Abstract
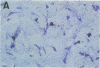
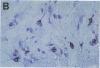
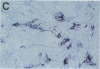
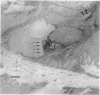
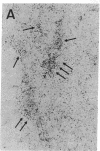
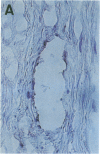
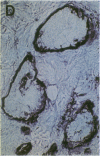
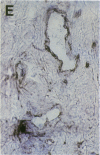
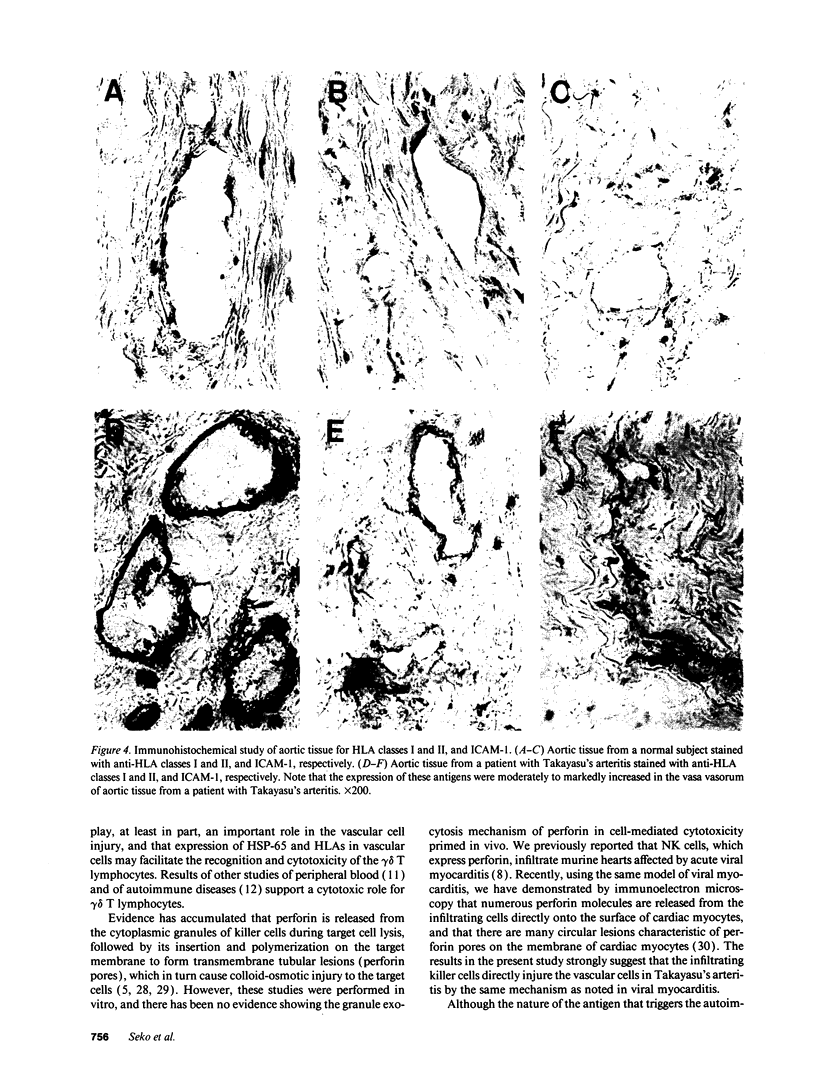
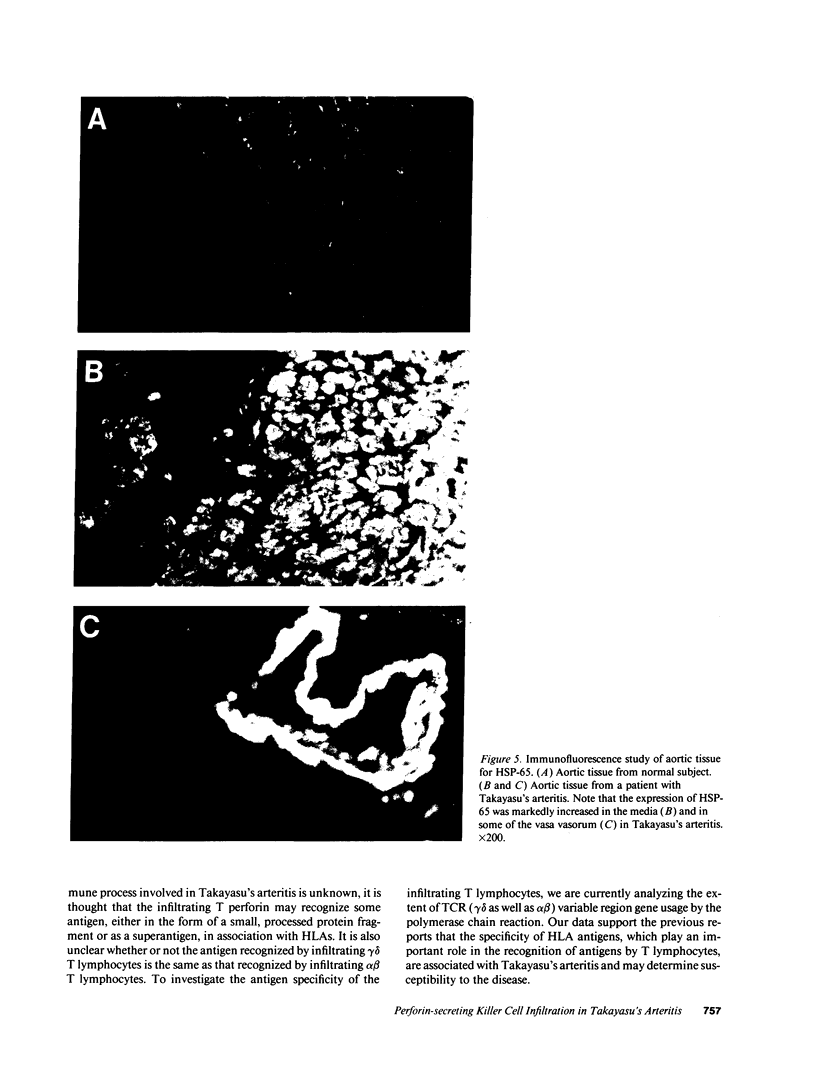
Cell-mediated autoimmunity has been strongly implicated in the pathogenesis of vascular cell injury in Takayasu's arteritis. To clarify the immunological mechanisms involved, we examined the expression of a cytolytic factor, perforin in infiltrating cells of aortic tissue samples from seven patients with Takayasu's arteritis. We also examined the expression of a 65-kD heat-shock protein (HSP-65), human leukocyte antigen classes I and II, and intercellular adhesion molecule-1 in the aortic tissue. Immunohistochemical studies showed that the infiltrating cells mainly consisted of gamma delta T lymphocytes, natural killer cells, macrophages, cytotoxic T lymphocytes and T helper cells, and that perforin was expressed in gamma delta T lymphocytes, natural killer cells, and cytotoxic T lymphocytes. In situ hybridization analysis also revealed expression of perforin mRNA in the infiltrating cells. Immunoelectron microscopic studies demonstrated that the infiltrating cells released massive amounts of perforin directly onto the surface of arterial vascular cells. We also found that expression of HSP-65, human leukocyte antigen classes I and II, and intercellular adhesion molecule-1 was strongly induced in the aortic tissue and might facilitate the recognition, adhesion and cytotoxicity of the infiltrating killer lymphocytes. These findings provide the first direct evidence that the infiltrating cells in the aortic tissue mainly consist of killer cells, and strongly suggest that these killer cells, especially gamma delta T lymphocytes, may recognize HSP-65 and play a critical role in the vascular cell injury of Takayasu's arteritis by releasing perforin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Engel A. G., Ii K., Harper M. C. Polymyositis mediated by T lymphocytes that express the gamma/delta receptor. N Engl J Med. 1991 Mar 28;324(13):877–881. doi: 10.1056/NEJM199103283241303. [DOI] [PubMed] [Google Scholar]
- Kanzaki S., Kanda S. Coombs' antibodies and rheumatoid factors in Takayasu's arteritis. JAMA. 1985 Jul 12;254(2):232–232. [PubMed] [Google Scholar]
- Kawasaki A., Shinkai Y., Kuwana Y., Furuya A., Iigo Y., Hanai N., Itoh S., Yagita H., Okumura K. Perforin, a pore-forming protein detectable by monoclonal antibodies, is a functional marker for killer cells. Int Immunol. 1990;2(7):677–684. doi: 10.1093/intimm/2.7.677. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Shinkai Y., Yagita H., Okumura K. Expression of perforin in murine natural killer cells and cytotoxic T lymphocytes in vivo. Eur J Immunol. 1992 May;22(5):1215–1219. doi: 10.1002/eji.1830220516. [DOI] [PubMed] [Google Scholar]
- Koizumi H., Liu C. C., Zheng L. M., Joag S. V., Bayne N. K., Holoshitz J., Young J. D. Expression of perforin and serine esterases by human gamma/delta T cells. J Exp Med. 1991 Feb 1;173(2):499–502. doi: 10.1084/jem.173.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Robbins E., Springer T. A., Burakoff S. J. LFA-1, LFA-2, and LFA-3 antigens are involved in CTL-target conjugation. J Immunol. 1984 May;132(5):2180–2182. [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- McHugh N. J., James I. E., Plant G. T. Anticardiolipin and antineutrophil antibodies in giant cell arteritis. J Rheumatol. 1990 Jul;17(7):916–922. [PubMed] [Google Scholar]
- Nakata M., Smyth M. J., Norihisa Y., Kawasaki A., Shinkai Y., Okumura K., Yagita H. Constitutive expression of pore-forming protein in peripheral blood gamma/delta T cells: implication for their cytotoxic role in vivo. J Exp Med. 1990 Dec 1;172(6):1877–1880. doi: 10.1084/jem.172.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numano F., Isohisa I., Kishi U., Arita M., Maezawa H. Takayasu's disease in twin sisters. Possible genetic factors. Circulation. 1978 Jul;58(1):173–177. doi: 10.1161/01.cir.58.1.173. [DOI] [PubMed] [Google Scholar]
- Numano F., Isohisa I., Maezawa H., Juji T. HL-A antigens in Takayasu's disease. Am Heart J. 1979 Aug;98(2):153–159. doi: 10.1016/0002-8703(79)90215-1. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Scott D. G., Salmon M., Scott D. L., Blann A., Bacon P. A., Walton K. W., Oakland C. D., Slaney G. F. Takayasu's arteritis: a pathogenetic role for cytotoxic T lymphocytes? Clin Rheumatol. 1986 Dec;5(4):517–522. [PubMed] [Google Scholar]
- Seko Y., Shinkai Y., Kawasaki A., Yagita H., Okumura K., Takaku F., Yazaki Y. Expression of perforin in infiltrating cells in murine hearts with acute myocarditis caused by coxsackievirus B3. Circulation. 1991 Aug;84(2):788–795. doi: 10.1161/01.cir.84.2.788. [DOI] [PubMed] [Google Scholar]
- Seko Y., Shinkai Y., Kawasaki A., Yagita H., Okumura K., Yazaki Y. Evidence of perforin-mediated cardiac myocyte injury in acute murine myocarditis caused by Coxsackie virus B3. J Pathol. 1993 May;170(1):53–58. doi: 10.1002/path.1711700109. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Takio K., Okumura K. Homology of perforin to the ninth component of complement (C9). Nature. 1988 Aug 11;334(6182):525–527. doi: 10.1038/334525a0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Yoshida M. C., Maeda K., Kobata T., Maruyama K., Yodoi J., Yagita H., Okumura K. Molecular cloning and chromosomal assignment of a human perforin (PFP) gene. Immunogenetics. 1989;30(6):452–457. doi: 10.1007/BF02421177. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Matsuki K., Saito Y., Sugimoto T., Juji T. HLA-D region genomic polymorphism associated with Takayasu's arteritis. Angiology. 1990 Jun;41(6):421–426. doi: 10.1177/000331979004100601. [DOI] [PubMed] [Google Scholar]
- Xu Q., Dietrich H., Steiner H. J., Gown A. M., Schoel B., Mikuz G., Kaufmann S. H., Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992 Jul;12(7):789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- Xu Q., Willeit J., Marosi M., Kleindienst R., Oberhollenzer F., Kiechl S., Stulnig T., Luef G., Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993 Jan 30;341(8840):255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- Yagita H., Nakata M., Kawasaki A., Shinkai Y., Okumura K. Role of perforin in lymphocyte-mediated cytolysis. Adv Immunol. 1992;51:215–242. doi: 10.1016/s0065-2776(08)60488-5. [DOI] [PubMed] [Google Scholar]
- Young J. D., Hengartner H., Podack E. R., Cohn Z. A. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986 Mar 28;44(6):849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Young J. D. Killing of target cells by lymphocytes: a mechanistic view. Physiol Rev. 1989 Jan;69(1):250–314. doi: 10.1152/physrev.1989.69.1.250. [DOI] [PubMed] [Google Scholar]
- Young L. H., Klavinskis L. S., Oldstone M. B., Young J. D. In vivo expression of perforin by CD8+ lymphocytes during an acute viral infection. J Exp Med. 1989 Jun 1;169(6):2159–2171. doi: 10.1084/jem.169.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. H., Peterson L. B., Wicker L. S., Persechini P. M., Young J. D. In vivo expression of perforin by CD8+ lymphocytes in autoimmune disease. Studies on spontaneous and adoptively transferred diabetes in nonobese diabetic mice. J Immunol. 1989 Dec 15;143(12):3994–3999. [PubMed] [Google Scholar]