Abstract
Activation of glutamate receptors and glial cells in the spinal dorsal horn are two fundamental processes involved in the pathogenesis of various pain conditions, including neuropathic pain induced by injury to the peripheral or central nervous systems. Numerous studies have demonstrated that minocycline treatment attenuates allodynic and hyperalgesic behaviors induced by tissue inflammation or nerve injury. However, the synaptic mechanisms by which minocycline prevents hyperalgesia are not fully understood. We recently reported that deficient glutamate uptake by glial glutamate transporters (GTs) is key for the enhanced activation of N-methyl-D-aspartate (NMDA) receptors in the spinal sensory synapses of rats receiving partial sciatic nerve ligation (pSNL). In this study, we investigated how minocycline affects activation of NMDA receptors in the spinal sensory synapses in rats with pSNL by whole cell recordings of NMDA currents in spinal laminea I and II neurons from spinal slices. The effects of minocycline treatments on the dorsal horn expression of glial GTs and astrocyte marker glial fibrillary acidic protein (GFAP) were analyzed by immunohistochemistry. We demonstrated that normalized activation of NMDA receptors in synapses activated by both weak and strong peripheral input in the spinal dorsal horn is temporally associated with attenuated mechanical allodynia in rats with pSNL receiving intraperitoneal injection of minocycline. Minocycline ameliorated both the downregulation of glial GT expression and the activation of astrocytes induced by pSNL in the spinal dorsal horn. We further revealed that preventing deficient glial glutamate uptake at the synapse is crucial for preserving the normalized activation of NMDA receptors in the spinal sensory synapses in pSNL rats treated with minocycline. Our studies suggest that glial GTs may be a potential target for the development of analgesics.
Keywords: glutamate transporters, glutamate receptors, spinal sensory processing, nociception, glia, pain
Introduction
Activation of glutamate receptors and glial cells in the spinal dorsal horn are two fundamental processes involved in the development and maintenance of pathological pain (hyperalgesia and allodynia) induced by tissue inflammation or injury, including neuropathic pain induced by injury to the peripheral or central nervous system (CNS) (Ren and Dubner, 2008). Numerous studies have demonstrated that glial inhibitors or modulators attenuate the pathogenesis of pain (Milligan and Watkins, 2009). Minocycline is a semisynthetic tetracycline antibiotic that inhibits glial activation. Systemic and intrathecal injection of minocycline attenuates the allodynic and hyperalgesic behaviors induced by tissue inflammation (Hua et al., 2005) or nerve injury (Mika et al., 2010; Raghavendra et al., 2003). The effects of minocycline are associated with the suppression of glial activation and pro-inflammatory cytokine expression in the spinal dorsal horn. However, the synaptic mechanisms by which minocycline prevents pathological pain induced by nerve injury are not fully understood.
Presynaptic glutamate concentrations and properties of postsynaptic glutamate receptors are major factors that determine the activation of neurons and influence neuronal excitability (Anderson and Swanson, 2000; Clements, 1996; Jonas, 2000). Because glutamate is not metabolized extracellularly, the presynaptic glutamate concentrations are determined not only by the amount of synaptically released glutamate but also by the rate at which glutamate is taken up by glutamate transporters (GTs) (Danbolt, 2001; Jonas, 2000; Trussell, 1998). GTs are located in the plasma membranes of glial cells and neurons that rapidly take up synaptically released glutamate from the extracellular space and maintain the homeostasis of extracellular glutamate concentrations (Danbolt, 2001; Jonas, 2000; Trussell, 1998). Two types of glial GTs (GLT-1 and GLAST) located on astrocytes and one type of neuronal GT exist in the spinal dorsal horn (Mao et al., 2002; Sung et al., 2003; Weng et al., 2005; Xin et al., 2009).
Glial GTs are the most dominant and widely distributed transporters in the central nervous system (CNS) and account for more than 90% of the glutamate uptake (Tanaka et al., 1997). In recent years, our group and others have demonstrated the crucial role of glial GTs in the spinal pain signaling system. Pharmacological blockade of GTs (including glial GTs) in the spinal cord results in a hyperalgesic state in awake animals (Liaw et al., 2005; Weng et al., 2006) and increases the activation of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), N-methyl-D-aspartate (NMDA) receptors (Nie and Weng, 2009; Weng et al., 2006; Weng et al., 2007), and group I metabotropic glutamate receptors (mGluRs) (Galik et al., 2008). Hyperalgesia induced by chronic nerve injury (Sung et al., 2003; Xin et al., 2009), chemotherapy (e.g., paclitaxel; (Weng et al., 2005), or opioids (Mao et al., 2002; Thomson et al., 2006) is associated with the downregulation of glial GT protein expression in the spinal dorsal horn. Furthermore, we recently revealed that deficient glutamate uptake by glial cells in the spinal sensory synapses, which results in extrasynaptic glutamate spillover and the activation of extrasynaptic NMDA receptors, is key for the enhanced NMDA receptor activation elicited by peripheral afferent inputs in neuropathic rats induced by partial sciatic nerve ligation (pSNL)(Nie and Weng, 2010).
These findings led us to hypothesize that preventing the impairment of glutamate uptake by glial GTs at the synapses is a key mechanism underlying the attenuation of mechanical allodynia by minocycline, a known glial inhibitor, in neuropathic rats. In this study, we demonstrated that normalized activation of NMDA receptors in the spinal dorsal horn is associated with attenuated mechanical allodynia in rats with pSNL receiving minocycline treatments, and that the preservation of glutamate uptake by glial GTs plays a key role in the normalized activation of NMDA receptors in the spinal sensory synapses in these rats.
EXPERIMENTAL PROCEDURES
Animals
Young adult male Sprague-Dawley rats (weight range, 150-220 g) were used for all experiments. All animal experiments were approved by The University of Texas M. D. Anderson Cancer Center Institutional Animal Care and Use Committee and were fully compliant with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
pSNL and behavioral tests
In rats anesthetized with isoflurane (2-3%), the left sciatic nerve at the upper thigh was exposed and tightly ligated with 5-0 silk sutures to approximately one-third to one-half its original thickness, as described by Seltzer et al. (1990). The wound was closed with muscle sutures and skin staples. In the sham-operated rats, the left sciatic nerve was exposed but not ligated. To verify the development of tactile allodynia after pSNL, behavioral tests were performed to measure the mechanical sensitivity of both hind paws prior to and after surgery (Weng et al., 2003). Briefly, the animals were placed on wire mesh, loosely restrained under a plexiglass cage (12 × 20 × 15 cm3), and allowed to accommodate for at least 15 min. Von Frey monofilaments with bending forces ranging from 0.1 to 8.0 g were applied from below through the mesh onto the mid-plantar area of each hind paw to evoke paw withdrawal responses. Each hind paw was stimulated 10 times with each von Frey monofilament, and the frequency (percentage) of paw withdrawal responses to 10 stimulations was recorded (Weng et al., 2003). The least bending force that evoked paw withdrawal in more than half the trials was assigned as the 50% withdrawal threshold (Cata et al., 2004; Weng et al., 2003).
Drug administration
Adult rats were randomly assigned to four experimental groups. Minocycline was given to both pSNL rats (minocycline-pSNL group) and sham-operated rats (minocycline-sham group). Another group of pSNL rats received a saline treatment (saline-pSNL group). Saline was also administered to a group of sham-operated rats (saline-sham group), which were used as controls. Following the scheme used by others (Mika et al., 2010; Zanjani et al., 2006; Raghavendra et al., 2003) and our previous study (Cata et al., 2008), both minocycline (50 mg/kg/day in 1 ml) and saline (1 ml/day) were administered intraperitoneally (i.p.) 1 h before the surgery and then every evening until day 10 to 12 post-surgery.
In vitro whole cell recordings
Spinal slice preparation
pSNL rats receiving minocycline (50 mg/kg/day, i.p.) 1 h before the surgery and then every evening until day 10 to 12 post-surgery were used. The animals were deeply anesthetized by isoflurane inhalation and the lumbar spinal cord was removed by laminectomy. The lumbar spinal cord section was placed in ice-cold sucrose artificial cerebrospinal fluid (aCSF) presaturated with 95% O2 and 5% CO2. The sucrose aCSF contained 234 mM sucrose, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 12.0 mM glucose, and 25.0 mM NaHCO3. The pia-arachnoid membrane was removed from the lumbar spinal cord. The L4-5 spinal segment, identified by the lumbar enlargement and large dorsal roots, was attached with cyanoacrylate glue to a cutting support, which was then glued onto the stage of a vibratome (Series 1000, Technical Products International, St. Louis, MO). Transverse spinal cord slices (400 μm) were cut in the ice-cold sucrose aCSF and then preincubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 35°C for at least 2 h before they were transferred to the recording chamber. The Krebs solution contained 117.0 mM NaCl, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 11.0 mM glucose, and 25.0 mM NaHCO3.
Whole cell voltage-clamp recordings
Following pre-incubation, a spinal cord slice was placed in the recording chamber (volume, 1.5 ml), perfused with Krebs solution at 35°C, and saturated with 95% O2 and 5% CO2. Borosilicate glass recording electrodes (resistance, 3-5 MΩ) were pulled and filled with an internal solution containing 110.0 mM Cs2SO4, 2.0 mM MgCl2, 0.5 mM CaCl2, 5.0 mM HEPES, 5.0 mM EGTA, 5.0 mM ATP-Mg, 0.5 mM Na-GTP, and 10.0 mM lidocaine N-ethyl bromide (QX314) adjusted to pH 7.2-7.4 using 1.0 M CsOH (290-300 mOsm). QX314 was added to the internal solution to suppress the generation of action-potentials in the recorded cells. Live dorsal horn neurons in the spinal laminae I and II region were visualized using an infrared Nomarski microscope system and approached using a three-dimensional motorized manipulator, and whole-cell configurations were established by applying moderate negative pressure after electrode contact (Nakatsuka et al., 2003). A seal resistance of at least 2 GΩ and an access resistance of 20-35 MΩ were considered acceptable (Weng et al., 2006; Wu et al., 2005). The series resistance was optimally compensated by at least 70% and constantly monitored throughout the experiments. Experiments showing any evidence of loss of voltage control were discarded. Signals were amplified using an Axopatch 700B amplifier (Molecular Devices, CA), digitized at 10 kHz, displayed and stored in a personal computer.
Excitatory postsynaptic currents (EPSCs) were evoked using constant-current electrical stimuli (0.2-ms duration repeated every 45 s) applied with a concentric bipolar stimulating electrode placed at the dorsal root entry zone (Weng et al., 2006; Yoshimura and Nishi, 1993). NMDA EPSCs were isolated by including 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM), bicuculline (10 μM), and strychnine (5 μM) in the external solution to block the non-NMDA glutamate (AMPA and kainate receptors), GABAA, and glycine receptors and holding the membrane potential at +40 mV to remove the voltage-dependent Mg2+ block from NMDA receptors. In a subset of experiments, NMDA currents evoked by exogenous L-glutamate (Glu-NMDA current) and those evoked by exogenous NMDA (Agonist-NMDA current) were recorded in the same neuron. This recording was achieved by puffing L-glutamate (50 μM, 20-ms duration) and NMDA (200 μM, 20-ms duration), respectively, onto the recording cells through a double-barrel pipette with an 8-12 μm tip opening (Nie and Weng, 2010).
Immunocytochemical analysis
Immunocytochemistry was used to analyze the expression of two glial GT proteins (GLT-1 and GLAST) and the glial fibrillary acidic protein (GFAP) in astrocytes in the spinal dorsal horns (Weng et al., 2005) from rats in all four experimental groups (4 to 5 rats/group). On day 10 post pSNL or sham operation, rats were deeply anesthetized with pentobarbital (60 mg/kg, i.p.), and 300 ml heparinized saline solution (4°C) was perfused intracardially followed by 500 ml of a solution of 4% paraformaldehyde and 0.1% picric acid in 0.1 M phosphate-buffered saline (PBS; 4°C). The spinal segments L4 and L5 were then removed and post-fixed for 24 h at 4°C in the same fixative and cryoprotected for 24 h at 4°C in 30% sucrose in 0.1 M PBS. Serial transverse sections, 30-μm thick, were cut with a freezing microtome (−20°C), collected in 0.1 M PBS (pH 7.4), and processed while free-floating. Spinal sections taken from all four groups of animals were processed at the same time to minimize experimental variations. The sections were rinsed in 0.1 M PBS three times (10 min each) and then blocked with 10% normal goat serum plus 0.3% Triton X-100 for 1 h at room temperature (20°C). The sections were incubated for 36 h at 4°C in 2% normal goal serum with the following antibodies: rabbit anti-GFAP (1:500), guinea pig anti-GLAST (1:2000), or anti-GLT-1 (1:2000). The sections were washed three times in 0.1 M PBS (10 min each) and then incubated for 2 h at room temperature with the corresponding FITC-conjugated secondary antibody (1:250, Chemicon) or Cy3-conjugated secondary antibody (1:500, Chemicon). After rinsing in 0.1 M PBS, the sections were mounted onto gelatin-coated slides, air-dried, and sealed with a coverslip and DPX mounting medium. To examine the specificity of the secondary antibodies, control sections were processed as described, but with the primary antibodies omitted.
For each rat, four to five nonadjacent sections from the L4 and L5 segments were selected randomly, and the immunostaining for each antibody in the spinal cord dorsal horn was recorded with a digital camera (CoolSnap of Photometrics, Roper Scientific, USA). The functional state of the astrocytes in the spinal dorsal horn was determined by the morphology of astrocytes stained by GFAP and the immunostaining densities of GFAP. The immunostaining densities of GFAP, GLT-1 and GLAST were calculated by subtracting the background staining in the spinal white matter from the optical density in spinal laminae I and II (Weng et al, 2005; Xin et al, 2009). The immunostaining densities in the tissue sections from the operated sides of the minocycline-pSNL group, minocycline-sham group, and saline-pSNL group were expressed as a percentage of the immunostaining densities in the tissue sections from the operated side of the control group (saline-sham group). All measurements were performed by evaluators blinded to the study groups.
DNQX, bicuculline, strychnine, L-glutamate, and tetrodotoxin (TTX) were obtained from Sigma-Aldrich, and dihydrokainic acid (DHK), and D-aminophosphonovaleric acid (D-AP5) were obtained from Tocris Bioscience. All pharmacological agents were applied to the recording chamber by perfusion.
Data analysis
The NMDA EPSCs or currents were analyzed off-line. The mean of three to four EPSCs or currents evoked by electrical stimulation or by puffed L-glutamate or NMDA at baseline and in the presence of tested drugs was measured. To determine time constants for the decay phase of NMDA EPSCs or currents, the decay phase was fitted with a monoexponential function (Weng et al., 2007). The Clampfit software program (version 10.2; Molecular Devices, CA, USA) was used to measure the peak latency (time from stimulation onset to peak), amplitude, duration, and time constant of the mean EPSCs or currents.
All data are presented as the mean ± S.E. Student's t-test was used to determine the statistical differences between data obtained with and without tested drugs (paired t-test) or between groups (nonpaired t-test). A P value less than 0.05 was considered statistically significant.
RESULTS
Minocycline prevents the development of pSNL-induced mechanical allodynia
To examine the effects of minocycline on the development of pSNL-induced mechanical allodynia, we performed behavioral tests in four groups of rats: saline-pSNL rats (n = 5), saline-sham rats (n = 4), minocycline-pSNL rats (n = 5), and minocycline-sham rats (n = 4). The mechanical thresholds (0.56 ± 0.06 g) for the hind paw ipsilateral to the pSNL in the saline-pSNL rats measured day 10 after surgery were significantly lower than those obtained before surgery (6.67 ± 0.52 g; P < 0.001) (Table 1). Consistent with findings by others (Mika et al., 2010; Raghavendra et al., 2003) the minocycline treatment significantly attenuated the development of pSNL-induced hypersensitivity to mechanical stimulation. While the mechanical thresholds prior to the surgery in the minocycline pSNL rats (6.80 ± 0.73 g) were similar to those in the saline-pSNL rats, the mechanical thresholds measured day 10 post surgery in the minocycline-pSNL rats (5.00 ± 0.95 g) were significantly higher than those in the saline-pSNL rats (0.56 g ± 0.06 g; P < 0.001) . The mechanical thresholds measured day 10 post surgery were similar to those obtained at baseline in the saline-sham rats (baseline: 6.20 ± 1.20 g; day 10: 6.80 ± 0.73 g) and minocycline-sham rats (baseline: 5.60 ± 0.87 g; day 10: 5.30 ± 0.70 g), indicating that minocycline had no effects on mechanical sensitivity in the normal control rats.
Table 1.
| Baseline | Day 10 post surgery | |
|---|---|---|
| Saline pSNL (n=5) | 6.67 ± 0.52 g |
|
| Minocycline pSNL (n=5) | 6.80 ± 0.73 g | |
| Minocycline sham (n=4) | 5.60 ± 0.87 g | 5.30 ± 0.70 g |
| Saline sham (n=4) | 6.20 ± 1.20 g | 6.80 ± 0.73 g |
Mechanical hindpaw withdrawal thresholds measured prior to surgery and day 10 postsurgery. *** P<0,001.
Minocycline ameliorates the pSNL-induced enhanced activation of NMDA receptors in spinal superficial dorsal horn neurons
We recently reported that rats with pSNL have enhanced activation of NMDA receptors in the superficial spinal dorsal horn neurons (Nie and Weng, 2010). To study the effect of minocycline on the activation of NMDA receptors in pSNL rats, we recorded NMDA EPSCs or currents from neurons in the spinal superficial dorsal horns (laminae I and II) of rats (n = 13) receiving minocycline (50 mg/kg/day, i.p.) 1 h before the surgery and then every evening until day 10 to 12 post-surgery. The mechanical thresholds (6.80 ± 0.49 g) measured immediately prior to the electrophysiological experiments were significantly higher than those in the saline-pSNL rats (0.56 g ± 0.06 g; P < 0.001).
In order to characterize responses of NMDA receptors in the synapses responding to weak peripheral input and synapses responding to strong peripheral input, we evoked NMDA EPSCs by electrically stimulating the spinal dorsal root entry zone at two stimulating intensities: one at twice the EPSC activation threshold (2T) and another at the intensity that evokes a maximum EPSC in the recorded neuron (maximum stimulation) (Nie and Weng, 2009; Nie and Weng, 2010). The results were then compared with the recently published data collected from normal control rats and untreated pSNL rats (Nie and Weng, 2010). We found that the amplitude, peak latency, duration, decay-time constant, and area of NMDA EPSCs evoked by 2T and maximum stimulation in the minocycline-pSNL group were similar to those collected from the control groups (Fig. 1). Compared with the results collected from the untreated pSNL rats (39 neurons), NMDA EPSCs evoked by weak (2T) stimulation in the minocycline-pSNL group (16 neurons) had shorter peak latencies (17.75 ± 1.60 ms versus 27.73 ± 2.52 ms; P < 0.05) and durations (594.25 ± 61.39 ms versus 1068.86 ± 51.49 ms; P < 0.001), faster decay-time constants (102.17 ± 13.74 ms versus 204.49 ± 20.26 ms; P < 0.001), and smaller areas (9340.62 ± 1601.17 pA.ms versus 24058.08 ± 3530.92 pA.ms; P < 0.001). Similarly, NMDA EPSCs evoked by maximum stimulation in the minocycline-pSNL group were similar to those found in the normal control rats, but had significantly shorter durations, faster decay-time constants, and smaller areas than those in the untreated pSNL rats (Fig. 1). No significant differences were found in the NMDA EPSC amplitudes between the minocycline-pSNL and untreated pSNLrats. These data indicated that the minocycline treatment ameliorates the pSNL-enhanced activation of NMDA receptors in the spinal sensory synapses, which is associated with attenuation of the pSNL-induced mechanical allodynia.
Fig. 1. Minocycline ameliorates the pSNL-induced enhanced activation of NMDA receptors in spinal superficial dorsal horn neurons.
Shown are samples of NMDA EPSCs evoked by graded stimulation (2T stim and Max stim) in normal control rats, untreated-pSNL rats, and minocycline-pSNLrats (50 mg/kg given i.p., starting 1 h prior to nerve injury and until day 10 to 12 post-surgery). Bar graphs show the average peak amplitude, peak latency, duration, decay-time constant, and area of NMDA EPSCs obtained from normal control rats, untreated-pSNL rats, and minocycline-pSNL rats. Statistical differences of the EPSCs between untreated pSNL rats and normal control rats or minocycline pSNL rats are marked by asterisks. *P < 0.05; **P < 0.01; ***P < 0.01.
Minocycline attenuates both the downregulation of glial GT protein expression and the activation of astrocytes induced by pSNL in the spinal dorsal horn
The normalized activation of glutamate receptors may be due to the correction of abnormalities in any or a combination of three basic factors: the amount of presynaptically released glutamate, the rate of glutamate uptake by GTs, and the properties of postsynaptic glutamate receptors. We recently revealed that deficient glutamate uptake by glial GTs is key for the abnormal activation of NMDA receptors in spinal sensory synapses (Nie and Weng, 2010). The fact that minocycline shortened the NMDA EPSC peak latency, duration, and decay-time constant in pSNL rats strongly suggests that minocycline treatment may preserve glutamate uptake at the synapses. Efficient glutamate uptake at the synapses reduces the EPSC peak latency and the EPSC duration by shortening the dwelling time of the glutamate exposed to the recorded neuron, resulting in the normalized activation of NMDA receptors. To test this notion, we first examined the expression of both glial GT proteins (GLT-1 and GLAST) in the spinal laminae I and II of saline-pSNL rats (n = 5), saline-sham rats (n = 4), minocycline-pSNL rats (n = 5), and minocycline-sham rats (n = 4) on day 10 post surgery (Fig. 2A, B). The behavioral data for these four groups of animals were presented in Table 1. Consistent with our previous study (Xin et al., 2009), the expression of GLT-1 and GLAST, as measured by the immunostaining densities in spinal laminae I and II ipsilateral to the pSNL, was 42.42% ± 9.66% (P < 0.001) and 35.55% ± 9.99% (P < 0.001), respectively, lower than those in saline-sham rats. As shown in Fig. 2, the reduction of glial GT expression was largely prevented in the pSNL rats receiving a 10-day treatment of minocycline. Minocycline had no effect on the expression of glial GTs in the spinal dorsal horn in the sham-operated rats (Fig. 2A, B).
Fig. 2. Minocycline attenuates the pSNL-induced reduction of glial GT protein expression in the spinal dorsal horn.
(A) Shown is the staining of glial GTs (GLT-1 and GLAST) in the spinal dorsal horn from saline-pSNL rats, minocycline-pSNL rats, minocycline-sham rats, and saline-sham rats day 10 after surgery. Scale bar: 200 μm. (B) Bar graphs show the relative mean (±S.E.) immunostaining densities of GLT-1 and GLAST in the dorsal horn ipsilateral to the operated side day 10 after surgery. The immunostaining densities from saline-pSNL rats, minocycline-pSNL rats, and minocycline-sham rats are presented as a percentage of the immunostaining densities obtained from the saline-sham rats. Asterisks denote the statistical comparisons between the three groups. ***P < 0.01; n.s, no significant difference.
It was demonstrated that effective glutamate uptake by glial cells depends not only on the number and function of GTs themselves but also on the availability of GTs near the synapse (Oliet et al., 2001). The number of GTs around the synapse is decreased following the retraction of astrocytic processes and the hypertrophy of astrocytic soma upon astrocytic reactivation (Oliet et al., 2001). Hence, we used GFAP as a marker for activation of astrocytes and analyzed the effects of minocycline on the functional state of astrocytes in the spinal dorsal horn in all four groups of rats on day 10 post surgery. Consistent with the results of numerous previous studies by others (Milligan and Watkins, 2009) and us (Xin et al., 2009), pSNL caused activation of astrocytes, which is characterized by hypertrophy with thicker processes and soma. Minocycline largely reduced the pSNL-induced activation of astrocytes (Fig. 3). When the immunostaining densities of GFAP were measured, the expression of GFAP in spinal laminae I and II ipsilateral to the operation side in saline-pSNL rats was significantly increased (252.6 ± 44.37% of saline-sham rats, P = 0.03, n=5, Fig. 3). The pSNL induced upregulation of GFAP was significantly attenuated in pSNL rats receiving a 10-day treatment of minocycline (143.10 ± 11.80% of saline-sham rats, P = 0.03, n=5) (Fig. 3). Minocycline had no effect on the expression of GFAP in the spinal dorsal horn in the sham-operated rats (107.46 ± 11.52% of saline-sham rats, n=4). Taken together, these data indicate that minocycline attenuates the deficient expression of glial GTs and astrocytic activation in the spinal dorsal horn of neuropathic rats. Moreover, the effects of minocycline on the expression of glial GTs and GFAP are concomitantly associated with the attenuation of mechanical hypersensitivity and normalized activation of NMDA receptors in the spinal sensory synapses responding to weak and strong peripheral input in pSNL rats.
Fig. 3. Minocycline attenuates the pSNL-induced activation of astrocytes in the spinal dorsal horn.
(A) Shown is the staining of GFAP for astrocytes in the spinal dorsal horn from saline-pSNL rats, minocycline-pSNL rats, minocycline-sham rats, and saline-sham rats. Note the characteristic hypertrophy with thicker processes and soma in the activated astrocytes on the ipsilateral dorsal horn of saline-pSNL rats. Minocycline treatments reduced the pSNL-induced activation of astrocytes. Insets show representative astrocytes in the saline-pSNL rats and minocycline-pSNL rats. Long scale bar: 80 μm; short scale bar: 10 μm. (B) Bar graphs show the relative mean (±S.E.) immunostaining densities of GFAP in the dorsal horn ipsilateral to the operated side day 10 after surgery. The immunostaining densities from saline-pSNL rats, minocycline-pSNL rats, and minocycline-sham rats are presented as a percentage of the immunostaining densities obtained from the saline-sham rats. Asterisks denote the statistical comparisons between the three groups. *P < 0.05.
Preservation of glutamate uptake by glial GTs at the synapse contributes to the normalized activation of NMDA receptors in the spinal sensory synapses in pSNL rats receiving minocycline
We next examined the function of glutamate uptake at the synaptic level. We recently reported that the enhanced activation of NMDA receptors induced by pharmacological blockade of glial GTs in normal control rats was precluded in pSNL-induced neuropathic rats (Nie and Weng, 2010). If the preservation of glutamate uptake by glial GTs is a key cause of the normalized activation of NMDA receptors in pSNL rats receiving minocycline, blockade of glial GTs should enhance the activation of NMDA receptors in these rats, because the effect of glial GT blockers is proportional to the number of functional glial GTs. Hence, in spinal slices from rats in the minocycline-pSNL group, we studied the NMDA currents elicited by exogenous L-glutamate (Glu-NMDA current) and NMDA (Agonist-NMDA current) in individual superficial dorsal horn neurons before and during bath perfusion of a selective glial GT blocker, DHK.
GTs selectively take up L-glutamate but not NMDA (Danbolt, 2001; Jabaudon et al., 1999). Because of the substrate selectivity of GTs, blockade of GTs affects the activation of NMDA receptors activated by L-glutamate, but not by agonist NMDA (Nie and Weng, 2010). Thus, recordings of currents evoked by agonist NMDA under the same conditions provide a reliable index to monitor any possible changes in postsynaptic NMDA receptors. We evoked Glu-NMDA and Agonist-NMDA currents in the same neuron by puffing L-glutamate (50 μM, 20-ms duration) and NMDA (200 μM, 20-ms duration), respectively, into the recorded cell through a double-barrel pipette. TTX (bath concentration, 1 μM) was added to prevent glutamate release from presynaptic neurons activated by puffed glutamate or NMDA (Nie and Weng, 2010). Although the puff duration was kept constant at 20 ms throughout these studies, the puff air pressure (3-6 psi) for each barrel was optimized so that the baseline amplitudes of NMDA currents evoked by puffing L-glutamate and NMDA were similar (Glu-NMDA current, 102.30 ± 7.22 pA; Agonist-NMDA current, 104.62 ± 5.74 pA, n = 6).
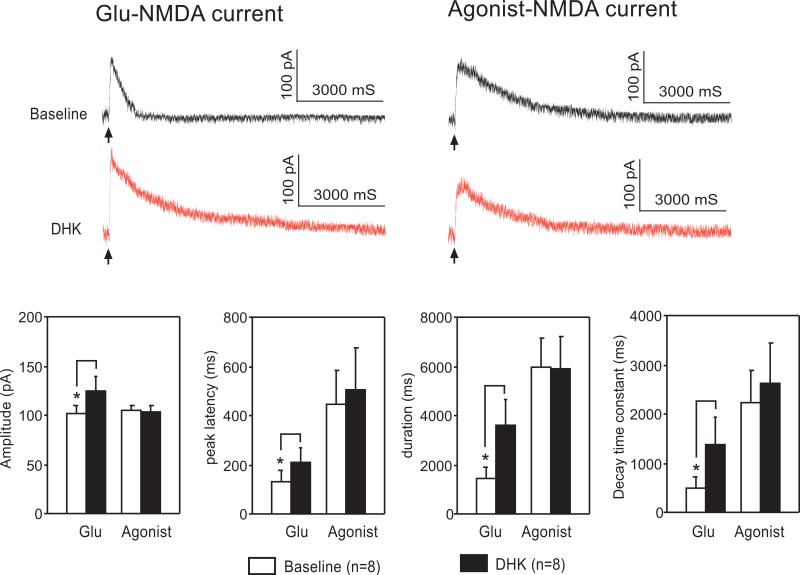
Bath perfusion of a selective glial GT blocker, DHK (300 μM, tested in six neurons) (Danbolt, 2001) significantly increased the Glu-NMDA current amplitude (from 102.30 ± 7.22 pA to 124.06 ± 15.40 pA; n = 6, P < 0.05), peak latency (from 134.79 ± 45.84 ms to 208.18 ± 61.36 ms; n = 6, P < 0.05), duration (from 1466.96 ± 442.104 ms to 3643.27 ± 1035.77 ms; n = 6, P = 0.01), and decay-time constant (from 503.10 ± 219.40 ms to 1396.30 ± 559.15 ms; n = 6, P = 0.02; Fig. 4). These data indicate that the preservation of glial glutamate uptake at the synapse contributes to the normalized activation of NMDA receptors in the spinal dorsal horn in pSNL rats receiving minocycline. Furthermore, we also found that Agonist-NMDA currents (n = 6) recorded simultaneously before and during perfusion of DHK were not significantly altered (Fig. 4), ruling out the possibility that changes in the number or properties of postsynaptic NMDA receptors contributed to the enhanced EPSCs induced by DHK.
Figure 4. Glutamate uptake by glial cells reduces the activation of NMDA receptors evoked by exogenous glutamate in pSNL rats receiving minocycline treatment.
Original recordings show the NMDA current evoked by puff application (20 ms duration) of L-glutamate (Glu-NMDA current) and NMDA (Agonist-NMDA current) recorded in the same neuron at baseline and during perfusion of a glial GT blocker, DHK (300 μM). Arrows denote the onset of puff application. Bath application of DHK increased the Glu-NMDA current but had no effect on the Agonist-NMDA current. The recordings were obtained for spinal superficial dorsal horn neurons from minocycline-pSNL rats in the presence of TTX (1 μM), DNQX (10 μM), bicuculline (10 μM), and strychnine (5 μM) at a holding potential of +40 mV. Bar graphs show the mean (+ S.E.) amplitude, peak latency, duration, and decay-time constant for the Glu-NMDA current and Agonist-NMDA current at baseline and during perfusion of DHK. Asterisks denote the statistical comparison of data obtained before and during DHK perfusion. *P < 0.05.
Finally, to specifically determine the function of glutamate uptake at synapses responding to weak and strong peripheral input, we examined the effect of DHK (300 μM) on NMDA EPSCs evoked by 2T and maximum stimulation of the spinal dorsal root entry zone in nine neurons from the minocycline-pSNL group. We found that the properties of the NMDA EPSCs evoked by 2T and maximum stimulation were significantly increased after perfusion of DHK (Fig. 5). For example, DHK significantly increased the maximum stimulation-evoked NMDA EPSC amplitude by 20.08% ± 17.83% (n = 9, P < 0.01), peak latency by 26.81% ± 6.89% (n = 9, P < 0.01), duration by 79.92% ± 26.32% (n = 9, P < 0.01), and decay-time constant by 49.56% ± 8.49% (n = 9, P < 0.01). Similarly, the responses of the NMDA receptors in synapses responding to 2T stimulation were also enhanced (Fig. 5). These findings are in contrast with the results collected from the same preparation in the untreated pSNL rats (Nie and Weng, 2010), where blockade of glial GTs with DHK (300 μM) did not significantly alter the NMDA currents evoked by peripheral synaptic input or exogenous glutamate. Taken together, we conclude that the preservation of glial glutamate uptake in the synapse plays a crucial role in the normalized activation of NMDA receptors in synapses responding to weak and strong peripheral input in pSNL rats treated with minocycline.
Figure 5. Preservation of glutamate uptake by glial GTs at the synapse contributes to the normalized activation of NMDA receptors in the spinal sensory synapses in pSNL rats receiving minocycline treatment.
Original recordings show NMDA EPSCs evoked by 2T and maximum stimulation recorded in the same neuron at baseline and during perfusion of DHK (300 μM). Blocking glial GTs with DHK resulted in enhanced activation of NMDA receptors evoked by 2T and maximum stimulation. Bar graphs show the mean (+ S.E.) amplitude, peak latency, duration, and decay-time constant of NMDA EPSCs at baseline and during perfusion of DHK. Asterisks denote the statistical comparison of data obtained before and during DHK perfusion. *P < 0.05; **P < 0.01; ***P < 0.01.
Discussion
Given the crucial role of NMDA receptors and glial cells in the induction and maintenance of pathological pain, identifying mechanisms by which glial cells regulate the activation of NMDA receptors has important implications for the development of analgesics. We recently reported that pSNL results in the enhanced activation of NMDA receptors in the spinal superficial dorsal horn neurons, which is, in part, due to the deficiency of glial glutamate uptake at the synapse. In this study, we further revealed that inhibition of glial activation with minocycline results in the attenuation of pSNL-induced mechanical allodynia, which is temporally accompanied by the normalized activation of NMDA receptors in spinal sensory synapses responding to both weak and strong peripheral synaptic input. We have provided evidence that the preservation of glial glutamate uptake at the synapse plays an important role in maintaining normal activation of NMDA receptors in pSNL rats treated with minocycline. Our results indicate that preventing deficient glial glutamate uptake is one mechanism by which minocycline prevents the development of pSNL-induced pathological pain.
Three essential factors govern activation of glutamatergic synapses: the amount of glutamate release from presynaptic terminals, the rate at which glutamate is removed by glutamate transporters, and the number and properties of glutamate receptors in postsynaptic neurons (Clements, 1996; Jonas, 2000). These three factors are regulated by the glial cells. For example, many bioactive substances (such as ATP, nitric oxide, glutamate) released from activated glial cells can regulate glutamate release from presynaptic terminals (Fiacco et al., 2008; Ikeda and Murase, 2004; Ikeda et al., 2007; Inoue et al., 2004; Newman, 2003). In addition, the number of postsynaptic AMPA and NMDA receptors is increased by proinflammatory cytokines (Choi et al., 2010; Kawasaki et al., 2008) and brain-derived neurotrophic factor released from activated glial cells (Lu et al., 2009). Importantly, glial cells directly regulate presynaptic glutamate concentrations through glial GTs, which actively take up glutamate from the extracellular space into the cell and maintain the homeostasis of extracellular glutamate (Danbolt, 2001).
The role of glial glutamate uptake in the spinal nociceptive process was recently demonstrated by us and others. Downregulation of GT protein expression in the spinal dorsal horn is associated with allodynia and hyperalgesia induced by chronic nerve injury (Sung et al., 2003; Xin et al., 2009), chemotherapy (e.g., paclitaxel) (Weng et al., 2005) or opioids (Mao et al., 2002; Thomson et al., 2006). We recently reported that prolonged activation of NMDA receptors in the spinal superficial dorsal horn in pSNL rats is due to extrasynaptic glutamate spillover caused by a deficiency of glial glutamate uptake at the synapses (Nie and Weng, 2010). When deficient glutamate uptake is mimicked by pharmacological blockade of GTs, activation of AMPA, NMDA receptors and group I mGluRs in spinal dorsal horn neurons are increased (Galik et al., 2008; Nie and Weng, 2009; Weng et al., 2006; Weng et al., 2007). The enhanced activation of glutamate receptors induced by deficient glutamate uptake could trigger signaling pathways in postsynaptic neurons, leading to further enhanced synaptic transmission. Activation of group I metabotropic glutamate receptors (mGluRs) results in increased activation of AMPA (Song et al., 2009) and NMDA receptors (Guo et al., 2004; Kalia et al., 2004). Increased activation of NMDA receptors further leads to the rapid exocytosis of AMPA receptors as well as a covalent modification of synaptic AMPA receptors (Nicoll, 2003). Because dorsal horn astrocytes also express glutamate receptors like mGluR5 (Gwak and Hulsebosch, 2005) and NMDA receptors (O'Donnell et al., 2004), the elevation of extracellular glutamate concentrations and extrasynaptic glutamate spillover induced by deficient glutamate uptake (Weng et al., 2006; Nie and Weng, 2010) could directly affect glial functions. For example, we recently reported that severe deficiency of glutamate uptake triggers glutamatergic communications between neurons and glial cells in the spinal dorsal horn. Consequently, glial cells are activated by peripheral synaptic input and release glutamate to activate postsynaptic neurons in spinal pain pathways (Nie et al., 2010).
Improvement in glutamate uptake or GT protein expression is associated with the amelioration of hyperalgesia induced by nerve injury or morphine tolerance. For example, hyperalgesia induced by nerve injury and the associated deficiency in glutamate uptake, as measured using synaptosome preparations, can be reversed with a cytosolic phospholipase A2 inhibitor (Sung et al., 2007). Prevention of GLT-1 downregulation by amitriptyline in the spinal dorsal horn is accompanied by an attenuation of morphine tolerance in rats (Lim et al., 2005; Tai et al., 2007). Furthermore, selective increased expression of GLT-1 by ceftriaxone treatment (Hu et al., 2010) or gene transfer (Maeda et al., 2008) significantly reduces hyperalgesia induced by nerve injury. However, the impact of increases in the expression of glial GTs or glutamate uptake measured in synaptosome preparations on the activation of glutamate receptors at synaptic levels remains unclear. Our study provides the first direct evidence that the reduced allodynia in pSNL induced by minocycline is at least in part attributable to the normalized activation of NMDA receptors in the superficial spinal dorsal horn, owing to the preservation of glial glutamate uptake at synaptic levels. It is conceivable that the preservation of glial glutamate uptake by minocycline would also lead to the “normalized” activation of AMPA receptors in neuropathic rats. Further investigation is needed to prove this notion.
Synaptic mechanisms underlying the minocycline-induced prevention of behavioral hypersensitivity induced by tissue inflammation and injury are not fully understood. Minocycline inhibits the activation of glial cells induced by tissue inflammation or injury. It suppresses the production or activities of many bioactive substances that are known to contribute to the development of behavioral hypersensitivity, which include proinflammatory cytokines (Ledeboer et al., 2005; Zanjani et al., 2006), inducible nitric oxide synthase (Wilkins et al., 2004), prostaglandin E2 (Patel et al., 1999), caspase-1 and 3 (Krady et al., 2005), matrix metalloproteinase-2 (MMP2) (Bhatt and Veeranjaneyulu, 2010) and MMP9 (Bhatt and Addepalli, 2010; Ji et al., 2009; Murata et al., 2008). Minocycline also prevents the upregulation of mGlu3 and mGlu5 receptors in the dorsal horn induced by chronic constriction of sciatic nerve (Osikowicz et al., 2009). Some of these inhibitory effects are caused by inhibition of p38 MAP kinase pathways (Hua et al., 2005). In agreement with studies by others in cultured cells (Rothstein et al., 2005), we found that 10 day treatment with minocycline itself did not change the expression of glial GTs in normal control rats. The prevention of downregulation of glial GTs by minocycline in neuropathic rats most likely results from the inhibition of glial activation and the subsequent release of bioactive substances from activated glial cells by minocycline. This notion is consistent with a recent study showing that downregulation of glial GT protein expression in the spinal dorsal horn and behavioral hypersensitivity induced by nerve injury was reversed by the glial modulator propentofylline (Tawfik et al., 2008). Further support is provided by studies of forebrain areas and cultured cells, where reduction of glial glutamate uptake is linked to signaling pathways triggered by substances released by activated glial cells. For example, proinflammatory cytokines (IL-1β and TNFα) reduce glial glutamate uptake through pathways related to the release of nitric oxide (Hu et al., 2000; Ye and Sontheimer, 1996) and activation of NF-κB (Han et al., 2001; Ye and Sontheimer, 1996; Zou and Crews, 2005). Peripheral nerve injury or tissue inflammation results in the activation of glial cells, increases levels of proinflammatory cytokines (Watkins et al., 2001), and activates NF-κB (Tegeder et al., 2004) and nitric oxide synthase (Infante et al., 2007; O'Rielly and Loomis, 2006) in the spinal dorsal horn (Milligan and Watkins, 2009). Therefore, we suggest that the inhibition of glial cell activation by minocycline and the subsequent suppression on both the production of proinflammatory cytokines and the activation of NF-κB and nitric oxide synthase result in the preservation of glial GT expression and function in the spinal dorsal horn.
Effective glutamate uptake by glial cells depends not only on the number and function of GTs themselves but also on the availability of GTs near the synapse (Oliet et al., 2001). Glial GTs are located on the plasma membrane of the astrocyte processes, which wrap around the synapse (Ye and Sontheimer, 1996). Such microgeographic organization allows astrocytes to rapidly take up glutamate released from neurons and to terminate the effects of released neurotransmitters. The coverage of synaptic structures by astrocytic processes is reduced following the retraction of astrocytic processes and the hypertrophy of astrocytic soma upon astrocytic reactivation (Oliet et al., 2001). Consequently, an abnormal spatiotemporal profile of presynaptic glutamate concentrations occurs because fewer glial GTs are available near the synapse, leading to prolonged EPSC kinetics (Gallo and Chittajallu, 2001; Halassa et al., 2007; Huang and Bergles, 2004; Oliet et al., 2001). Minocycline is known to reduce the reactivation of astrocytes induced by nerve injury (Liu et al., 2009; Mika et al., 2010; Raghavendra et al., 2003), which was also confirmed in this study. Thus, another mechanism by which minocycline treatment causes normalized activation of NMDA receptors may be related to the preservation of the intimate relationship between the synapses and astrocyte processes, which maintains the availability of GTs to presynaptically released glutamate.
In conclusion, we have identified glial GT as one of the functional molecules by which minocycline attenuates behavioral hypersensitivity in neuropathic rats. Minocycline preserves the normalized activation of glutamate receptors and synaptic transmission by maintaining both the expression (number) of glial GTs and the availability of glial GTs to presynaptically released glutamate in the spinal dorsal horn. Our studies further support the idea that glial GTs may be a potential target for the development of analgesics.
ACKNOWLEDGMENTS
This project was supported by the NIH RO1 grant (NS064289) to H.R.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. GLIA. 2000;32:1–14. [PubMed] [Google Scholar]
- Bhatt LK, Addepalli V. Attenuation of diabetic retinopathy by enhanced inhibition of MMP-2 and MMP-9 using aspirin and minocycline in streptozotocin-diabetic rats. Am J Transl Res. 2010;2:181–189. [PMC free article] [PubMed] [Google Scholar]
- Bhatt LK, Veeranjaneyulu A. Minocycline with aspirin: a therapeutic approach in the treatment of diabetic neuropathy. Neurol Sci. 2010 doi: 10.1007/s10072-010-0243-3. [DOI] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res. 2008;1229:100–110. doi: 10.1016/j.brainres.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata JP, Weng H-R, Dougherty PM. Cyclooxygenase inhibitors and thalidomide ameliorate vincristine-induced hyperalgesia in rats. Cancer Chemother Pharmacol. 2004;54:391–397. doi: 10.1007/s00280-004-0809-y. [DOI] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010 doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, McCarthy KD. Sorting out Astrocyte Physiology from Pharmacology. Annu Rev Pharmacol Toxicol. 2008 doi: 10.1146/annurev.pharmtox.011008.145602. [DOI] [PubMed] [Google Scholar]
- Galik J, Youn DH, Kolaj M, Randic M. Involvement of group I metabotropic glutamate receptors and glutamate transporters in the slow excitatory synaptic transmission in the spinal cord dorsal horn. Neuroscience. 2008;154:1372–1387. doi: 10.1016/j.neuroscience.2008.04.059. [DOI] [PubMed] [Google Scholar]
- Gallo V, Chittajallu R. Neuroscience. Unwrapping glial cells from the synapse: what lies inside? Science. 2001;292:872–873. doi: 10.1126/science.1060854. [DOI] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Upregulation of Group I metabotropic glutamate receptors in neurons and astrocytes in the dorsal horn following spinal cord injury. Exp Neurol. 2005;195:236–243. doi: 10.1016/j.expneurol.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001;108:425–435. doi: 10.1172/JCI12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- Hu Y, Li W, Lu L, Cai J, Xian X, Zhang M, Li Q, Li L. An anti-nociceptive role for ceftriaxone in chronic neuropathic pain in rats. Pain. 2010;148:284–301. doi: 10.1016/j.pain.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Murase K. Glial nitric oxide-mediated long-term presynaptic facilitation revealed by optical imaging in rat spinal dorsal horn. J Neurosci. 2004;24:9888–9896. doi: 10.1523/JNEUROSCI.2608-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Tsuda M, Inoue K, Murase K. Long-term potentiation of neuronal excitation by neuron-glia interactions in the rat spinal dorsal horn. Eur J Neurosci. 2007;25:1297–1306. doi: 10.1111/j.1460-9568.2007.05386.x. [DOI] [PubMed] [Google Scholar]
- Infante C, Diaz M, Hernandez A, Constandil L, Pelissier T. Expression of nitric oxide synthase isoforms in the dorsal horn of monoarthritic rats: effects of competitive and uncompetitive N-methyl-D-aspartate antagonists. Arthritis Res Ther. 2007;9:R53. doi: 10.1186/ar2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, Koizumi S. ATP- and adenosine-mediated signaling in the central nervous system: Chronic pain and microglia: Involvement of the ATP receptor P2X4 . J Pharmacol Sci. 2004;94:112–114. doi: 10.1254/jphs.94.112. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci U S A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Wang XY, Lo EH. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci. 2009;30:336–340. doi: 10.1016/j.tips.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P. The Time Course of Signaling at Central Glutamatergic Synapses. News Physiol Sci. 2000;15:83–89. doi: 10.1152/physiologyonline.2000.15.2.83. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr., Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lim G, Wang S, Mao J. cAMP and protein kinase A contribute to the downregulation of spinal glutamate transporters after chronic morphine. Neurosci Lett. 2005;376:9–13. doi: 10.1016/j.neulet.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Liu XD, Wang JJ, Sun L, Chen LW, Rao ZR, Duan L, Cao R, Wang MQ. Involvement of medullary dorsal horn glial cell activation in mediation of masseter mechanical allodynia induced by experimental tooth movement. Arch Oral Biol. 2009;54:1143–1150. doi: 10.1016/j.archoralbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, Colmers WF, Dawbarn D, Ballanyi K, Smith PA. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587:1013–1032. doi: 10.1113/jphysiol.2008.166306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kawamoto A, Yatani Y, Shirakawa H, Nakagawa T, Kaneko S. Gene transfer of GLT-1, a glial glutamate transporter, into the spinal cord by recombinant adenovirus attenuates inflammatory and neuropathic pain in rats. Mol Pain. 2008;4:65. doi: 10.1186/1744-8069-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika J, Rojewska E, Makuch W, Przewlocka B. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience. 2010;165:1420–1428. doi: 10.1016/j.neuroscience.2009.11.064. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Tsuzuki K, Ling JX, Sonobe H, Gu JG. Distinct roles of P2X receptors in modulatiing glutamate release at different primary sensory synapses in rat spinal cord. J Neurophysiol. 2003;89:3243–3252. doi: 10.1152/jn.01172.2002. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos Trans R Soc Lond B Biol Sci. 2003;358:721–726. doi: 10.1098/rstb.2002.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Weng HR. Glutamate Transporters Prevent Excessive Activation of NMDA Receptors and Extrasynaptic Glutamate Spillover in the Spinal Dorsal Horn. J Neurophysiol. 2009;101:2041–2051. doi: 10.1152/jn.91138.2008. [DOI] [PubMed] [Google Scholar]
- Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. J Neurophysiol. 2010;103:2570–2580. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zhang H, Weng HR. Bidirectional neuron-glia interactions triggered by deficiency of glutamate uptake at spinal sensory synapses. J Neurophysiol. 2010 doi: 10.1152/jn.00282.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell R, Molon-Noblot S, Laroque P, Rigby M, Smith D. The ultrastructural localisation of the N-methyl-D-aspartate NR2B receptor subunit in rat lumbar spinal cord. Neurosci Lett. 2004;371:24–29. doi: 10.1016/j.neulet.2004.08.082. [DOI] [PubMed] [Google Scholar]
- O'Rielly DD, Loomis CW. Increased expression of cyclooxygenase and nitric oxide isoforms, and exaggerated sensitivity to prostaglandin E2, in the rat lumbar spinal cord 3 days after L5-L6 spinal nerve ligation. Anesthesiology. 2006;104:328–337. doi: 10.1097/00000542-200602000-00019. [DOI] [PubMed] [Google Scholar]
- Oliet SHR, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–929. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Osikowicz M, Skup M, Mika J, Makuch W, Czarkowska-Bauch J, Przewlocka B. Glial inhibitors influence the mRNA and protein levels of mGlu2/3, 5 and 7 receptors and potentiate the analgesic effects of their ligands in a mouse model of neuropathic pain. Pain. 2009;147:175–186. doi: 10.1016/j.pain.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Patel RN, Attur MG, Dave MN, Patel IV, Stuchin SA, Abramson SB, Amin AR. A novel mechanism of action of chemically modified tetracyclines: inhibition of COX-2-mediated prostaglandin E2 production. J Immunol. 1999;163:3459–3467. [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes HM, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Song JH, Park ES, Han SM, Han SR, Ahn DK, Youn DH. Signal transduction mechanisms underlying group I mGluR-mediated increase in frequency and amplitude of spontaneous EPSCs in the spinal trigeminal subnucleus oralis of the rat. Mol Pain. 2009;5:50. doi: 10.1186/1744-8069-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B, Wang S, Zhou B, Lim G, Yang L, Zeng Q, Lim JA, Wang JD, Kang JX, Mao J. Altered spinal arachidonic acid turnover after peripheral nerve injury regulates regional glutamate concentration and neuropathic pain behaviors in rats. Pain. 2007;131:121–131. doi: 10.1016/j.pain.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YH, Wang YH, Tsai RY, Wang JJ, Tao PL, Liu TM, Wang YC, Wong CS. Amitriptyline preserves morphine's antinociceptive effect by regulating the glutamate transporter GLAST and GLT-1 trafficking and excitatory amino acids concentration in morphine-tolerant rats. Pain. 2007;129:343–354. doi: 10.1016/j.pain.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Regan MR, Haenggeli C, Lacroix-Fralish ML, Nutile-McMenemy N, Perez N, Rothstein JD, DeLeo JA. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience. 2008;152:1086–1092. doi: 10.1016/j.neuroscience.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Niederberger E, Schmidt R, Kunz S, Guhring H, Ritzeler O, Michaelis M, Geisslinger G. Specific Inhibition of IkappaB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats. J Neurosci. 2004;24:1637–1645. doi: 10.1523/JNEUROSCI.3118-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson LM, Zeng J, Terman GW. Differential effect of glutamate transporter inhibition on EPSCs in the morphine naive and morphine tolerant neonatal spinal cord slice. Neurosci Lett. 2006;407:64–69. doi: 10.1016/j.neulet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Trussell L. Control of time course of glutamatergic synaptic currents. Prog Brain Res. 1998;116:59–69. doi: 10.1016/s0079-6123(08)60430-6. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Weng HR, Aravindan N, Cata JP, Chen JH, Shaw AD, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci Lett. 2005;386:18–22. doi: 10.1016/j.neulet.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience. 2006;138:1351–1360. doi: 10.1016/j.neuroscience.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Pan ZZ, Nie H. Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons. Neuroscience. 2007;149:898–907. doi: 10.1016/j.neuroscience.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Weng H-R, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003;103:131–138. doi: 10.1016/s0304-3959(02)00445-1. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Nikodemova M, Compston A, Duncan I. Minocycline attenuates nitric oxide-mediated neuronal and axonal destruction in vitro. Neuron Glia Biol. 2004;1:297–305. doi: 10.1017/S1740925X05000104. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Zhao MG, Toyoda H, Ko S, Zhuo M. Kainate receptor-mediated synaptic transmission in the adult anterior cingulate cortex. J Neurophysiol. 2005 doi: 10.1152/jn.00091.2005. [DOI] [PubMed] [Google Scholar]
- Xin WJ, Weng HR, Dougherty PM. Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation. Mol Pain. 2009;5:15. doi: 10.1186/1744-8069-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport. 1996;7:2181–2185. doi: 10.1097/00001756-199609020-00025. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]
- Zanjani TM, Sabetkasaei M, Mosaffa N, Manaheji H, Labibi F, Farokhi B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. Eur J Pharmacol. 2006;538:66–72. doi: 10.1016/j.ejphar.2006.03.063. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]