Abstract
Objective
To examine maternal pre-pregnancy (preconception) predictors of birthweight and fetal growth for singleton live births occurring over a 2-year period in a prospective study.
Methods
Data are from a population-based cohort study of 1,420 women who were interviewed at baseline and 2-years later; self-report data and birth records were obtained for incident live births during the followup period. The analytic sample includes 116 singleton births. Baseline preconception maternal health status and health-related behaviors were examined as predictors of birthweight and fetal growth, controlling for prenatal and sociodemographic variables, using multiple regression analysis.
Results
Preconception BMI (overweight or obese) and vegetable consumption (at least one serving per day) had statistically significant independent and positive effects on birthweight and fetal growth. Maternal weight gain during pregnancy, a prenatal variable, was an additional independent predictor of birthweight and fetal growth. Sociodemographic variables were not significant predictors after controlling for preconception and prenatal maternal characteristics.
Conclusions
Findings confirm that preconception maternal health status and health-related behaviors can affect birthweight and fetal growth independent of prenatal and socioeconomic variables. Implications for preconception care are discussed.
Keywords: Preconception health, Birthweight, Body mass index, Nutrition, Cohort study
To reduce adverse pregnancy outcomes such as preterm birth and low birthweight, recent recommendations have called for expanded health services and preventive interventions to improve women's health prior to pregnancy [1, 2]. The rationale is that once women are pregnant, it may be too late to address maternal health problems or risks, and many women do not initiate prenatal care before critical phases of fetal development have occurred. Nevertheless, the evidence for associations between pre-pregnancy health indicators and pregnancy outcomes is not extensive, and there have been few field trials of preventive preconception interventions [3].
Most of the research addressing maternal risks for adverse pregnancy outcomes has relied on data from women who are pregnant or postpartum. In such studies, retrospective measures of pre-pregnancy risks may be subject to recall bias, and retrospective data obtained during pregnancy may be influenced by attitudinal or behavioral changes associated with risk reduction after a woman has become pregnant [4, 5]. Little prospective research has enrolled women prior to pregnancy and linked pre-pregnancy health status or health-related behaviors with subsequent pregnancy outcomes. In addition, few existing studies provide assessments of the relative importance of various mutable risk factors in predicting pregnancy outcomes [6]. Better information about risk patterns for adverse pregnancy outcomes prior to pregnancy would be useful for targeting preconception interventions to those at highest risk or to those with specific types of risks.
Linking pre-pregnancy health status and risks with pregnancy experiences and outcomes is consistent with a lifespan perspective on women's health. In this framework, the cumulative effects of health and life experiences are viewed as determinants of later reproductive outcomes [7–9]. Thus the period of potential impact on a woman's pregnancy is extended to her entire life prior to that pregnancy, including her socioeconomic origins, childhood, adolescence, and pregnancy history. Unfortunately, few available data sets provide lifecourse measures of women's health and social determinants of health, or even pre-pregnancy maternal health measures that can be linked with pregnancy outcomes.
This paper uses a unique data source—a population-based prospective cohort study conducted as part of the Central Pennsylvania Women's Health Study (CePAWHS)—to examine maternal pre-pregnancy predictors of subsequent birth outcomes for pregnancies occurring over a 2-year follow-up period. The primary hypothesis is that preconceptional maternal health characteristics—including health status and health-related behaviors—will be significantly associated with birthweight and fetal growth, after controlling for prenatal characteristics and sociodemographics.
Methods
Study Design and Sample
A population-based cohort study of reproductive-age women was conducted in a 28-county region of Central Pennsylvania. This region was chosen because it is diverse with respect to socioeconomic status and includes urban as well as rural and semi-rural locations. The study was conducted in accord with prevailing ethical principles and was approved by the Institutional Review Board of the Penn State College of Medicine.
The baseline random-digit-dial (RDD) telephone survey was conducted between September 2004 and March 2005 on a representative sample of 2,002 English- and Spanish-speaking women ages 18−45. The purpose of the survey was to provide estimates of the prevalence of risk factors for adverse pregnancy outcomes such as preterm birth and low birthweight in the general population; to identify subpopulations at greatest risk; and to provide a baseline for longitudinal follow-up. The RDD sample was highly representative of the target population with respect to age, race/ethnicity, educational level, and poverty status. Details of the sampling strategy, response rate, and sample representativeness have been previously published [10]. The 30-minute interview included questions about physical and mental health status, pregnancy history, health-related behaviors, stress and exposures, health care access, and sociodemographics. At the end of the interview, women were asked to consent to follow-up contacts, and 90% did so.
Those consenting to follow-up were re-contacted for a second telephone interview at the 2-year anniversary of their baseline interview, with a 79% response rate (n = 1,420). The main reason for non-response was failure to locate women who had changed residences after the baseline survey; only 5% refused the interview. Participants in the follow-up survey were compared with non-participants, and significant response bias was found according to most sociodemographic variables, in expected directions. Women who were older at baseline (ages 35−45), college-educated, married or cohabitating, not in poverty, and non-Hispanic white were more likely to participate in the follow-up survey. There was no significant difference in response by residence along the rural-urban continuum.
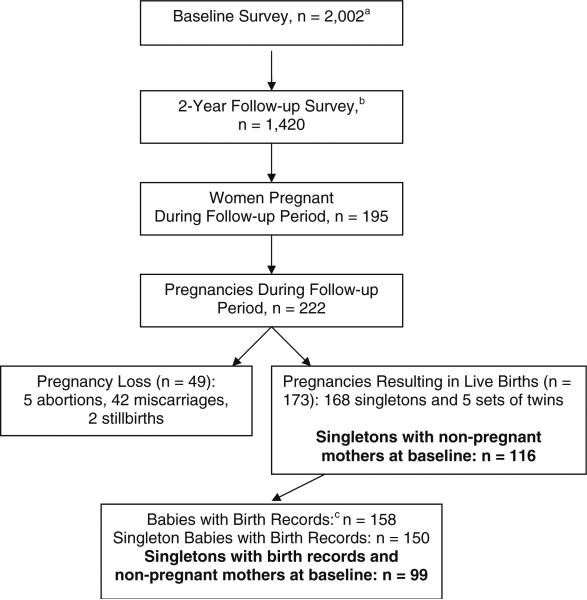
In the follow-up survey, 195 women reported at least one pregnancy during the 2-year follow-up period (6.9% annual pregnancy rate), for a total of 222 pregnancies; women who were pregnant at the time of the follow-up interview were re-contacted to ascertain the outcome of the pregnancy, so the outcomes of all pregnancies that began during the follow-up period are known. (See Fig. 1.) Twenty-two percent of these pregnancies resulted in fetal loss (abortion, miscarriage, stillbirth), and 78% resulted in live births, for a total of 178 babies, including 169 singletons and five sets of twins. The percentage of live births in our sample is higher than the percentage of live births for pregnancies in women ages 18 and over nationally (65%), based on data from the National Survey of Family Growth for 1990−2004 [11].
Fig. 1.
Study sample. a 90% of baseline respondents consented to followup contact. b Participation rate = 79% of those consenting at baseline to followup contact (see text). c 16 mothers declined consent to access Pennsylvania birth certificate records. Birth records could not be matched for two of the births
For this analysis, we excluded twins because of the well-established increased risk of lower birthweight and gestational age for multiples. We also excluded 52 babies whose mothers were pregnant at the time of the baseline interview; these exclusions were necessary because the mother's baseline characteristics were not “preconceptional.” Thus, the analytic sample includes 116 singleton births for which self-reported longitudinal survey data were obtained, including pre-pregnancy measures.
We obtained consent from 90% of the women who gave birth to access electronic birth records from the Pennsylvania Department of Health, and birth record matching was successful for all but two of the births (n = 99 birth records). The birth records allow us to confirm self-reported gestational age and birthweight.
Measures
Dependent Variables
For incident pregnancies occurring during the followup period, measures of gestational age and birthweight were obtained both in the follow-up survey through self-report and from the birth records. Here we analyze the determinants of birthweight (converted to grams) and of birth-weight adjusted for gestational age, an indicator of fetal growth. Adverse categorical outcomes such as preterm birth (<37 completed weeks gestation), low birthweight (<2,500 g), macrosomia (>4,000 g), abnormal conditions of the newborn, and abnormal Apgar scores did not occur with sufficient frequency for analysis.
Preconception Predictors
The primary predictors are pre-pregnancy measures of maternal health status and specific health-related behaviors expected to be predictive of birthweight. These variables were measured in the baseline survey. A single-item measure of perceived health status from the SF-12 Health Survey (“In general, would you say your health is excellent, very good, good, fair or poor?”) assessed overall health status [12]; this is a widely used indicator of overall health status. It is scored so that a higher score indicates higher health status: 31% rated their health as excellent, 41% as very good, 27% as good, and 1% as fair or poor, for a mean score of 4.03. Because the scores are skewed, the measure was dichotomized to contrast those with excellent or very good health with all others.
Body mass index (BMI) was computed from self-reported height and weight at baseline (weight in kilograms divided by height in meters squared); the mean BMI was 25.6, with a standard deviation of 5.4 (BMI values ranged from 17.5 to 54.1). For analysis, BMI is categorized to contrast the overweight (BMI = 25−29.9) and obese (BMI ≥ 30) with the underweight (BMI < 18.5, n = 1) and normal (BMI = 18.5−24.9). Psychosocial stress was measured using the 12-item Psychosocial Hassles Scale that assessed the degree to which common hassles (such as money worries, problems with friends) were perceived as stressful (on a 4-point scale ranging from “no stress” to “severe stress”) during the past 12 months; the scale was adapted from the Prenatal Psychosocial Profile Hassles Scale, which referred to stress during pregnancy [13], which in turn was adapted from the stress subscale of the Prenatal Psychosocial Profile developed by Curry and colleagues [14]. Because scale scores were highly skewed, they were dichotomized at the median for analysis, with 46% of respondents classified as high stress.
Health-related behaviors included current cigarette smoking (22%); any alcohol use in the past month (54%); daily use of a multivitamin containing folic acid (30%); vegetable consumption (not counting green salad, carrots, or potatoes) at least one serving per day (41%); fruit consumption (not counting fruit juice) at least one serving per day (34%); and physical activity levels during the past month that meet current recommendations of 30 minutes or more of moderate or vigorous intensity on most, if not all, days of the week (22%) [15–17]. All of these behaviors reflect healthy lifestyle and/or have been directly linked with adverse pregnancy outcomes.
Prenatal Variables
Prenatal variables are possible mediators of the effects of preconception variables on birth outcomes. Three maternal health-related behaviors measured preconceptionally (smoking cigarettes, alcohol use, and folic acid supplementation) also were measured prenatally for the incident pregnancy: 6% reported smoking during pregnancy, 4% reported using alcohol, and 79% reported folic acid supplementation. The preconception and prenatal measures were not colinear, and all three behaviors showed a statistically significant tendency to improve between baseline and pregnancy. Because too few women reported using alcohol during pregnancy, only prenatal smoking and folic acid use are examined as possible mediators of the relationship between preconception variables and pregnancy outcomes. An additional mediator considered is maternal weight gain during the index pregnancy. On average, women gained 32.8 pounds (standard deviation = 16.4).
Other Covariates
Sociodemographics of the analytic sample were measured in the baseline interview. Age was measured continuously in years, with an average of 28.9 years in the analytic sample. Marital status was dichotomized to contrast women who are married or cohabitating (88%) with women not currently married or partnered (12%). Educational level, an indicator of socioeconomic status, was dichotomized to contrasting women with high school educations or less (34%) with women with at least some college education or more (66%). Race/ethnicity was dichotomized to contrast non-Hispanic White women (89%) with all others (11%); the “other” category is comprised of 62% African Americans, 23% Hispanic, and 15% other or mixed race. This distribution reflects the population of Central Pennsylvania. We also explored a measure of whether or not the incident birth is the woman's first or higher-order birth because first births are expected to predict lower gestational age and lower birthweight [18, 19]. Among the incident pregnancies, 35% were first births. However, this variable had no relationship with the dependent variables and therefore was dropped from further analyses.
Analyses
Bivariate associations between independent and dependent variables were first examined using ANOVA f-tests, t-tests, and Pearson correlations, as appropriate. Independent variables were examined for multicollinearity. A hierarchical series of ordinary least squares regressions with robust standard errors (using the new SAS version 9.1 proc “glimmix”) were then fit to the data in order to examine the predictive strength of preconceptional, prenatal and demographic characteristics on birth weight outcomes. At each step, the independent variables in the regression were examined for multicollinearity. In addition, sensitivity analyses assessing the robustness of results against influential points and departures from normality assumptions were also conducted.
In the multiple regression analysis of birthweight, all preconception variables were entered in the first step. On the second step, prenatal variables were entered to examine whether they mediated relationships between the preconception variables and the outcomes. On the third step, sociodemographic variables were entered. In the regression analysis of fetal growth (birthweight adjusted for gestational age), only the significant preconception predictors from the birthweight analysis were included to produce a parsimonious model.
Results
Table 1 shows the distribution of gestational age and birthweight for all singleton births occurring during the 2-year follow-up period, by data source. The two sets of measures were highly correlated: Pearson correlations between survey and birth records were 0.93 (P < .0001) for gestational age and 0.96 (P < .0001) for birthweight. Accordingly, and because the sample size for the survey is larger than the subsample with birth record data, only results using the survey data are presented here.
Table 1.
Gestational age and birthweight, by data source, for singleton births
| Surveya (n = 116 singletons) | Birth recordb (n = 99 singletons) | |
|---|---|---|
| Gestational age, in weeks | ||
| Mean | 38.93 | 38.83 |
| Standard deviation | 2.64 | 2.37 |
| Range | 25.00−42.00 | 25.00−41.00 |
| Birthweight, in grams | ||
| Mean | 3391.10 | 3351.28 |
| Standard deviation | 674.78 | 649.70 |
| Range | 652.04−5414.76 | 660.49−5421.00 |
Based on mother's self-report in the follow-up survey
Based on Pennsylvania birth record
Table 2 shows the bivariate associations between the preconception variables and birthweight as reported in the survey. Preconception BMI is significantly associated with birthweight, with overweight and obese women having higher birthweight babies than normal weight women. Consuming at least one serving of vegetables daily also was significantly associated with higher birthweight. There were no other statistically significant bivariate associations.
Table 2.
Bivariate associations between preconception variables and birthweight (n = 115)
| Preconception variables | % (n) | Mean birthweight (SD) | P-valuea |
|---|---|---|---|
| Perceived health status | |||
| Excellent, very good | 72% (84) | 3385.1 (681.5) | 0.8755 |
| Good, fairb | 28% (32) | 3407.4 (667.1) | |
| BMI categories | |||
| Normal or underweight | 52% (60) | 3219.1 (656.8) | 0.0157 |
| Overweight | 33% (38) | 3575.0 (724.2) | |
| Obese | 15% (18) | 3587.0 (456.7) | |
| Psychosocial Hassles scale | |||
| Low stress | 54% (63) | 3457.3 (694.1) | 0.2571 |
| High stress | 46% (53) | 3313.7 (649.4) | |
| Smokes cigarettes | |||
| Yes | 22% (25) | 3203.5 (664.9) | 0.1165 |
| No | 78% (91) | 3443.2 (671.9) | |
| Uses alcohol | |||
| Yes | 54% (62) | 3380.9 (589.7) | 0.7199 |
| No | 46% (52) | 3428.1 (768.6) | |
| Uses daily multivitamin with folic acid | |||
| Yes | 30% (35) | 3443.6 (571.7) | 0.5661 |
| No | 70% (81) | 3369.0 (715.8) | |
| Consumes vegetables at least once/day | |||
| Yes | 41% (48) | 3586.8 (544.2) | 0.0054 |
| No | 59% (68) | 3250.9 (726.5) | |
| Consumes fruit at least once/day | |||
| Yes | 34% (39) | 3389.3 (694.3) | 0.9838 |
| No | 66% (77) | 3392.0 (669.5) | |
| Physical activity level meets recommendationsc | |||
| Yes | 22% (25) | 3533.5 (730.4) | 0.2346 |
| No | 78% (91) | 3351.5 (657.3) | |
SD standard deviation
One case had missing birthweight
Based on ANOVA f-test for BMI categories and t-tests for all other variables
No respondents reported “poor” health status
Thirty minutes or more of moderate or vigorous physical activity on most, if not all, days of the week (see text)
Table 3 shows the results of multiple regression analysis of birthweight. In step one, in which the preconception variables are entered, BMI and vegetable consumption were significantly (P < 0.05) and positively associated with birthweight. These effects persist in steps 2 and 3, in which the only other significant predictor is weight gain during pregnancy (positively associated with birthweight). The interaction between preconception BMI and weight gain during pregnancy was tested but did not have a significant effect on birthweight.
Table 3.
Multiple regression analysis of birthweight (regression coefficients and 95% confidence intervals; base n = 115)
| Step 1 | Step 2 | Step 3 | |
|---|---|---|---|
| Preconception variables | |||
| Perceived health status (high) | −86.3 (−388.4,215.8) | −19.5 (−311.7, 272.9) | −29.8 (−370.3, 310.7) |
| BMI (overweight/obese) | 327.8 (73.3, 582.4) | 400.1 (138.5, 661.6) | 370.9 (89.4, 652.4) |
| Psychosocial Hassles (high) | −155.7 (−399.4, 87.9) | −138.7 (−370.0, 92.7) | −106.3 (−357.0, 144.4) |
| Smokes cigarettes | −152.2 (−479.1,174.8) | −135.4 (−519.6, 248.7) | −141.9 (−569.3, 285.5) |
| Uses alcohol | −14.5 (−266.1, 237.0) | −89.9 (−315.6, 135.8) | −76.5 (−320.7, 167.7) |
| Uses daily multivitamin, folic acid | 98.3 (−169.5, 366.0) | 42.8 (−218.0, 303.6) | 33.3 (−231.5, 298.1) |
| Consumes vegetables daily | 295.0 (24.5, 565.5) | 351.7 (80.4, 622.9) | 345.6 (37.6, 653.6) |
| Consumes fruit daily | −147.9 (−493.0, 197.1) | −152.9 (−484.8, 179.0) | −165.0 (−538.8, 208.8) |
| Physical activity level meets recommendations | 261.8 (−70.0, 593.6) | 292.2 (−48.4, 632.7) | 319.5 (−30.3, 669.4) |
| Prenatal variables | |||
| Smokes cigarettes | −225.4 (−898.2, 447.5) | −217.9 (−859.2, 423.5) | |
| Uses daily multivitamin, folic acid | 167.6 (−211.3, 546.5) | 130.6 (−270.6, 531.7) | |
| Pregnancy weight gain (pounds) | 15.8 (7.1, 24.4) | 15.7 (6.6, 24.8) | |
| Sociodemographics | |||
| Age (years) | 14.5 (−11.6, 40.7) | ||
| Marital status (married/cohabitating) | −41.7 (−461.9, 378.5) | ||
| Education (some college or more) | 58.2 (−200.5, 316.9) | ||
| White, non-Hispanic | −46.6 (−626.8, 533.5) | ||
| Adjusted R2 | 0.10 | 0.23 | 0.21 |
Predictors with a small fraction of missing values (alcohol use at baseline and both folic acid use variables) were imputed by the mean values. Significant coefficients (P < 0.05) are in bold
Table 4 examines the effects of BMI and vegetable consumption on birthweight adjusted for gestational age (fetal growth), controlling for prenatal variables. The other preconception variables and all of the sociodemographic variables are dropped from this model because they neither contributed independently to the outcomes nor appeared to confound or mediate the effects of the significant preconception variables (BMI and vegetable intake). Results confirm significant independent effects of BMI and vegetable consumption on fetal growth. Among the prenatal variables, prenatal weight gain also is significant. As in the model of birthweight, the interaction between BMI and weight gain during pregnancy was not significant.
Table 4.
Multiple regression analysis of fetal growth: significant preconception predictors controlling for prenatal variables (n = 115)
| Regression coefficients | 95% Confidence intervals | |
|---|---|---|
| Preconception variables | ||
| BMI (overweight/obese) | 259.6 | (52.6, 466.7) |
| Consumes vegetables daily | 250.7 | (39.1, 462.3) |
| Prenatal variables | ||
| Smokes cigarettes | −196.9 | (−701.2, 307.4) |
| Uses daily multivitamin, folic acid | 55.2 | (−200.4, 310.7) |
| Pregnancy weight gain (pounds) | 12.0 | (6.6, 17.4) |
| Adjusted R2 | 0.41 | |
Multiple regression of birthweight adjusting for gestational age. Significant coefficients (P < 0.05) are in bold
Discussion
This study is one of the first to explore preconception predictors of birth outcomes using a population-based prospective data set. For all of the live births in this study, measures of maternal health status and health-related behaviors were obtained from a survey before the woman became pregnant; these measures were analyzed as predictors of birthweight and fetal growth for pregnancies that occurred during a 2-year follow-up period. The findings indicate that two preconception variables, maternal BMI and consumption of at least one serving per day of vegetables, had statistically significant and sizable independent effects on birthweight and fetal growth, controlling for prenatal variables and sociodemographics. Among the prenatal variables, maternal weight gain also predicted birthweight and fetal growth. None of the sociodemographic variables had significant effects on birthweight or fetal growth after controlling for preconception and prenatal maternal characteristics.
Previous research has found a relationship between pre-pregnancy BMI and birthweight, although not necessarily in longitudinal designs. For example, Frederick and colleagues [20] found that pre-pregnancy BMI was an independent predictor of and positively associated with infant birthweight in the Omega Study, in which pre-pregnancy BMI was based on self-reported weight and height measured retrospectively reported by pregnant women. Consistent with our study, the Omega study found no significant interaction of preconception BMI and maternal weight gain in relation to infant birthweight. Although it is biologically plausible that maternal pre-pregnancy BMI impacts fetal growth, the mechanism by which this occurs is still unknown. Birthweight is likely influenced by a complex interaction of genetics, maternal weight status, nutrition, physical activity, and other factors in need of further investigation.
No prior studies to our knowledge have examined pre-pregnancy dietary measures in relation to pregnancy outcomes. Our finding that preconception vegetable consumption predicts higher birthweight and fetal growth, independent of other maternal health status indicators and health behaviors, is new. Furthermore, the magnitude of the effect is substantial, with women consuming at least one serving of vegetables per day having birth weights on average approximately 350 g larger. In our sample, women who consumed at least one serving of vegetables a day also tended to consume fruit daily, to engage in regular physical activity, to have at least some college education, and to be non-Hispanic white. Vegetable consumption was not associated with other variables in this analysis, including preconceptional BMI and pregnancy weight gain. Thus, vegetable consumption could be a marker for other healthy behaviors, or it could reflect greater health awareness or an unmeasured psychosocial factor. Alternatively, the nutritional benefits of vegetables other than green salad, carrots, and potatoes could be directly impacting birthweight and fetal growth. This finding should be explored further in future research.
The implications of these findings for preconception care are important, particularly in light of recommendations to improve preconception care [1]. Preventing low birthweight should include attention to women's nutritional habits prior to pregnancy, including vegetable consumption. Nutritional information and food preparation techniques for vegetables could be incorporated into educational materials and public health messages. In addition, weight loss or weight maintenance prior to pregnancy may be needed to assure that women are not overweight or obese when they become pregnant. Although we found that overweight/obese weight status was associated with higher birthweight and greater fetal growth, high pre-pregnancy BMI is nevertheless of concern due to the elevated risk of macrosomia (infant birth-weight > 4,000 g), polycystic ovary syndrome, and other related pregnancy complications such as gestational diabetes, preeclampsia, and higher rate of cesarean section. In our sample, for example, 12 infants were born with macrosomia, ten of whom were born to women who were overweight or obese prior to pregnancy. Preconceptional women who are overweight or obese therefore require information about BMI, nutrition, physical activity, stress management, and other factors likely to be associated with their weight status and with the likelihood of effective weight reduction.
The major limitation of this study is the small sample of preconceptional women that yielded live births for this analysis. Despite the strength of the longitudinal design, the number of incident singleton births observed over the 2-year follow-up period was limited by the baseline sample size. Another limitation is possible response bias in the longitudinal survey affecting the variables of interest, for example, fewer birthweights in the low birthweight range. The main implication for future research is that larger prospective studies are needed that follow women from preconception through delivery.
The findings of this prospective study confirm that preconception maternal health status and health-related behaviors can affect birthweight and fetal growth. In particular, maternal BMI and vegetable consumption significantly predict birthweight and fetal growth, after controlling for possible prenatal mediators and sociodemographic variables.
Acknowledgments
This project was funded, in part, under grant number 4100020719 with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The Penn State Survey Research Center conducted the telephone surveys. The Pennsylvania Department of Health provided the birth records.
Contributor Information
Carol S. Weisman, College of Medicine, Pennsylvania State University, 600 Centerview Drive, A210, Hershey, PA 17033, USA e-mail: cweisman@psu.edu
Dawn P. Misra, Wayne State University School of Medicine, Detroit, MI, USA
Marianne M. Hillemeier, College of Health and Human Development, Pennsylvania State University, University Park, PA, USA
Danielle Symons Downs, College of Health and Human Development, Pennsylvania State University, University Park, PA, USA.
Cynthia H. Chuang, College of Medicine, Pennsylvania State University, 600 Centerview Drive, A210, Hershey, PA 17033, USA
Fabian T. Camacho, College of Medicine, Pennsylvania State University, 600 Centerview Drive, A210, Hershey, PA 17033, USA
Anne-Marie Dyer, College of Medicine, Pennsylvania State University, 600 Centerview Drive, A210, Hershey, PA 17033, USA.
References
- 1.Centers for Disease Control and Prevention Recommendations to improve preconception health and health care—United States: A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR. 2006;55(RR-6) inclusive page numbers. [PubMed] [Google Scholar]
- 2.Atrash HK, Johnson K, Adams M, Cordero JF, Howse J. Preconception care for improving perinatal outcomes: The time to act. Maternal and Child Health Journal. 2006;10:S3–S11. doi: 10.1007/s10995-006-0100-4. doi:10.1007/s10995-006-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korenbrut CC, Steinberg A, Bender C, Newberry S. Preconception care: A systematic review. Maternal and Child Health Journal. 2002;6:75–88. doi: 10.1023/a:1015460106832. doi:10.1023/A:1015460106832. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JE, Ebrahim S, Floyd L, Atrash H. Prevalence of risk factors for adverse pregnancy outcomes during pregnancy and the preconception period–United States, 2002−2004. Maternal and Child Health Journal. 2006;10:S101–S106. doi: 10.1007/s10995-006-0093-z. doi: 10.1007/s10995-006-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas JS, Fuentes-Afflick E, Stewart AL, Jacskon RA, Dean ML, Brawarsky P, et al. Prepregnancy health status and the risk of preterm delivery. Archives of Pediatrics and Adolescent Medicine. 2005;159:58–63. doi: 10.1001/archpedi.159.1.58. doi:10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Alexander GR. Prematurity at birth: determinants, consequences and geographic variation. In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. National Academies Press; Washington, D.C.: 2006. [Google Scholar]
- 7.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal. 2003;7:13–30. doi: 10.1023/a:1022537516969. doi:10.1023/A:1022537516969. [DOI] [PubMed] [Google Scholar]
- 8.Misra DP, Guyer B, Allston A. Integrated perinatal health framework: A multiple determinants model with a life span approach. American Journal of Preventive Medicine. 2003;25:65–75. doi: 10.1016/s0749-3797(03)00090-4. doi:10.1016/S0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 9.Misra DP, Grason J. Achieving safe motherhood: applying a life course and multiple determinants perinatal health framework in public health. Women's Health Issues. 2006;16:159–175. doi: 10.1016/j.whi.2006.02.006. doi:10.1016/j.whi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Weisman CS, Hillemeier MM, Chase GA, Dyer AM, Baker SA, Feinberg M, et al. Preconceptional health: Risks of adverse pregnancy outcomes by reproductive life stage in the central Pennsylvania women's health study (CePAWHS). Women's Health Issues. 2006;16:216–224. doi: 10.1016/j.whi.2006.01.001. doi:10.1016/j.whi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Ventura SJ, Abma JC, Mosher WD, Henshaw SK. Estimated pregnancy rates by outcome for the United States, 1990−2004. National Vital Statistics Reports. 2008;56(15):1–25. [PubMed] [Google Scholar]
- 12.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to score version 2 of the SF-12 Health Survey. QualityMetric Incorporated; Lincoln, RI: 2002. [Google Scholar]
- 13.Misra D, O'Campo P, Strobino D. Testing a sociomedical model for preterm birth. Paediatric and Perinatal Epidemiology. 2001;15:110–122. doi: 10.1046/j.1365-3016.2001.00333.x. doi:10.1046/j.1365-3016.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 14.Curry MA, Campbell RA, Christian M. Validity and reliability testing of the prenatal psychosocial profile. Research in Nursing and Health. 1994;17:127–135. doi: 10.1002/nur.4770170208. doi:10.1002/nur.4770170208. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists Exercise during pregnancy and the postpartum period (Committee Opinion No 267). Obstetrics and Gynecology. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. doi:10.1016/S0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 16.American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription. 6th ed. Lippincott, Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- 17.United States Department of Health and Human Services 2008 Physical Activity Guidelines for Americans. 2008 Available at: www.health.gov/paguidelines.
- 18.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. doi:10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 19.Wen SW, Smith G, Yang Q, Walker M. Epidemiology of preterm birth and neonatal outcome. Seminars in Fetal and Neonatal Medicine. 2004;9:429–435. doi: 10.1016/j.siny.2004.04.002. doi:10.1016/j.siny.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Frederick IO, Williams MA, Sales AE, Martine DP, Killien M. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Maternal and Child Health Journal. 2008;12:557–567. doi: 10.1007/s10995-007-0276-2. doi:10.1007/s10995-007-0276-2. [DOI] [PubMed] [Google Scholar]