Abstract
Recent experiments point to two predominant forms of fatigue microdamage in bone: linear microcracks (tens to a few hundreds microns in length) and “diffuse damage” (patches of diffuse stain uptake in fatigued bone comprised of clusters of sublamellar-sized cracks). The physiological relevance of diffuse damage in activating bone remodeling is not known. In this study microdamage amount and type were varied to assess whether linear or diffuse microdamage have similar effects on the activation of intracortical resorption. Activation of resorption was correlated to the number of linear microcracks (Cr.Dn) in the bone (R2=0.60, p<0.01). In contrast, there was no activation of resorption in response to diffuse microdamage alone. Furthermore, there was no significant change in osteocyte viability in response to diffuse microdamage, suggesting that osteocyte apoptosis, which is know to activate remodeling at typical linear microcracks in bone, does not result from sublamellar damage. These findings indicate that inability of diffuse microdamage to activate resorption may be due to lack of a focal injury response. Finally, we found that duration of loading does not affect the remodeling response. In conclusion, our data indicate that osteocytes activate resorption in response to linear microcracks but not diffuse microdamage, perhaps due to lack of a focal injury-induced apoptotic response.
INTRODUCTION
Healthy bone has the capability of bearing daily loads and resisting fracture. However, like most composite materials it has a low fracture initiation toughness, implying that it damages easily as a result of wear and tear (48,53). Microscopic cracking or microdamage is the microstructural consequence of fatigue in bone. Unlike engineered materials, bone has an ability to repair microdamage that is essential for the maintenance of its structural integrity and quality. Frost in1960 first hypothesized that microscopic cracks in bone, on the order of 30-100 μm in length, result from fatigue in vivo (32). Moreover, Frost suggested that these microcracks activate bone remodeling as an internal repair process, such that osteoclasts will target and remove the damaged bone and osteoblasts replace the area with new bone. Over the last decade and a half, this hypothesis of “targeted remodeling” has been widely confirmed in a number of animal models, both by inducing microcracks in live bone and studying their repair, and by pharmacologically suppressing remodeling and observing the resulting accumulation of microcracks in vivo. (3,13,15,38,39,51,52,55,56, 65-71, 76,77).
Linear microcracks are just one of several matrix damage types in bone that result from fatigue. Numerous recent studies have described a range of “small-crack”-type damage processes that occur with fatigue (3, 7, 19, 26, 30, 45, 59, 75). These small crack processes produce what has come to be termed “diffuse” matrix microdamage, owing to the diffuse pooling of histological stains seen under the light microscope in such damaged regions. Confocal microscopy studies demonstrate that these diffuse staining regions in fatigued bone are composed of clusters of very small (sublamellar) sized bone matrix cracks, on the order of 1 μm or smaller (7, 26, 75). Typical linear fatigue microcracks lead to degraded bone material properties, with decreases in stiffness, strength and fracture toughness (12, 68, 74, 75). However, intriguing recent work by Diab and Vashishth (21-23) shows that diffuse damage production (and its presence) is a hallmark of bones with improved fracture resistance.
Whether living bone remodeling responds similarly to diffuse damage as it does to linear microcracks, i.e. by activation of bone targeted remodeling, is not known. In studies of rat ulnae fatigue in vivo, Bentolila et al (3) found that diffuse damage removal by intracortical remodeling was significantly less effective than linear microcrack removal, providing intriguing preliminary evidence that the response to diffuse damage and linear microcracks may differ. However, their experiments were not designed to specifically differentiate the biological consequences of diffuse matrix damage and more typical linear microcracks. In the current study we directly tested whether diffuse matrix damage and more typical linear microcracks differ in their ability to activate bone remodeling.
MATERIALS AND METHODS
Experimental approach
To test the hypothesis that there is a differential relationship between microdamage type and activation of resorption, we systematically varied fatigue damage levels in rat ulnae by applying different numbers of loading cycles (1500, 3000, and 4500 cycles) to 3 cohorts of rats. Fatigue-induced losses of bone stiffness were allowed to vary freely in these animals. An additional group of ulnae were loaded to a fixed mechanical degradation level (23% stiffness loss from baseline) that was used in previous studies in order to provide a reference value.
In Vivo Fatigue Loading
In vivo fatigue loading was performed to induce matrix damage in ulnae of adult female Sprague-Dawley rats (283 ± 10 grams, 4-5 months old, Charles River). Under isoflurane anesthesia (1-3%), right ulnae were subjected to fatigue loading in end-load bending via the olecranon and flexed carpus as previously described (3,15,77,78). Briefly, loading was carried out under load control using a closed-loop servo-hydraulic materials testing system (Instron Model 8841, Instron Corp., Canton, MA, USA) at a frequency of 2 Hz, using a mean peak load magnitude of 16N corresponding to 3800±500 μstrain. This approximates the peak physical strains reported in racehorses and military recruits under vigorous physical activity (11, 25, 50, 58). In the first phase of these studies, a group of animals (n=7/group) were fatigue loaded to a single endpoint based on whole bone stiffness loss (23% decrease in secant structural stiffness) to provide an identical reference point to previous studies with this model by our laboratory (3, 15, 76, 77) and others (19, 29, 39, 44). This fatigue level induces bone microcracks, focal osteocyte apoptosis and subsequent targeted intracortical remodeling in rat ulnae (3, 15, 76, 77). Three additional groups of rats (n=22) were fatigue loaded in vivo as described above for fixed numbers of cycles, based on the mean number of cycles needed to reach the fatigue endpoint for animals in the first phase of this study. This allowed us to produce bones that sampled a range of damage patterns and levels throughout the typical fatigue history of these bones in vivo. Accordingly ulnae in the fixed cycle groups were loaded for either 1500, 3000 or 4500 load cycles, respectively. After loading, rats recovered from anesthesia and resumed normal cage activity for 14 days with access to food and water ad libitum. At this point, which reflects the peak intracortical resorption phase in the remodeling response of fatigued rat ulnae, the contralateral ulnae were loaded identically in order to define initial baseline levels of fatigue microdamage within each animal prior to bone remodeling (3). Rats were immediately sacrificed without recovery from anesthesia and both the post-loading bones (“survival bones”) and the contralateral baseline bones (“Non-survival bones”) were harvested. Ulnae from independent, non-loaded control rats were examined as well.
Following euthanasia, forelimbs were immediately removed and bones dissected free of soft tissues. Ulnae were fixed in neutral buffered formalin, stained in basic fuchsin for microdamage assessment (3,10), and embedded undecalcified in polymethylmethacrylate (46). Using a low speed circular diamond saw, cross-sections (150 μm thick) were cut from the ulnar mid-diaphyseal region and polished to 70 μm for fluorescence and confocal microscopy studies. This is the site at which where microdamage concentrates with fatigue loading in these bones (3, 44).
Histomorphometric Analysis
Specimens were analyzed for intracortical remodeling sites (Rs.N, #/mm2) and microdamage. Linear and diffuse microcracks were identified using fluorescence microscopy based on their patterns of staining with basic fuchsin, which cannot penetrate mineralized matrix that is devoid of cavities or microdamage. (7, 10, 32). Confocal microscopy was used to examine damage at higher magnification for qualitative studies and illustrative purposes. Microdamage content was measured from number density of typical linear microcracks (Cr.Dn, #/mm2) and from area fraction occupied by patches of diffuse basic fuchsin staining within the cortex (Diff.dx.Ar/B.Ar, mm2/mm2), following methods detailed elsewhere (3, 7). Briefly,linear microcracks, on the size order of 10's to approximately 100 μm, were distinguished by linear regions of basic fuchsin uptake with sharp boundaries. Diffuse microdamage was visualized as pooled basic fuchsin that formed a “diffuse staining pattern” in regions of fatigued ulnae that did not exhibit this staining pattern in control bones. A “diffuse pattern” was defined as one in which clusters of microcracks were too small to be distinguished from one another (i.e., <1-2 μm). Linear microcracks and diffuse damage regions were measured using a 10 mm × 10 mm eyepiece grid reticule at 400x magnification. Measurements were made by a single observer (BCH) who was blinded to specimen identification, with confirmation by a secondary observer (MBS) on randomly chosen sections.
Osteocyte Integrity
Osteocyte apoptosis has been shown to be a key control point in fatigue-induced bone remodeling (15, 76, 77). However, specific molecular markers of apoptosis could not be studied directly in the thick, undecalcified sections that were needed in this experiment to image diffuse matrix damage. Therefore, we adapted the approach of Bentolila et al (3) and Cardoso et al (15) who used osteocyte morphology as an index of osteocyte integrity. Specifically, Verborgt et al (76) confirmed that the number of osteocytes with pyknotic nuclei correlated strongly with the numbers of apoptotic osteocytes in fatigued ulnae as determined by immunohistochemistry and TUNEL staining. Accordingly, measurements were made as follows: normal osteocytes (fully occupying lacunae, N.Ot/B.Ar, #/mm2) and atypical appearing osteocytes (retracted or pyknotic, At.N.Ot/B.Ar, #/mm2) were counted (Figure 1). For linear microcracks, this area was within ± 80 μm on either side of the linear microcrack. For diffuse microdamage, the area included the entire region of diffuse microdamage and extended to ± 80 μm on either side of the damaged region. The tissue was sampled in the same region of interest (mid-diaphysis) as for the microdamage quantification, and measurements were made using brightfield microscopy at 400X magnification. The measurements were repeated three times using the grid reticule as described above.
Figure 1.
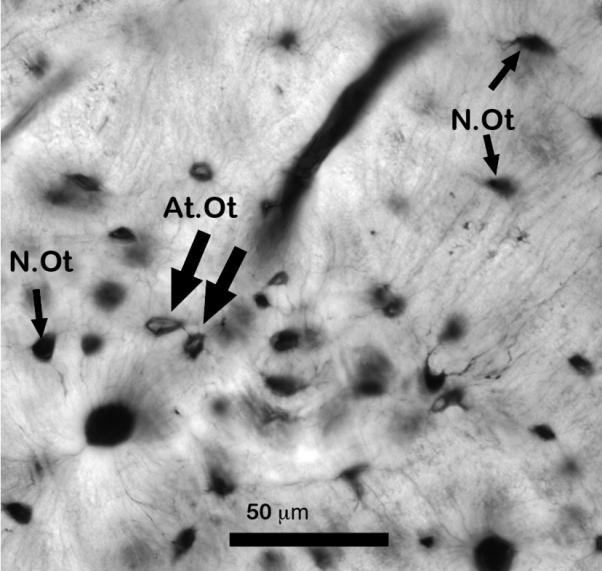
Photomicrograph showing examples of osteocyte integrity at assessed from the thick basic fuchsin stained sections used in this study. Normal osteocytes (N.Ot) fully occupying lacunae and atypical-appearing osteocytes (At.Ot) appears retracted or pyknotic (Scale bar = 50 μm).
Statistical analyses
Two-way ANOVA was used to analyze stiffness loss as a function of both duration of loading and damage type. Multiple comparison tests were performed with the post-hoc Dunn Procedure (GraphPad Instat For MacIntosh, GraphPad Software, San Diego, CA, USA). Plots of resorption number (Rs.N/B.Ar) versus linear (Cr.Dn) or diffuse (Diff.Dx.Ar/B.Ar) damage content were analyzed using regression analysis. χ2 analysis with equations for three-dimensional contingency analysis (82) was used to determine if activation of resorption depended on the number of loading cycles and/or the microdamage type. Osteocyte viability was analyzed using Kruskal-Wallis ANOVA with the post-hoc Dunn Procedure (GraphPad Instat For MacIntosh, GraphPad Software, San Diego, CA, USA).
RESULTS
In Vivo Fatigue
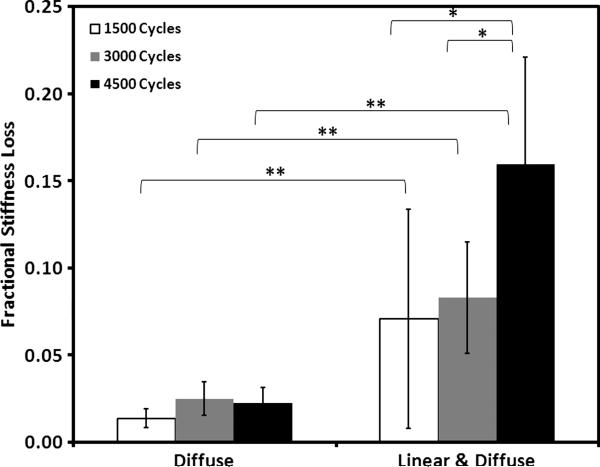
Fatigued, non-survival ulnae containing only diffuse microdamage exhibited low stiffness loss even at high loading cycles (Figure 2 and Table 1). In contrast, fatigued, non-survival ulnae containing both linear microdamage and diffuse microdamage sustained significantly higher stiffness loss at all loading cycles than the fatigued, non-survival ulnae containing diffuse microdamage alone (Figure 2, p<0.005). Moreover, in ulnae exhibiting solely diffuse microdamage, loading duration did not significantly affect stiffness loss (Figure 2, p=0.3).
Figure 2.
Linear microdamage in bone was associated with stiffness loss, as shown by fractional stiffness loss (stiffness after loading divided by initial stiffness) of rat ulnae loaded for 1,500, 3,000 or 4,500 cycles in vivo (rats immediately euthanized after loading). Post-hoc observation of these ulnae allowed us to group them into two cohorts: bones showing only diffuse microdamage and bones showing diffuse and linear microdamage (no specimens were observed with solely linear microdamage). Note that bones with solely diffuse microdamage sustained little stiffness loss despite higher numbers of loading cycles. Stiffness loss was markedly higher in ulnae containing both linear and diffuse microdamage than in ulnae loaded to the same number of cycles but exhibiting only diffuse microdamage. Stiffness loss did not depend on loading cycles for ulnae exhibiting solely diffuse microdamage. In contrast, in ulnae containing both diffuse and linear microdamage, increased numbers of loading cycles tended to increase stiffness loss (*p<0.05, **p<0.005).
Table 1.
Summary data for linear microcracks (Cr.Dn), diffuse microdamaae (Diff.Dx.Ar/B.Ar) content and intracortical resorption space number (Rs.N) for acutely fatigued (Non-survival) and Survival (Fatigue + 14 days) ulnae at each fatigue levels examined.
| Fatigue loading levels | ||||||
|---|---|---|---|---|---|---|
| 4500 Cycles | 3000 Cycles | 1500 Cycles | ||||
| Non-survival | Survival | Non-survival | Survival | Non-survival | Survival | |
| Diff.Dx.Ar/B.Ar (mm2/mm2) | 0.085 ± 0.039 | 0.071 ± 0.029 | 0.054 ± 0.027 | 0.049 ± 0.029 | 0.055 ± 0.030 | 0.051 ± 0.038 |
| Cr.Dn (#/mm2) | 1.97 ± 0.52 | 1.18 ± 0.31* | 1.51 ± 0.43 | 0.95 ± 0.33* | 1.88 ± 0.76* | 1.09 ± 0.41* |
| Rs.N (#/mm2) | 0 | 1.14 ± 0.61 | 0 | 1.16 ± 0.68 | 0 | 1.23 ± 0.52 |
P<0.025 for Non-survival vs. survival ulnae within a loading group
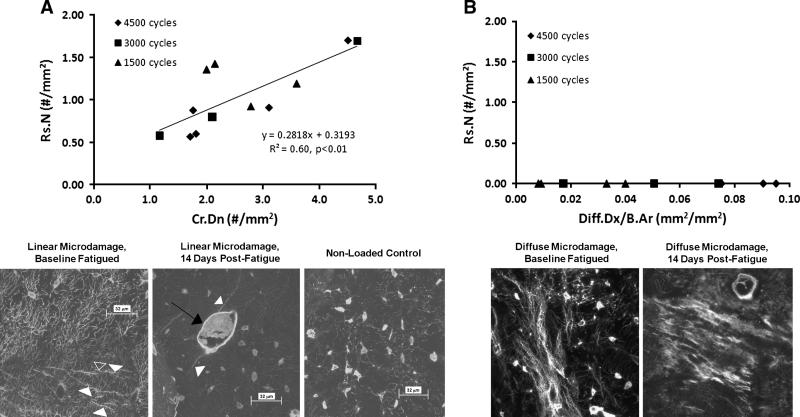
Linear microdamage content and number of resorption spaces were correlated (Figure 3A, r2 = 0.60, p<0.01). In contrast, there was no activation of resorption in the ulnae that had only diffuse microdamage (Figure 3B). Bones that exhibited linear microdamage also had diffuse microdamage. In those bones with both damage types, resorption spaces were absent in diffuse damage regions that did not also contain adjacent linear microcracks. Some ulnae in the high cycle load group (4500 cycles) sustained only diffuse damage, while some in the low (1500 cycle) load group showed both linear microcracks and diffuse damage. However, there was no relationship between activation of resorption and duration of loading; i.e. bones that sustained large numbers of loading cycles with only diffuse damage did not activate intracortical remodeling. Contingency analysis of resorption, linear microdamage, and duration of loading (number of cycles or time) shows that activation of resorption depends on the presence of linear microdamage but not on duration of loading (Overall: χ2 = 25.9, p<0.001; Activation of Resorption Vs. Duration of Loading: χ2 = 2.41, p=0.3; Activation of Resorption Vs. Presence of Linear Microdamage: p<0.001, Fisher's Exact Test).
Figure 3.
Panel A that intracortical remodeling activity (Rs.N) at 14 days after fatigue in vivo was linearly dependent on the amount of linear microdamage in the ulna. Duration of loading did not affect activation of resorption. The confocal photomicrographs below the plot show examples of linear microcracks (left, acute fatigue, non survival), a resorption space at a microcrack (14 days post fatigue loading) and nonloaded control bone. Panel B shows the differential response to diffuse microdamage. Diffuse (sublamellar) microdamage did not evoke an intracortical remodeling response at any level of damage or number of loading cycles. (Scale bars on confocal photomicrographs = 32 μm)
Osteocyte Integrity
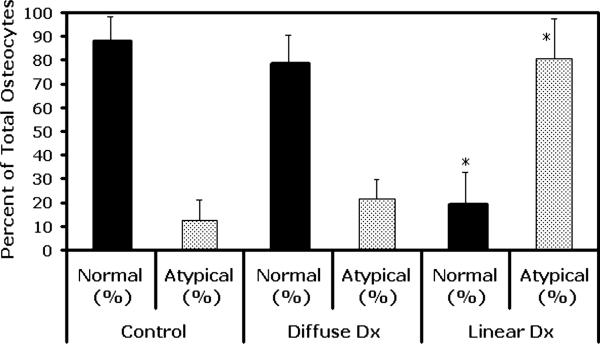
The numbers of normal and pyknotic osteocytes in diffuse microdamage sites were similar to control bones (Figure 4, p=0.23). This was true for both bones that showed only diffuse damage regions (i.e., lower damage levels) and discrete diffuse damage regions in bones that also had linear microcracks (i.e., higher damage levels). In contrast, the number of atypical osteocytes in association with linear microcrack sites was increased dramatically (by more than 600%) over that in control bones (Figure 4, p<0.0001).
Figure 4.
Osteocyte integrity (normal and atypical appearing osteocytes N.Ot/mm2 and At.Ot/mm2, respectively, see text for definition]) in association with different bone microdamage types. There was no significant change of osteocyte integrity in diffuse microdamage regions (p=0.23 vs. non-loaded control bone); however, linear microdamage resulted in a significant loss of normal osteocyte integrity. (* p<0.0001 relative to non-loaded control).
DISCUSSION
The results of this study indicate that linear microcracks provide a physiologically relevant stimulus for bone remodeling, as previously established (3, 15, 55, 55, 76, 77). However, there was no bone remodeling activity associated with diffuse microdamage foci. Moreover, the observed remodeling responses were due to the presence of linear microcracks and not to the cumulative loading history itself; bones that sustained large numbers of loading cycles with only diffuse damage did not activate intracortical remodeling.
Previous studies testing the effects of microdamage on bone remodeling activation did not differentiate between linear and diffuse microdamage. Bentolila et al (3), in the first description of the rat ulnar fatigue model, observed that diffuse damage removal by intracortical resorption was significantly less effective than linear microcrack removal, hinting that the response to diffuse damage and linear microcracks was different. However, because of the high fatigue levels examined in their study, the ulnae possessed both damage types, making it impossible to separate completely the effects of these two damage types. In the current studies we built on the recent observations from our laboratory and others showing that small-crack-type (i.e., diffuse) damage occurs earlier in bone's fatigue loading history than linear microcracks and also in different areas from linear microcracks (3, 7, 21-23, 69, 70, 75). Diffuse damage leads to changes in local material properties. A recent study using probabilitistic failure analysis suggests that diffuse damage can be viewed as occurring when debonding at mineral-collagen interfaces takes place at a large scale (24). If debonding at mineral-collagen interfaces is non-existent or limited in scope relative to the location of the defect, the formation of a linear microcrack would be probabilistically favored based on finite element analysis. Diffuse damage can also be a precursor to linear microdamage (23).
There is inherent variability in fatigue life of rat ulnae resulting from inter-animal differences in diaphyseal cross-sectional geometry. For example the coefficient of variation for cortical bone area in rodent long bones is in the range of 10-15% (42, 64). As area weighted moments of inertia, these small differences in bone geometry will lead to different structural stiffnesses among bones such that stresses applied to the ulnae (and resulting local strains) will differ among animals for the same applied load magnitude. This mechanical variability is typical of load controlled structural tests in engineering and biomechanics, where structures are similar but not identical. By taking advantage of this inherent variation and examining ulnae loaded to varying numbers of cycles, rather than to a specific level of mechanical degradation as in previous studies, we were able to generate bones that contained only sublamellar microdamage and also some bones containing combinations of both diffuse matrix damage and typical linear microcracks. This approach also produced bones at the higher loading cycles end of the experiment that developed only diffuse damage, and also some with both damage types. Similarly, some bones at the low cycle end of the experiment had diffuse damage only and others had both types.
This uncoupling of damage types from the number of cycles (or duration of loading) allowed us to directly test in Survival bones the relationships among damage type, bone resorption and the duration of loading (number of cycles or time). Remodeling was not activated in any bone that contained solely diffuse matrix damage regardless of the duration of loading. Moreover, there was no relationship between activation of resorption and duration of loading. Some bones sustained large numbers of loading cycles and developed only diffuse damage, but did not activate intracortical remodeling. Conversely, intracortical remodeling was activated in bones that contained linear microcracks, independent of the number of load cycles.
Typical linear microcracks in bone causes localized osteocyte apoptosis around the crack. This region of apoptosis co-localizes the region of bone that will subsequently undergo osteoclastic resportion (3, 15, 55, 56, 76, 77). Moreover, Cardoso et al recently demonstrated that this osteoctye apoptosis plays a direct, controlling role in the activation and targeting of the focal remodeling-repair response to microdamage that follows fatigue in vivo (15). We found that pharmacological suppression of this osteocyte apoptosis after fatigue completely prevented the activation of intracortical remodeling. The results of the current study indicate that diffuse matrix damage does not alter osteocyte viability, nor does it activate focal bone remodeling activities to remove and replace the damaged region. Thus, it seem reasonable to posit that the absence of osteocyte apoptosis in zones of diffuse matrix damage may explain why diffuse damage does not evoke a targeted remodeling response seen with other bone microdamage.
In the current studies, we examined osteocyte apoptosis and intracortical remodeling in the ulnar cortex at only a single time period (14 days) after fatigue. Whether osteocytes in diffuse damage zones might die much later than we have examined in this study is certainly possible and awaits future studies. However, previous studies have shown that at linear microcrack sites, osteocyte apoptosis occurs very rapidly, within 1-2 days, after loading. Thus the absence of osteocyte death in diffuse damage zones at 2 weeks after loading strongly argues that the response of osteocytes to diffuse damage is different from the response to linear microcracks. The absence of any intracortical resorption in diffuse damage zones at 2 weeks after loading – the timing for peak resorption at linear microcracks – reinforces the argument that bone's response of to diffuse damage is fundamentally different from its response to linear microcracks
The long-term fate of this diffuse microdamage in living bone remains unclear. Without the associated local osteocyte apoptosis, diffusely damaged matrix does not appear to be specifically targeted for remodeling and removal. One possibility is that it may remain substantially unresolved or unrepaired over time. However, Bentolila et al (3) reported a small, non-significant reduction of diffuse damage in fatigue loaded rat ulnae by two weeks after loading, compared to a multifold increase in resorption of linear microcracks that resulted in a 50% reduction in microcrack content within the same time frame. Given that linear microcracks and diffuse damage region were co-localized at the higher fatigue level examined in the Bentolila study, the diffuse damage could have been removed incidentally, and not particularly effectively, by the other targeted remodeling sites nearby. An intriguing, alternative speculation is that diffuse microdamage may be dealt with in an entirely different and hitherto largely unappreciated manner - perhaps by a direct matrix repair mechanism effected through the osteocytes (8). Tissue repair by remineralization occurs routinely in enamel, where mineral damage from wear or acid is reversed by a physico-chemical process dependent on the pH, protein and ionic composition of saliva (1, 14, 20, 36, 61, 72). In bone, these micro-environmental properties would be directly dependent on the behavior of the osteocytes. Furthermore, osteocytes have been shown to produce a range of matrix proteins and regulators of bone mineralization that might also participate in direct matrix repair (4-6, 27, 33, 35, 37, 40, 41, 43, 54, 79, 80). Given the extensive infiltration of osteocyte process and canalculi thought the bone tissue, no area of the bone matrix is located farther than about 1 μm from an osteocyte process (78), thus, providing a close spatial relationship between cells and matrix that could enable osteocytes to repair sublamellar size cracks in bone through local biochemical processes.
The reason why diffuse damage does not cause osteocyte apoptosis is currently unknown. However, several recent studies provide intriguing insights. Tami et al showed that linear microcracks impair local canalicular fluid transport between osteocytes (71) and this loss of canalicular fluid transport due to linear microdamage may lead to osteocyte apoptosis by causing hypoxic stress on cells (38). In addition, linear microcracks that directly transect osteocytes likely cause necrosis of those few cells involved. This may drive apoptosis of adjacent osteocytes, analogous to other focal injury systems, such as focal heart or brain ischemia and also focal trauma, where the small core of necrotic cells at the center of the injury induces a much larger zone of apoptosis in neighboring cells (2, 17, 18, 28, 34, 62). We speculate that sublamellar (diffuse) damage does not similarly impair the effectiveness of local fluid and solute transport to and from osteocytes and thus does not cause metabolic stress on the osteocytes that leads to cell death. Another possibility is that submicron-sized cracks in sublamellar damage may be too small to directly injure osteocytes and activate a focal injury response as the size of sublamellar cracks is on the order of <1 μm, compared to the length of a typical osteocyte cell process (~8-10 μm) and the diameter of an osteocyte (~10-15 μm).
The design of the current study and nature of the animal model itself impose several limitations that will require future studies to resolve. Stiffness loss in ulnae exhibiting solely diffuse microdamage was less than in ulnae exhibiting both diffuse and linear microdamage (9.8 ± 5.8% vs. 20.6 ± 4.4%, respectively), as the linear microcracks appear at a later stage of fatigue than does diffuse damage. It is possible that the relatively greater overall deformations of osteocytes resulting from this lower stiffness in fatigued bone would contribute to cell injury. However, osteocyte integrity was normal in discrete diffuse damage sites in bones that also had linear microcracks in other areas, arguing that the higher tissue deformations resulting from lowered stiffness in fatigue bone did not contribute significantly to cell injury. The nature of the local stresses causing each of these damage types may have also influenced the degree of local osteocyte death and activation of bone remodeling. Typical linear microcracks in this model occur largely in compressive cortices, while diffuse damage is characteristic of tensile regions (3, 7, 23, 75). Whether the chronic compression of osteocytes in one bone damage region differs in its effects from chronic tensile loading in other damage regions is not known and requires further study. As the intracortical remodeling activated by microdamage stimulus originates principally from periosteal and endocortical surfaces, it is possible that the differential response to the microdamage types observed may also be influenced by location of the damage type. The linear microdamage observed in this study was located mostly in the compressively-loaded regions of the ulnar diaphysis (44, 69, 70) nearer to the periosteal surfaces, while the diffuse damage characteristically occurred in tensile-loaded regions of the ulnar cortex and was distributed throughout the cortex. However, in previous canine (9, 51) fatigue studies, both linear microdamage and the associated intracortical remodeling activity were observed away from bone surfaces, indicating that it is unlikely that location of microdamage plays a major role in governing the remodeling response to the overuse stimulus. Lastly, intriguing recent studies by Diab and Vashishth (21-23) show that linear microcracks and diffuse damage have differential biomechanical response/outcomes: diffuse damage is hallmark of bones with better fracture resistance, i.e. unlike linear microcracks diffuse damage allows bones to dissipate energy and postpone fracture. Thus, it seems reasonable to speculate that bone has evolved fundamentally different matrix-level repair responses to deal with different damage types with different mechanical consequences.
In conclusion, these studies demonstrate that bone responds differently to linear microcracks and diffuse damage. Typical linear microcracks affect surrounding osteocytes in a profound manner, such that the surrounding osteocytes undergo apoptosis that in turn provides the tissue focus for the activation and targeting of subsequent intracortical remodeling. In contrast, diffuse microdamage does not induce osteocyte apoptosis nor does it elicit an intracortical remodeling response. Regardless of the mechanisms of cellular injury, or the lack thereof in the case of diffuse damage, the current studies show that diffuse damage after fatigue does not result in osteocyte death, and this appears to be key to explaining why remodeling does not occur in these regions in response to diffuse matrix damage.
Acknowledgments
Research supported through NIH grant AR 41210
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Altenburger MJ, Schirrmeister JF, Lussi A, Klasser M, Hellwig E. In situ fluoride retention and remineralization of incipient carious lesions after the application of different concentrations of fluoride. Eur J Oral Sci. 2009;117(1):58–63. doi: 10.1111/j.1600-0722.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins AL, 3rd, Vallabhajosyula P. Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery. 1998;42(2):352–60. doi: 10.1097/00006123-199802000-00091. [DOI] [PubMed] [Google Scholar]
- 3.Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23:275–81. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 4.Boskey AL. Bone Mineralization. In: Cowin SC, editor. Bone Mechanics Handbook. 2 ed. CRC Press; New York: 2001. [Google Scholar]
- 5.Boskey AL, Wright TM, Blank RD. Collagen and bone strength. J Bone Miner Res. 1999;14:330–5. doi: 10.1359/jbmr.1999.14.3.330. [DOI] [PubMed] [Google Scholar]
- 6.Bonewald LF. Osteocyte biology: its implications for osteoporosis. J Musculoskelet Neuronal Interact. 4:101–4. l2004. [PubMed] [Google Scholar]
- 7.Boyce TM, Fyhrie D, Glotkowski MC, Radin EL, Schaffler MB. Damage type and strain mode associations in human compact bone bending fatigue. J Orthop Res. 1998;16(3):322–9. doi: 10.1002/jor.1100160308. [DOI] [PubMed] [Google Scholar]
- 8.Boyde A. The real response of bone to exercise. J Anat. 2003;203(2):173–89. doi: 10.1046/j.1469-7580.2003.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18:189–200. doi: 10.1016/0021-9290(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 10.Burr DB, Stafford T. Validity of the bulk-staining technique to separate artifactual from in vivo bone microdamage. Clin Orthop. 1990;260:305–8. [PubMed] [Google Scholar]
- 11.Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18:405–10. doi: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 12.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 13.Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- 14.Cai F, Shen P, Walker GD, Reynolds C, Yuan Y, Reynolds EC. Remineralization of enamel subsurface lesions by chewing gum with added calcium. J Dent. 2009;37(10):763–8. doi: 10.1016/j.jdent.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso L, Herman BC, Verborgt O, Laudier DM, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24(4):597–605. doi: 10.1359/JBMR.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter DR. Mechanical loading histories and cortical bone remodeling. Calcif Tissue Int. 1984;36(Suppl 1):S19–24. doi: 10.1007/BF02406129. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Deshmukh M, D'Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Holtzman DM. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101(9):1992–9. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004;6(2):277–87. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- 19.Colopy SA, Benz-Dean J, Barrett JG, Sample SJ, Lu Y, Danova NA, Kalscheur VL, Vanderby R, Markel MD, Muir P. Response of the osteocyte syncytium adjacent to and distant from linear microcracks during adaptation to cyclic fatigue loading. Bone. 2004;35(4):881–91. doi: 10.1016/j.bone.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds EC. Physicochemical characterization of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J Biol Chem. 2005;280(15):15362–9. doi: 10.1074/jbc.M413504200. [DOI] [PubMed] [Google Scholar]
- 21.Diab T, Sit S, Kim D, Rho J, Vashishth D. Age-dependent fatigue behaviour of human cortical bone. Eur J Morphol. 2005;42(1-2):53–9. doi: 10.1080/09243860500095539. [DOI] [PubMed] [Google Scholar]
- 22.Diab T, Condon KW, Burr DB, Vashishth D. Age-related change in the damage morphology of human cortical bone and its role in bone fragility. Bone. 2006;38(3):427–31. doi: 10.1016/j.bone.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Diab T, Vashishth D. Morphology, localization and accumulation of in vivo microdamage in human cortical bone. Bone. 2007;40:612–8. doi: 10.1016/j.bone.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong XN, Guda T, Millwater HR, Wang X. Probabilistic failure analysis of bone using a finite element model of mineral-collagen composites. J Biomech. 2009;42(3):202–9. doi: 10.1016/j.jbiomech.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekenman I, Milgrom C, Finestone A, Begin M, Olin C, Arndt T, Burr D. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30:866–70. doi: 10.1177/03635465020300061801. [DOI] [PubMed] [Google Scholar]
- 26.Fazzalari NL, Kuliwaba JS, Forwood MR. Cancellous bone microdamage in the proximal femur: influence of age and osteoarthritis on damage morphology and regional distribution. Bone. 2002;31(6):697–702. doi: 10.1016/s8756-3282(02)00906-7. [DOI] [PubMed] [Google Scholar]
- 27.Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y. The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res. 2003;82(10):776–80. doi: 10.1177/154405910308201003. [DOI] [PubMed] [Google Scholar]
- 28.Fisher M. The ischemic penumbra: identification, evolution and treatment concepts. Cerebrovasc Dis. 2004;17(Suppl 1):1–6. doi: 10.1159/000074790. [DOI] [PubMed] [Google Scholar]
- 29.Follet H, Li J, Phipps RJ, Hui S, Condon KW, Burr B. Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone. 2007;40(4):1172–7. doi: 10.1016/j.bone.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 30.Frank JD, Ryan M, Kalscheur VL, Ruaux-Mason CP, Hozak RR, Muir P. Aging and accumulation of microdamage in canine bone. Bone. 2002;30(1):201–6. doi: 10.1016/s8756-3282(01)00623-8. [DOI] [PubMed] [Google Scholar]
- 31.Frost HM. The Laws of Bone Structure. Charles C. Thomas; Springfield, Illinois: 1964. [Google Scholar]
- 32.Frost HM. Presence of microscopic cracks in vivo in bone. Henry Ford Hospital Medical Bulletin. 1960;8:25–35. [Google Scholar]
- 33.Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res. 2003;18(5):807–17. doi: 10.1359/jbmr.2003.18.5.807. [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94(4):1621–8. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/ osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278(3):1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 36.Hara AT, Gonzalez-Cabezas C, Creeth J, Zero DT. The effect of human saliva substitutes in an erosion-abrasion cycling model. Eur J Oral Sci. 2008;116(6):552–6. doi: 10.1111/j.1600-0722.2008.00575.x. [DOI] [PubMed] [Google Scholar]
- 37.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003;2(8):552–8. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 38.Herman BC, Laudier DM, Cardoso L, Zhu L-L, Li Y, Majeska RJ, Sun HB, Schaffler MB. Acute osteocyte response to fatigue Microdamage: Production of HIF-1α and VEGF-A. Orthop Trans. 2007;32:118. [Google Scholar]
- 39.Hsieh YF, Silva MJ. In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. J Orthop Res. 2002;20(4):764–71. doi: 10.1016/S0736-0266(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 40.Igarashi M, Kamiya N, Ito K, Takagi M. In situ localization and in vitro expression of osteoblast/ osteocyte factor 45 mRNA during bone cell differentiation. Histochem J. 2002;34(5):255–63. doi: 10.1023/a:1021745614872. [DOI] [PubMed] [Google Scholar]
- 41.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O'Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291–7. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jee WSS, Li XJ, Schaffler MB. Adaptation of diaphyseal structure with aging and increased mechanical usage in the adult rat: a histomorphometrical and biomechanical study. Anat Rec. 1991;230(3):332–8. doi: 10.1002/ar.1092300306. [DOI] [PubMed] [Google Scholar]
- 43.Komaba H, Fukagawa M. FGF23: a key player in mineral and bone disorder in CKD. Nefrologia. 2009;29(5):392–396. doi: 10.3265/Nefrologia.2009.29.5.5400.en.full. [DOI] [PubMed] [Google Scholar]
- 44.Kotha SP, Hsieh YF, Strigel RM, Muller R, Silva MJ. Experimental and finite element analysis of the rat ulnar loading model-correlations between strain and bone formation following fatigue loading. J Biomech. 2004;37:541–8. doi: 10.1016/j.jbiomech.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Lee TC, Myers ER, Hayes WC. Fluorescence-aided detection of microdamage in compact bone. J Anat. 1998;193(Pt 2):179–84. doi: 10.1046/j.1469-7580.1998.19320179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li CY, Schaffler MB, Wolde-Semait HT, Hernandez CJ, Jepsen KJ. Genetic background influences cortical bone response to ovariectomy. J Bone Miner Res. 2005;20:2150–8. doi: 10.1359/JBMR.050819. [DOI] [PubMed] [Google Scholar]
- 47.Martin RB. Is all cortical bone remodeling initiated by microdamage? Bone. 2002;30(1):8–13. doi: 10.1016/s8756-3282(01)00620-2. [DOI] [PubMed] [Google Scholar]
- 48.Martin RB. Fatigue microdamage as an essential element of bone mechanics and biology. Calcif Tissue Int. 2003;73(2):101–7. doi: 10.1007/s00223-002-1059-9. [DOI] [PubMed] [Google Scholar]
- 49.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15(4):613–20. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 50.Milgrom C, Finestone A, Shlamkovitch N, Giladi M, Radin E. Anterior knee pain caused by overactivity: a long term prospective followup. Clin Orthop Relat Res. 1996;331:256–60. doi: 10.1097/00003086-199610000-00036. [DOI] [PubMed] [Google Scholar]
- 51.Mori S, Burr DB. Increased intracortical remodeling following fatigue damage. Bone. 1993;14:103–9. doi: 10.1016/8756-3282(93)90235-3. [DOI] [PubMed] [Google Scholar]
- 52.Muir P, Johnson KA, Ruaux-Mason CP. In vivo matrix microdamage in a naturally occurring canine fatigue fracture. Bone. 1999;25(5):571–6. doi: 10.1016/s8756-3282(99)00205-7. [DOI] [PubMed] [Google Scholar]
- 53.Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO. Mechanistic aspects of fracture and R-curve behavior in human cortical bone. Biomaterials. 2005;26:217–31. doi: 10.1016/j.biomaterials.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Nampei A, Hashimoto J, Hayashida K, Tsuboi H, Shi K, Tsuji I, Miyashita H, Yamada T, Matsukawa N, Matsumoto M, Morimoto S, Ogihara T, Ochi T, Yoshikawa H. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J Bone Miner Metab. 2004;22(3):176–84. doi: 10.1007/s00774-003-0468-9. [DOI] [PubMed] [Google Scholar]
- 55.Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lanyon LE. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284(4):C934–43. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 56.Noble B. Microdamage and apoptosis. Eur J Morphol. 2005;42(1-2):91–8. doi: 10.1080/09243860500096248. [DOI] [PubMed] [Google Scholar]
- 57.Norman TL, Wang Z. Microdamage of human cortical bone: incidence and morphology in long bones. Bone. 1997;20:375–9. doi: 10.1016/s8756-3282(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 58.Nunamaker DM, Butterweck DM, Provost MT. Fatigue fractures in thoroughbred racehorses: relationships with age, peak bone strain, and training. J Orthop Res. 1990;8:604–11. doi: 10.1002/jor.1100080417. [DOI] [PubMed] [Google Scholar]
- 59.O'Brien FJ, Hardima DA, Hazenberg JG, Mercy MV, Mohsin S, Taylor D, Lee TC. The behaviour of microcracks in compact bone. Eur J Morphol. 2005;42:71–9. doi: 10.1080/09243860500096131. [DOI] [PubMed] [Google Scholar]
- 60.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5(11):611–9. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds EC. Calcium phosphate-based remineralization systems: scientific evidence? Aust Dent J. 2008;53(3):268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez M, Lucchesi BR, Schaper J. Apoptosis in myocardial infarction. Ann Med. 2002;34(6):470–9. doi: 10.1080/078538902321012414. [DOI] [PubMed] [Google Scholar]
- 63.Saito T, Nishii Y, Yasuda T, Ito N, Suzuki H, Igarashi T, Fukumoto S, Fujita T. Familial hypophosphatemic rickets caused by a large deletion in PHEX gene. Eur J Endocrinol. 2009;161(4):647–51. doi: 10.1530/EJE-09-0261. [DOI] [PubMed] [Google Scholar]
- 64.Schaffler MB, Li XJ, Jee WS, Ho SW, Stern PJ. Skeletal tissue responses to thermal injury: an experimental study. Bone. 1988;9:397–406. doi: 10.1016/8756-3282(88)90122-6. [DOI] [PubMed] [Google Scholar]
- 65.Schaffler MB, Pitchford WC, Choi K, Riddle JM. Examination of compact bone microdamage using back-scattered electron microscopy. Bone. 1994;15:483–8. doi: 10.1016/8756-3282(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 66.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–25. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 67.Schaffler MB. Musculoskeletal Fatigue and Stress Fractures. CRC Press; Boca Raton: 2001. Bone Fatigue and Remodeling in the Development of Stress Fractures. pp. 161–182. [Google Scholar]
- 68.Schaffler MB. Role of bone turnover in microdamage. Osteoporos Int. 2003;14:73–80. doi: 10.1007/s00198-003-1477-1. [DOI] [PubMed] [Google Scholar]
- 69.Silva MJ, Uthgenannt BA, Rutlin JR, Wohl GR, Lewis JS, Welch MJ. In vivo skeletal imaging of 18F-fluoride with positron emission tomography reveals damage- and time-dependent responses to fatigue loading in the rat ulna. Bone. 2006;39(2):229–36. doi: 10.1016/j.bone.2006.01.149. [DOI] [PubMed] [Google Scholar]
- 70.Silva MJ, Touhey DC. Bone formation after damaging in vivo fatigue loading results in recovery of whole-bone monotonic strength and increased fatigue life. J Orthop Res. 2007;25(2):52–61. doi: 10.1002/jor.20320. [DOI] [PubMed] [Google Scholar]
- 71.Tami AE, Nasser P, Verborgt O, Schaffler MB, Knothe Tate ML. The role of interstitial fluid flow in the remodeling response to fatigue loading. J Bone Miner Res. 2002;17:2030–7. doi: 10.1359/jbmr.2002.17.11.2030. [DOI] [PubMed] [Google Scholar]
- 72.ten Cate JM. Remineralization of deep enamel dentine caries lesions. Aust Dent J. 2008;53(3):281–5. doi: 10.1111/j.1834-7819.2008.00063.x. [DOI] [PubMed] [Google Scholar]
- 73.Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res. 2005;20(8):1372–80. doi: 10.1359/JBMR.050326. [DOI] [PubMed] [Google Scholar]
- 74.Turner CH. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int. 2002;13:97–104. doi: 10.1007/s001980200000. [DOI] [PubMed] [Google Scholar]
- 75.Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, Schaffler MB, Fyhrie DP. In vivo diffuse damage in human vertebral trabecular bone. Bone. 2000;26:147–52. doi: 10.1016/s8756-3282(99)00253-7. [DOI] [PubMed] [Google Scholar]
- 76.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–7. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 77.Verborgt O, Tatton NA, Majeska RJ, Schaffler MB. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17:907–14. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- 78.Wang L, Wan Y, Han Y, Henderson SC, Majeska RJ, Weinbaum S, Schaffler MB. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci. 2005;102(33):11911–6. doi: 10.1073/pnas.0505193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. Embo J. 2003;22:6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int. 2003;14(Suppl 3):S35–42. doi: 10.1007/s00198-002-1342-7. [DOI] [PubMed] [Google Scholar]
- 81.Zar JH. Biostatistical Analysis. 4th ed. Prentice Hall; Upper Saddle River: 1999. [Google Scholar]