factor (BDNF) levels in patients with major depression and the expression of BDNF mRNA in limbic structures of rats.
Objectives
This study addressed whether the SSRI escitalopram increases serum BDNF levels in subjects with PTSD and whether BDNF levels are associated with treatment response.
Methods
Medically healthy male subjects (N=16) with chronic PTSD completed a 12-week open label trial of flexible dose (5–20mg/day) escitalopram monotherapy. BDNF levels were obtained at baseline, and at weeks 4, 8 and 12.
Results
PTSD symptoms significantly declined over the course of the 12 week escitalopram treatment. Despite a substantial improvement in PTSD symptoms, there was virtually no change in BDNF levels over time. Nevertheless, mean BDNF levels across the trial were strongly correlated with the slope of PTSD symptoms over the 12 weeks (r = 0.58, p= 0.018). Lower mean BDNF was associated with a greater decrease in PTSD symptoms over the course of the trial.
Conclusions
PTSD subjects with low BDNF levels demonstrated the largest treatment response from an agent with putative neurotrophic effects.
Keywords: BDNF, biomarker, escitalopram, posttraumatic stress disorders, predictor of response
1. Introduction
Brain-Derived Neurotrophic Factor (BDNF), a member of the neurotrophin family, is a basic homodimeric protein that promotes neuronal survival and regulates the proliferation and differentiation of nerve cells in the peripheral and central nervous systems (Aydemir et al. 2006;Lindsay et al. 1994). BDNF is highly expressed in hippocampus, one of the few areas of active neurogenesis in human adult brains (Lie et al. 2004). In diverse animal models, stress can decrease the synthesis of hippocampal BDNF, the growth of new dentate granule cells, and induce atrophy of apical dendrites of CA3 neurons (Gould et al. 1997;Gould et al. 1998;Magarinos and McEwen 1995). In humans, stressful life events have been associated with low peripheral levels of BDNF (Grassi-Oliveira et al. 2008;Kauer-Sant’Anna et al. 2007). In addition, a recent meta-analysis showed that, before treatment, patients with major depressive disorder (MDD) present lower levels of serum BDNF than healthy subjects (Sen et al. 2008). Conversely, chronic administration of antidepressants appears to increase the expression of BDNF mRNA in limbic structures of rats (Hashimoto et al. 2004). Some studies have demonstrated that treatment with various antidepressant drugs can normalize serum BDNF in patients with MDD (Sen et al. 2008). Consistent with these observations, other authors showed that antidepressant medications increase neural progenitor cells in the hippocampus in animal and humans models (Boldrini et al. 2009).
BDNF is also thought to be involved in the pathogenesis of several neuropsychiatric disorders, including MDD and Posttraumatic Stress Disorder (PTSD) (Broekman et al. 2007). BDNF contributes to learning and memory (Lommatzsch et al. 2005), which are hippocampus-related functions (Felmingham et al. 2009) frequently impaired in PTSD patients (Vermetten et al. 2003). Patients with PTSD exhibit smaller hippocampal volumes than healthy controls, exposed or not to traumatic events (Karl et al. 2006;Vermetten et al. 2003), and duration of PTSD has been negatively correlated with right hippocampal volume (Felmingham et al. 2009). Accordingly, chronic treatment with paroxetine seems to reverse the hippocampal atrophy observed in PTSD patients (Vermetten et al. 2003). Surprisingly, there are only three studies investigating the relationship between peripheral BDNF levels and PTSD in humans (Dell’Osso et al. 2009;Hauck et al. 2009;Hauck et al. 2010). In a case report, a patient with PTSD who was already taking sertraline, showed higher serum BDNF levels than matched controls at first evaluation, and these levels decreased during treatment with sertraline and psychotherapy (Hauck et al. 2009). In a cross-sectional study, patients with PTSD and acute stress disorder (ASD) who had their first traumatic event in the year before assessment showed higher serum BDNF levels than age and gender controls. Further, this study showed that serum BDNF levels of patients with PTSD who experienced their first traumatic event more than four years before assessment was no different than matched controls (Hauck et al. 2010). However, in another cross-sectional study, drug-free patients with PTSD showed lower plasma BDNF levels than matched controls, independent of the length of time between the traumatic events experienced by the former and the assessment (Dell’Osso et al. 2009). Therefore, at present, the role of BDNF in PTSD is far from being understood.
Considering that response to escitalopram was associated with hippocampal neurogenesis in a stress-induced rat model of depression (Jayatissa et al. 2006), the low response rates to pharmacological therapy in PTSD (Berger et al. 2009), and the dearth of evidence of biomarkers of treatment response for PTSD, we sought to determine whether serum levels of BDNF predict response to escitalopram in patients with PTSD.
2. Methods
2.1 Patients and recruitment
Sixteen medically healthy male subjects with chronic PTSD, who were medication-free for at least 2 weeks, were recruited from internet advertisement in the community and the PTSD Program at the San Francisco Veterans Affairs Medical Center. PTSD was diagnosed if individuals met criteria for DSM-IV TR (American Psychiatric Association 2000) and a total score ≥ 40 on the Clinician Administered PTSD Scale (CAPS) (Blake et al. 1995). The Structured Clinical Interview for DSM-IV (SCID-I) (First et al. 1996) was administered to assess comorbidity, and subjects were excluded if they had a lifetime history of bipolar or any psychiatric disorder with psychotic features; prominent suicidal or homicidal ideation; history of alcohol or drug abuse/dependence within the past six months; history of neurological disorder; current systemic illness affecting central nervous system function; history of myocardial infarction in the past year; and subjects who planned to start a new form of psychotherapy during the protocol. Concomitant psychosocial treatment was limited only to ongoing therapy initiated at least 2 months prior to the trial. In addition, subjects who had taken any psychiatric medication two weeks before enrollment, those who were prescribed citalopram or escitalopram within the past 6 months, and those who had a trial of fluoxetine or antipsychotic medication in the past 6 weeks were also excluded.
After the procedures and possible adverse effects were fully explained, all participants gave informed consent. The study protocol and consent form was approved by the Committee on Human Research at the University of California, San Francisco (UCSF). This study was performed according to the ethical standards of the Declaration of Helsinki.
2.2 Procedure
The first phase of the study included a laboratory screening for medical illness and urine toxicology. This was an open-label, longitudinal, dose-escalating study. Subjects satisfying the inclusion criteria were started on 5mg escitalopram. Medication was increased as tolerated in weekly 2.5, 5 or 10 mg increments, until a maximally tolerated dosage or a clinical response was achieved. The final dosage ranged from 5 to 20 mg/day (mean 12.5 ± 5.08). The active treatment phase consisted of a 12-week trial. No concurrent psychotropic or hypnotic medications were allowed during the course of the study. Compliance was assessed by pill count check.
2.3 Measures
The symptoms of PTSD were assessed by the CAPS, a structured clinical interview, at baseline and week 12 and by the PTSD Checklist (PCL) (Blanchard et al. 1996), a 17-item self-report questionnaire, every 2 weeks. The response criteria with respect to PTSD symptoms was a reduction ≥ 30% in CAPS scores between baseline and endpoint, and full remission was defined as CAPS score ≤ 20 at week 12. Depressive symptoms were measured with the Hamilton Depression Rating Scale (HAM-D) (Hamilton 1960), a 17-item interviewer rated scale, at baseline and week 12. For MDD symptoms, response was defined as a reduction ≥ 50% in the HAM-D total scores between baseline and week 12, and remission as a HAM-D total score ≤ 7 at endpoint.
2.4 Blood Processing and Assay Methods
Blood for BDNF was collected into serum separator tubes baseline prior to treatment and at weeks 4, 8, and 12 during the trial. After sitting at room temperature for one hour to allow clotting, followed by one hour at 4° C for platelet activation (Karege et al. 2002a), blood was centrifuged at 4° C 2200rpm for 10 min, and serum was separated and stored at −80° C until assay. Serum was assayed for BDNF in duplicate, using a commercial BDNF ELISA assay kit (R&D Systems, Minneapolis, MN, USA). To evaluate inter-assay variability, an internal control consisting of serum obtained from one individual, frozen in multiple aliquots, was run on each plate processed. BDNF concentrations of this control sample were measured on several different days and multiple 96-well plates. The R&D Systems Human BDNF Quantikine ELISA Kit was found to have an acceptable 8–14% inter-assay variability. Intra-assay CV was <10%, or samples were re-assayed. Subjects’ baseline, 4, 8, and week 12 samples, were run in the same assay.
2.5 Data Analyses
Primary outcome analysis was focused on serum BDNF and changes in clinician administered and self-report measures of stress specific symptoms from baseline, weeks 4 and 8, and completion of 12 weeks of treatment. Specifically, separate linear mixed effects models were fitted to the CAPS (administered at baseline and endpoint) and PCL scores (administered at baseline and every four weeks, until week 12), which were the primary outcome measures for assessing the effect of escitalopram treatment. Analyses of depression symptoms were designated as secondary. We followed the recommendations of Speer (Speer 1992) in determining clinically significant change for each individual.
The specific form of the mixed model was a random coefficients model, specifying random (subject-specific) intercepts and, for variables measured at more than two time points, random slopes (Brown and Prescott 1999). For outcomes measured only at baseline and end of treatment, this analysis is essentially equivalent to a repeated measures ANOVA. For variables measured at 3 or more time points, the mixed model analysis is preferable to repeated measures ANOVA in that it relaxes the assumption of heterogeneity of variance and handles within-subjects correlations more realistically. It also accommodates randomly missing data. An important feature of the random coefficients model is that it models individual subjects’ slopes, and thus can generate subject-specific slope estimates to describe individual treatment responses over time. These slope estimates can then be used in further analyses to examine individual differences in treatment response (Gibbons et al. 1993). Since PCL-C was administered in more than two time points, this measurement was preferred to CAPS in the correlation analysis between treatment response and serum BDNF levels.
3. Results
Fifteen of the 16 subjects completed a 12-week open label trial of escitalopram monotherapy. One subject dropped out after week 8 due to personal reasons (not related to lack of efficacy or adverse effects), but his assessments at week 8 were used in the analyses. Table 1 depicts the patients’ demographic characteristics and comorbidities. Escitalopram significantly reduced PTSD symptoms over the course of treatment in both civilian (N= 7) and veterans (N= 9), as measured by CAPS and PCL (table 2). In addition, out of the 15 patients with PTSD who completed the study, 11 (73.3%) were considered treatment responders and 7 (46.7%) achieved full remission. As expected, depressive symptoms also decreased significantly over the trial, according to the HAM-D scores. Of the 8 patients diagnosed with MDD at baseline, 7 (87.5%) responded to escitalopram treatment and 5 (62.5%) attained remission at endpoint. Despite a substantial drop in PTSD and depressive symptoms, there was no appreciable change in serum BDNF levels over time (Table 2). However, the average serum BDNF levels across all collection points were strongly correlated with the slope of PTSD symptoms over the 12 weeks (r = 0.58, p = 0.018). Specifically, lower mean serum BDNF levels over time were associated with a greater decrease in PTSD symptoms over the course of the trial (Figure 1). Baseline serum BDNF levels were not correlated with CAPS or PCL-C scores (r = 0.25, p = 0.36 and r = −0.09, p = 0.73, respectively).
Table 1.
Demographic characteristics and comorbidities.
| Range | Mean (SD) | |
|---|---|---|
| Age (years) | 21–60 | 42.3 (14.50) |
| Education (years) | 12–18 | 15.2 (2.22) |
| Duration of PTSD (years) | 0.66–38 | 20.9 (13.68) |
| N (%) | ||
| Race | ||
| Caucasian | 8 (50.00) | |
| African American | 6 (37.50) | |
| Asian American | 1 (6.20) | |
| Hispanic | 1 (6.20) | |
| Marital status | ||
| Married/Living together | 9 (56.20) | |
| Divorced | 4 (25.00) | |
| Never married | 3 (18.70) | |
| Military status | ||
| Veteran | 12 (75.00) | |
| Civilian | 4 (25.00) | |
| Trauma | ||
| Combat | 9 (56.25) | |
| Witness death | 3 (18.75) | |
| Assault | 2 (12.50) | |
| Childhood physical/sexual abuse | 1 (6.25) | |
| Witness domestic violence between parents | 1 (6.25) | |
| Comorbidities | ||
| Current depression | 8 (50.00) | |
| Lifetime depression | 5 (31.25) | |
| Lifetime alcohol abuse or dependence | 10 (62.50) | |
| Lifetime drug abuse or dependence | 5 (31.25) | |
| Other* | 5 (31.25) | |
SD: Standard Deviation
Includes: Panic disorder, generalized anxiety disorder, social phobia, dysthymia, anxiety disorder not otherwise specified.
Table 2.
Escitalopram doses and treatment outcomes.
| Medication | Range | Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Escitalopram (mg/day) | 5–20 | 12.5 ± 5.08* | |||||||
| Measures | Baseline | 2 week | 4 week | 6 week | 8 week | 12 week | F1 | d.f.2 | p1 |
| BDNF levels (ng/ml) | 16.90 ± 3.91* | NA | 16.32 ± 3.17* | NA | 17.08 ± 3.85* | 14.93 ± 3.68* | 2.27 | 1, 45.5 | 0.138 |
| CAPS scores | 61.81 ± 14.62* | NA | NA | NA | NA | 28.86 ± 17.91* | 38.61 | 1, 14.8 | < 0.001 |
| PCL scores | 56.00 ± 11.96* | 44.81 ± 13.05* | 41.56 ± 12.06* | 37.50 ± 11.45* | 35.87 ± 11.69* | 31.47 ± 13.26* | 70.50 | 1, 78.1 | < 0.001 |
| HAM-D scores | 18.19 ± 7.29* | NA | NA | NA | NA | 6.13 ± 4.61* | 57.64 | 1, 14.6 | < 0.001 |
Values are given as mean ± standard deviation.
CAPS: Clinician Administered PTSD Scale; PCL: PTSD Checklist; HAM-D: Hamilton Depression scale; NA: Not Available.
Linear change over time based on mixed model analysis of all available time points.
Denominator degrees of freedom are Satterthwaite estimates from the mixed model, and need not be integer values.
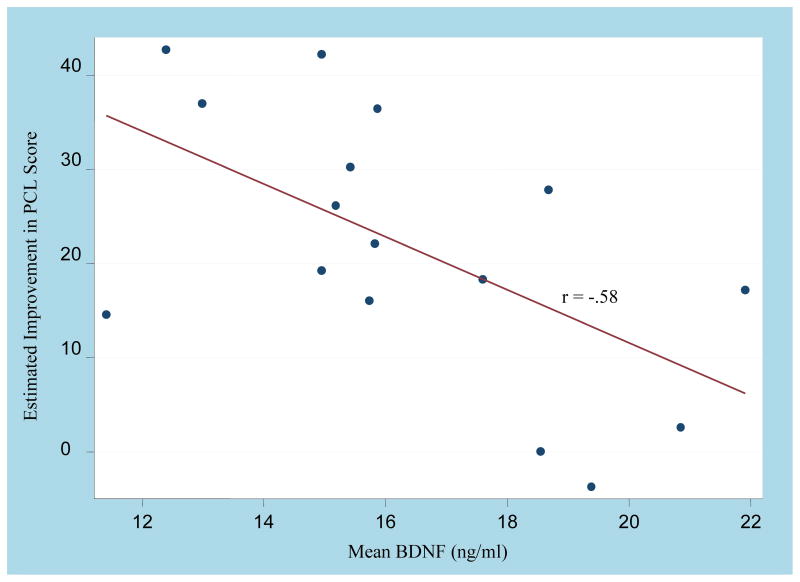
Figure 1.
Relationship between PTSD symptoms response to escitalopram and mean BDNF levels. Lower mean BDNF was associated with a greater decrease in PTSD symptoms (PCL) over the course of 12-week open trial (p= 0.018).
4. Discussion
Besides one case report (Hauck et al. 2009), this is the first study to longitudinally evaluate the relationships between serum BDNF levels and treatment response in patients suffering from PTSD. Despite a substantial decrease of PTSD and comorbid depressive symptoms over the course of an open label trial of escitalopram monotherapy, no significant changes in peripheral BDNF levels were detected throughout the 12 week treatment. Nevertheless, lower mean serum BDNF levels over the trial were a strong predictor of good PTSD response to escitalopram, even after adjusting for the age of participants and for baseline PTSD and depression severity. This suggests that low serum BDNF is a biomarker predicting a favorable response to an intervention that is presumed to potentiate neurogenesis. This finding will need to be confirmed by larger studies.
Our finding that lower mean serum BDNF levels over the trial predicted a good PTSD response to escitalopram might be explained by the effects of BDNF in the mesolimbic dopamine pathway. In contrast to the role that BDNF has in the hippocampus, high levels of BDNF in the mesolimbic dopamine pathway may be crucial to the development and maintenance of PTSD. The mesolimbic dopamine pathway is composed of dopaminergic neurons in the midbrain ventral tegmental area (VTA) and their projections to the nucleus accumbens (NAc) (Berton et al. 2006). It has been hypothesized that dopaminergic signaling to the NAc is involved in the appraisal and response to threats from the social environment (Insel and Fernald 2004). BDNF is a potent excitatory neurotransmitter evoking rapid postsynaptic depolarization (Lommatzsch et al. 2005; Kafitz et al. 1999), and BDNF activation of tropomyosin-related kinase B (TrkB) receptors may promote dopamine release in the NAc (Graham et al. 2009). In mice, intact BDNF function in the mesolimbic dopamine pathway is necessary for the development and the maintenance of social avoidance behavior (a core symptom of PTSD in humans) induced by defeat stress paradigm. Accordingly, the inhibition of BDNF activity in the VTA-NAc pathway exerts an antidepressant-like activity in rodents and reverses the social avoidance behavior caused by defeat stress paradigm (Berton et al. 2006; Eisch et al. 2003). From this perspective, high BDNF activity in this pathway might be a source of treatment resistance. Thus, if peripheral BDNF provides an indicator of activity in the VTA-NAc, then higher levels could be a predictor of poor response to treatment.
Our finding that the SSRI escitalopram ameliorated PTSD symptoms without increasing serum BDNF levels is similar to some, but not all of those reported in the field of depression. Matrisciano et al. (2009) demonstrated that sertraline, venalfaxine and escitalopram for 24 weeks had shown comparable efficacy in 21 depressed patients (10 women); however, only escitalopram did not induce an increase in serum BDNF levels (Matrisciano et al. 2009). On the other hand, Aydemir et al. (2006) showed that the treatment for 6 weeks with escitalopram was effective in 20 women with MDD and led to an increase in serum BDNF levels. Although the absence of studies correlating the use of escitalopram and alterations in serum BDNF levels of patients with PTSD limits us to compare our findings to those of studies on depression, these comparisons may not be valid. PTSD and depression seem to exhibit distinct neurobiological alterations (Yehuda 2002), and gender differences have been associated with peripheral BDNF levels (Lommatzsch et al. 2005). The fact that we enrolled only men in our study and the two above mentioned studies on depression employed both men and women or only women limits the comparability of these studies. Although most studies on MDD have shown that serum BDNF levels increase in association with the therapeutic effects of antidepressants (Sen et al. 2008), different antidepressant drugs have variable effects on serum BDNF levels (Matrisciano et al. 2009). In the field of PTSD, our results accord with those of the only study investigating the effects of treatment with a SSRI on serum BDNF. In a case report of Hauck et al. (2009) treated a patient with chronic PTSD with sertraline and psychotherapy for six weeks, and, despite the reduction in PTSD symptoms, no increase in serum BDNF levels was observed.
Despite some authors have advocated that plasma BDNF might represent a more reliable and sensitive peripheral marker of BDNF variations occurring in the brain (Dell’Osso et al. 2009; Lommatzsch et al. 2005) than serum BDNF, we decided to investigate the latter because plasma BDNF levels show diurnal variation (Piccinni et al. 2008; Begliuomini et al. 2008) and a high inter-individual variability (Grassi-Oliveira et al. 2008; Karege et al. 2005; Lee et al. 2007), therefore becoming unsuitable for a clinically feasible biomarker for treatment response. Moreover, increases in serum BDNF levels in treatment-responsive depressed patients but not in nonresponders suggests a relationship between central processes and BDNF measured peripherally (Vinogradov et al. 2009). Animal models demonstrated that brain and serum BDNF levels change in parallel during maturation and aging; and serum and cortical BDNF levels are positively correlated (Karege et al. 2002; Vinogradov et al. 2009).
Although no study has demonstrated that the treatment with SSRIs increases BDNF levels in patients with PTSD, evidence suggests that the chronic use of paroxetine promotes neurogenesis in the hippocampus of these patients. In an open-label study using magnetic resonance imaging (MRI), Vermetten et al. (2003) showed an increase of 4.6% in mean hippocampal volume of 20 patients with PTSD after treatment with paroxetine for 9–12 months (Vermetten et al. 2003). However, it is likely that there were multiple mechanisms beyond BDNF release that were involved in this observed increase in hippocampal volume. For example, high concentrations of glutamate can damage neurons in human hippocampus, and the stress-related neurodegeneration of hippocampus can be prevented by blocking the glutamate-activated NMDA receptors (Moghaddam 2002). Studies have shown that treatment with SSRIs reduces plasma levels of glutamate in depressed patients (Küçükibrahimoglu et al. 2009), and that treatment with paroxetine decreases central levels of glutamate in children with obsessive-compulsive disorder (Rosenberg et al. 2000). A recent MRI study showed that the reduction in hippocampal volume seen in patients with PTSD is restricted to the dentate gyrus (DG) and CA3 subfields (Wang et al. 2010). Studies from animal models suggest that while the DG subfield is a key site of neurogenesis (mediated by some neurotrophins, including BDNF), the CA3 is a major target of glucocorticoids, a class of steroid hormones that are elevated under conditions of stress and promote neuronal death (Wang et al. 2010). In addition, a study in rats showed that the administration of fluoxetine or desipramine leads to an increase in hippocampal expression of fibroblast growth factor 2, a neurotrophic factor underexpressed in hippocampus of depressed patients (Bachis et al. 2008). Finally, studies on depressed patients show that paroxetine can effectively ameliorate symptoms without increasing serum BDNF levels (Hellweg et al. 2008) similar to our results.
Like Hauck et al. (2010), we found no correlation between serum BDNF levels and severity of PTSD. However, studies comparing peripheral BDNF levels in patients with PTSD and controls show less consistent results. Hauck et al. (2010) found that patients with PTSD (and ASD) due to recent traumas (occurred in the year of assessment), had higher serum BDNF levels than matched controls, but this difference was not significant in patients with PTSD of longer duration (i.e. greater than 4 years). This contrasts with results reported by Dell’Osso et al. (2009) who showed that PTSD patients had lower plasma BDNF levels than matched controls independent to duration of illness. However, differences in the medication status of participants, and the type of BDNF levels measured (i.e. serum vs. plasma) in these studies could explain these apparently contradictory results. In a case report, Hauck and colleagues (2009) also found higher serum BDNF levels in a patient with chronic PTSD at baseline, compared to healthy controls. It is noteworthy that in this the patient was already taking sertraline for 15 days before the first assessment. Thus, due to the discrepancy in the results of the few studies investigating the role of BDNF in patients with PTSD, the differences in their methods and the small sample sizes preclude any definitive conclusion about this issue.
The present work is the second to investigate the efficacy of escitalopram in treating PTSD patients. In our sample, the assessments at week 12 demonstrate that both combat and non-combat PTSD subjects showed a significant decrease in PTSD and MDD symptoms, the former evaluated by self-report and clinician-report ratings. Regarding PTSD symptoms, at the end of our study, 73.3% of subjects were considered responders to escitalopram and 46.7% achieved full remission. These results are in agreement with another 12-week open-label trial where 24 veterans with PTSD were treated with 20 mg/day of escitalopram (Robert et al. 2006). In that study, the mean CAPS score decreased from 79.42 to 61.21, with 37.5% of participants being considered responders (characterized as a reduction ≥ 20% on CAPS scores, a more permissive criteria than ours) (Robert et al. 2006). Although we have found a greater response to escitalopram treatment in our study, it must be noted that the subjects included in Robert et al.’s work were all veterans, a group believed to be more refractory to treatment (Brady et al. 2000;Davidson et al. 2001), and had higher mean CAPS score at baseline (79.42 vs. 61.81).
4.1 Limitations
Although our results suggest that serum BDNF levels during treatment are a potential prognostic biomarker for response to SSRI therapy in PTSD, the present work must be interpreted in the light of some limitations. First, our data are from an open-label trial with a small sample size. The second limitation is that we used flexible doses of escitalopram (5 to 20 mg/day) in our patients. Third, it is still not known if serum BDNF is a good proxy for brain BDNF concentrations in humans. However, studies in rats show that BDNF readily crosses the blood–brain barrier in both directions through a high-capacity saturable transport system (Vega et al. 2006), and there is a strong correlation (r=0.81) between serum and cortical BDNF (Karege et al. 2002b). Furthermore, even if we assume that peripheral BDNF is a reliable indicator of the cortical levels (Karege et al. 2002b), the effects of BDNF seems to vary depending on the specific site of action in the brain. For instance, in mice targeted ablation of the BDNF gene within the dentate gyrus, but not within the CA1 region of the hippocampus, results in an attenuated response to antidepressants (Adachi et al. 2008). Fourth, neurogenesis is a very complex phenomenon that is influenced by many other unmeasured factors that play an important role in mood and anxiety, through direct actions in the hippocampus and other limbic system structures (Duman and Monteggia 2006). Fifth, this study was comprised only of male subjects in order to minimize heterogeneity. Sixth, although we have statistically controlled for depression symptoms, our small sample and the high comorbidity rates (Table 1) found in our study preclude us to completely rule out the influence of major depression disorder in serum BDNF levels. On the other hand, the high comorbidity rates found in our study are similar to those reported in general population (roughly 80%) (Kessler et al. 1995; Creamer et al. 2001), a fact that enhances the generalizability of our results. Finally, pill count is an imperfect measure of treatment adherence.
5. Conclusions
It appears that subjects with comparatively low serum BDNF levels during SSRI treatment have the most to gain from a treatment that has putative neurotrophic effects. However, the linkage of the mechanism of action of antidepressants with neurogenesis in hippocampus remains controversial (Bachis et al. 2008). Apparently, the effects of antidepressants on BDNF levels vary depending on the drug administered, and neurogenesis can be enhanced by other paths besides increasing BDNF levels. Further, BDNF elicits opposite effects on mesolimbic dopamine pathway and hippocampus, and although the mechanisms of action of antidepressants in PTSD seem to be related to BDNF activity, it is unlikely to involve a simple linear effect. In PTSD, there are a wide range of different pharmacological options, with limited efficacy and evidence for choosing among the available medications for a given patient (Berger et al. 2009;Evans et al. 2006). Understanding the biological differences between patients who will and will not respond to treatment can shorten patients’ suffering, help to elucidate the biological mechanisms of available treatments (Bryant et al. 2008;Etkin et al. 2005), and reduce costs to patients and governments. This study suggests that peripheral BDNF may be a promising predictor of a positive treatment response to antidepressant medication. This will need to be confirmed in larger controlled trials in both men and women. In addition, future post-mortem studies with brains from controls and patients with PTSD (treated and untreated) are necessary for a better understanding of the role of BDNF in different brain areas in the pathogenesis and treatment of PTSD.
Acknowledgments
This work was supported by the National Institutes of Health (TCN: MH057157), Forest Laboratories (TCN), the Sierra Pacific Mental Illness and Education Clinical Center (MIRECC), and the CNPq (National Research Council) - Federal Government of Brazil.
ABBREVIATIONS
- ANOVA
analysis of variance
- ASD
acute stress disorder
- BDNF
brain derived neurotrophic factor
- CAPS
Clinician Administered PTSD Scale
- DG
dentate gyrus
- HAM-D
Hamilton Depression Rating Scale
- MDD
major depressive disorder
- NAc
nucleus accumbens
- NMDA
N-methyl-D-aspartic acid
- PCL
Posttraumatic Stress Disorder Checklist
- PTSD
posttraumatic stress disorder
- SCID-I
Structured Clinical Interview for DSM-IV
- SSRI
selective serotonin reuptake inhibitor
- VTA
ventral tegmental area
Footnotes
Disclosure/Conflicts of Interest: Dr. Neylan received research funding from Forest Laboratories to conduct this clinical trial reported here. In the past 2 years Dr. Neylan has served on an advisory board for Pfizer, and has received research support from Actelion and Glaxo Smith Kline. Drs Berger, Mehra, Lenoci, Metzler, Otte, Tarasovsky, Mellon, Wolkowitz, and Marmar have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatry Press; 2000. Revised. [Google Scholar]
- Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, et al. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog in Neuropsychopharmacology Biol Psychiatry. 2006;30:1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55:1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, Pluchino N, et al. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J Endocrinol. 2008;197:429–435. doi: 10.1677/JOE-07-0376. [DOI] [PubMed] [Google Scholar]
- Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: A systematic review. Prog in Neuropsychopharmacology Biol Psychiatry. 2009;33:169–180. doi: 10.1016/j.pnpbp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: A randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- Broekman BFP, Olff M, Boer F. The genetic background to PTSD. Neurosci Biobehav Rev. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied mixed models in medicine. New York: J. Wiley & Sons; 1999. [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, et al. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med. 2008;38:555–561. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- Creamer M, Burgess P, McFarlane AC. Post-Traumatic Stress Disorder: Findings from The Australian National Survey of Mental Health and Well-Being. Psychol Med. 2001;31:1237–1247. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- Davidson JRT, Rothbaum BO, Van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58:485–492. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- Dell’Osso L, Carmassi C, Del Debbio A, Dell’Osso MC, Bianchi C, da Pozzo E, et al. Brain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorder. Prog in Neuropsychopharmacology Biol Psychiatry. 2009;33:899–902. doi: 10.1016/j.pnpbp.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A Neurotrophic Model for Stress-Related Mood Disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54(10):994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Etkin A, Pittenger C, Polan HJ, Kandel ER. Toward a neurobiology of psychotherapy: Basic science and clinical applications. J Neuropsychiatry Clin Neurosci. 2005;17:145–158. doi: 10.1176/jnp.17.2.145. [DOI] [PubMed] [Google Scholar]
- Evans K, Dougherty D, Pollack M, Rauch S. Using neuroimaging to predict treatment response in mood and anxiety disorders. Ann Clin Psychiatry. 2006;18:33–42. doi: 10.1080/10401230500464661. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 2009;20:1402–1406. doi: 10.1097/WNR.0b013e3283300fbc. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RI, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders Patient edition. New York: Biometrics Research institute, New York State Psychiatric Institute; 1996. [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: Application to the NIMH Treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, et al. Tropomyosin-Related Kinase B in the Mesolimbic Dopamine System: Region-Specific Effects on Cocaine Reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low Plasma Brain-Derived Neurotrophic Factor and Childhood Physical Neglect Are Associated with Verbal Memory Impairment in Major Depression-A Preliminary Report. Biol Psychiatry. 2008;64:281–285. doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Hauck S, Gomes F, de Moura Silveira E, Jr, Almeida E, Possa M, Ceitlin LHF. Serum levels of brain-derived neurotrophic factor in acute and posttraumatic stress disorder: A case report study. Rev Bras Psiquiatr. 2009;31:48–51. doi: 10.1590/s1516-44462009000100012. [DOI] [PubMed] [Google Scholar]
- Hauck S, Kapczinski F, Roesler R, de Moura Silveira E, Jr, Magalhães PV, Kruel LRP, et al. Serum brain-derived neurotrophic factor in patients with trauma psychopathology. Prog in Neuropsychopharmacology Biol Psychiatry. 2010;34:459–462. doi: 10.1016/j.pnpbp.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Hellweg R, Ziegenhorn A, Heuser I, Deuschle M. Serum concentrations of nerve growth factor and brain-derived neurotrophic factor in depressed patients before and after antidepressant treatment. Phamacopsychiatry. 2008;41:66–71. doi: 10.1055/s-2007-1004594. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased Serum Brain-Derived Neurotrophic Factor Levels in Major Depressed Patients. Psychiatry Res. 2002a;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002b;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kauer-Sant’Anna M, Tramontina J, Andreazza AC, Cereser K, da Costa S, Santin A, et al. Traumatic life events in bipolar disorder: Impact on BDNF levels and psychopathology. Bipolar Disord, Supplement. 2007;9:128–135. doi: 10.1111/j.1399-5618.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Küçükibrahimoglu E, Saygm MZ, Çahskan M, Kaplan OK, Ünsal C, Gören MZ. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur J Clin Pharmacol. 2009;65:571–577. doi: 10.1007/s00228-009-0650-7. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J Affect Disord. 2007;101:239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song HJ, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: From molecule to man. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res. 2009;43:247–254. doi: 10.1016/j.jpsychires.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Piccinni A, Marazziti D, Del Debbio A, Bianchi C, Roncaglia I, Mannari C, et al. Diurnal Variation of Plasma Brain-Derived Neurotrophic Factor (BDNF) in Humans: An Analysis of Sex Differences. Chronobiol Int. 2008;25:819–826. doi: 10.1080/07420520802387773. [DOI] [PubMed] [Google Scholar]
- Robert S, Hamner MB, Ulmer HG, Lorberbaum JP, Durkalski VL. Open-label trial of escitalopram in the treatment of posttraumatic stress disorder. J Clin Psychiatry. 2006;67:1522–1526. doi: 10.4088/jcp.v67n1005. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer DC. Clinically significant change: Jacobson and Truax (1991) revisited. J Consult Clin Psychol. 1992;60:402–408. doi: 10.1037//0022-006x.60.3.402. [DOI] [PubMed] [Google Scholar]
- Vega SR, Strüder HK, Wahmann BV, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is Serum Brain-Derived Neurotrophic Factor a Biomarker for Cognitive Enhancement in Schizophrenia? Biol Psychiatry. 2009;66:549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic Resonance Imaging of Hippocampal Subfields in Posttraumatic Stress Disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]