Abstract
Tissue stem cells have been linked to cancers of epithelial origin including the prostate. There are three relevant issues concerning stem cells and cancer that rely solely on functional studies: 1. Are there tissue‐regenerating stem cells in the adult organ? 2. Can tissue‐regenerating cells serve as targets for transformation? 3. Do primary tumors contain tumor‐propagating (cancer stem) cells? We will review the recent literature with respect to these critical issues to provide a direct link between primitive cells and prostate cancer.
Keywords: Prostate cancer, Cancer‐initiation, Stem cell, Tissue‐regeneration, Castration‐resistance
Abbreviations
- Sca-1
Stem Cell Antigen-1
- AR
Androgen Receptor
- NE
neuroendocrine
- K5
Keratin 5
- K8
Keratin 8
- UGSM
Urogenital Sinus Mesenchyme
- PSA
prostate-specific antigen
- PIN
prostatic intraepithelial neoplasia
- PI3K
Phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homolog
- CARN
castration-resistant Nkx3-1-expressing cell
1. Introduction
The fields of stem cell biology and cancer biology are closely linked. Approximately 1 in 6 men will be diagnosed with prostate cancer in his lifetime (American Cancer Society Statistics, 2009). The estimated incidence of prostate cancer continues to rise (Greenlee et al., 2000), and the field of prostate cancer research is similarly growing. However, issues related to stem cells have garnered little agreement among researchers. The cellular location of stem and progenitor cells, the cell‐of‐origin for prostate cancer, and the existence of cancer stem cells are all relevant issues currently being debated in the field. For each of these topics, we will discuss the experimental evidence to support each side of the debate.
In the hematopoietic field, cells are named for their functional activity, such that a cell capable of generating monocytes/macrophages and granulocytes is termed the granulocyte/macrophage lineage‐restricted progenitor (GMP) (Akashi et al., 2000). Recently this approach has been extended to epithelial tissue in the mammary system, with the identification of the mammary repopulating unit capable of regenerating mammary gland structures when transplanted into the cleared fat pad (Stingl et al., 2006), and the luminal progenitor capable of forming luminal‐restricted structures (Asselin‐Labat et al., 2007; Lim et al., 2009). While numerous models describing the prostate epithelial hierarchy have been proposed based on a variety of correlative data, we will concentrate solely on functional evidence to determine the hierarchical relationships between different cell types in the prostate epithelium. Finally, we will discuss how studying stem cells can be most useful for understanding and treating prostate cancer.
2. Adult stem cells in the prostate and their localization within the epithelium
Hormonal‐dependence distinguishes epithelial tissues like the prostate and the mammary gland from other adult organs. To account for changes in hormone levels, the prostate stem cell should be responsive to, but not dependent on, androgen for survival. This property is referred to as castration‐resistance. Prostate organogenesis occurs during embryonic development and organ maturation is completed by the end of puberty. Unlike the intestinal epithelium that is constantly being turned over (Barker et al., 2007), the adult prostate does not require rapidly cycling stem cells to replenish the organ every few days. Occasionally, cells in the prostate luminal layer will undergo apoptosis and shedding into the lumen. Therefore, prostate stem cells should have tissue‐regenerative capacity to replenish the gland after routine cell death. In contrast to the hematopoietic stem cell that must generate a vast array of mature lineages (Morrison et al., 1995), the prostate stem cell is responsible for generating a relatively simple double‐layered epithelium (Figure 1). Finally, the prostate stem cell must self‐renew to meet the needs of the organ over the course of a man's lifetime. We will evaluate the experimental evidence to support the existence of primitive prostate cells with the properties of castration‐resistance, tissue‐regeneration and self‐renewal.
Figure 1.
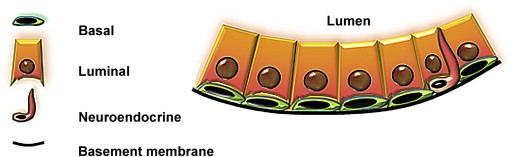
Three cell types in adult prostate epithelium. Basal cells (green) line the outside of the gland and reside against the basement membrane (black). Luminal cells (orange) contact the basal layer and the fluid‐filled lumen. Rare neuroendocrine cells (red) are typically found in the basal layer with neurite‐like extensions that can approach the luminal layer.
2.1. In vivo evidence for castration‐resistant cells
The first line of evidence to support a stem‐like cell in the prostate comes from studies demonstrating organ responsiveness to androgen. These findings began with Huggins and Hodges' discovery that the majority of prostate cancer cells are hormone‐dependent (Huggins and Hodges, 1941), leading many to hypothesize that prostate cancer could be treated with androgen ablation therapy (Huggins, 1943). Elimination of androgen (castration) or androgen receptor function provides temporary benefits to the patient; however the persistence of castration‐resistant cells within prostate tumors and their ability to proliferate in the absence of androgen causes the lethal hormone‐refractory phase of prostate cancer (Scher and Sawyers, 2005), termed castration‐resistant prostate cancer.
Castration‐resistance is also a property of the normal non‐cancerous prostate. Isaacs (1987) demonstrated that the normal rodent gland involutes after androgen withdrawal, accompanied by massive apoptosis of androgen‐dependent cells. Androgen add‐back is sufficient to stimulate the remaining castration‐resistant cells to regenerate the gland (Isaacs, 1987). Wilson's group demonstrated that cycles of gland involution and regeneration in response to androgen withdrawal and subsequent androgen addition can be repeated up to thirty times (Tsujimura et al., 2002), proving the existence of long‐term castration‐resistant cells in the normal rodent prostate. Since the cells that survive castration are capable of regenerating the remaining cells of the gland after addition of androgen, evidence suggests that a fraction of the castration‐resistant cells must have tissue‐regenerating activity (Figure 2).
Figure 2.
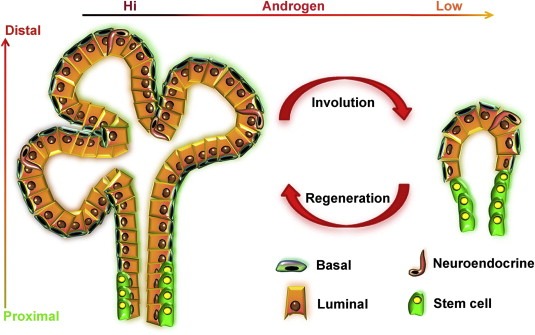
Response of prostate epithelium to castration and androgen addition. Androgen withdrawal causes massive apoptosis in the prostate epithelium, leaving behind only castration‐resistant cells. Upon addition of androgen, castration‐resistant cells are capable of regenerating the gland. The cycle of involution and regeneration can be repeated in the rodent prostate almost indefinitely.
2.2. In vivo evidence for tissue‐regenerating cells
Cunha and Lung (1978) developed a transplantation system where castration is not required, combining tissue fragments of murine fetal prostate mesenchyme with fetal epithelial components under the kidney capsule of immunodeficient mice. Upon transplantation, the embryonic mesenchyme (Urogenital Sinus Mesenchyme/UGSM) can induce the embryonic epithelium to generate functional (secretion‐producing) prostatic‐like glands (Cunha and Lung, 1978). These studies demonstrate that normal non‐castrated prostate tissue is capable of tissue‐regeneration in vivo. Instead of using tissue fragments, our group developed an alternative approach where both epithelium and inductive mesenchyme are dissociated to single cells. Adult epithelial cells can be combined with dissociated UGSM cells to demonstrate that single cells from the adult prostate gland are capable of regenerating prostatic tubules (Xin et al., 2003). Extending the system to dissociated adult prostate cells enables the subsequent purification of tissue‐regenerating cells from mouse and human adult prostate epithelium, and allows for genetic manipulation of both epithelial and mesenchymal components.
2.3. Self‐renewal of primitive prostate cells
One universal property of stem cells, both embryonic and adult, is self‐renewal. While adult stem cells are capable of differentiating into their mature progeny to fulfill the needs of the gland, tissue‐regenerating cells are required to produce more of themselves to sustain the tissue over a long period of time. Assays to measure self‐renewal activity need to be developed to determine if the prostate contains primitive self‐renewing cells. Since the prostate‐regeneration assay takes approximately 8 weeks for the development of primary outgrowths and requires the use of immunodeficient mice, in vivo self‐renewal studies are time‐intensive, expensive and technically challenging. As an alternative, in vitro sphere‐forming assays have been developed to study primitive cells from the prostate (Garraway et al., 2009; Goldstein et al., 2008; Lawson et al., 2007; Shi et al., 2007; Xin et al., 2007), similar to those developed to study the neural system (Reynolds and Weiss, 1996), and mammary gland (Dontu et al., 2003). A sub‐fraction of the human and mouse prostate basal compartment can generate spheres that self‐renew in a three‐dimensional semi‐solid structure comprised of extracellular matrix components, resembling the native laminin and collagen‐rich microenvironment (Goldstein et al., 2008). The development of this assay allows for the identification of pathways that regulate self‐renewal activity.
In the prostate sphere assay, primitive cells retain their ability to generate daughter spheres for more than 10 successive passages and a subset of sphere cells retain the capacity to generate prostatic tubules when transplanted in vivo (Xin et al., 2007). A greater number of cells have sphere‐forming activity than prostate‐regenerating activity, suggesting that the sphere assay measures both progenitor cell and stem cell function. One must critically evaluate these findings and ask if the in vitro assay is a true surrogate for stem cell self‐renewal in vivo. In the blood system, the hematopoietic stem cell has the capacity for long‐term multi‐lineage reconstitution in irradiated recipient mice (Morrison and Weissman, 1994) and can serially reconstitute irradiated recipients (Kiel et al., 2005), demonstrating in vivo self‐renewal activity. Does such an in vivo self‐renewing cell exist in the normal prostate? To tackle this issue, we generated primary outgrowths from a murine prostate stem cell‐enriched population [Trop2hi basal cells, described in Section 2.4], and found that cells with the same phenotypic profile could be re‐isolated with the capacity to generate secondary outgrowths indistinguishable from primary tubules (Goldstein et al., unpublished results). Wang et al. utilized a castration and regeneration model to demonstrate in vivo self‐renewal of a stem cell population [CARN cells, described in Section 2.5]. Our group has also combined the two approaches, tissue‐regeneration and castration/regeneration, to demonstrate in vivo self‐renewal (Lukacs et al., 2008). Primary cells are isolated from a transgenic mouse carrying luciferase under the androgen‐regulated Probasin promoter. Under normal conditions, luciferase signal can be detected from under the kidney capsule of recipient mice. Castration leads to a loss of signal and androgen add‐back restores bioluminescence, and the cycle can be repeated several times (Lukacs et al., 2008). While in vivo self‐renewal studies are not ideal for investigating a broad range of pathways implicated in regulating stem cell activity, they are useful to demonstrate that the prostate contains self‐renewing cells in the normal and castrated/regressed prostate.
2.4. Evidence for stem cells with a basal location
Stem cells in many epithelial tissues reside in the basal layer along the basement membrane (Stingl et al., 2006; Chan et al., 2009; Eirew et al., 2008; Rock et al., 2009; Shackleton et al., 2006; Blanpain et al., 2004; Cotsarelis et al., 1989; Hong et al., 2004; Iwai et al., 2008), close to the growth factors secreted by stromal cells. Early evidence suggested the basal layer as a niche for prostate stem cells. English et al. (1987) determined that basal cells preferentially survive androgen ablation, and possess the highest proliferation rate in the gland. Tsujimura et al. (2002) showed that proximally‐located basal cells preferentially retain BrdU label. Wilson's group and our group showed that high expression of Sca‐1 (stem cell antigen‐1) could enrich for prostate‐regenerating cells in the tissue‐regeneration assay (Burger et al., 2005; Xin et al., 2005). We further demonstrated that the epithelial cells in the Sca‐1+ fraction co‐express CD49f (integrin alpha 6) at high levels, and correspond to cells in the basal compartment. Upon transplantation, only the Sca‐1+ CD49fhi basal cells are capable of prostate‐regeneration (Goldstein et al., 2008; Lawson et al., 2010). We have since refined this population to show that Sca‐1+ CD49fhi basal cells expressing high levels of Trop2, a type I transmembrane protein related to EpCAM, are highly enriched for tissue‐regenerative activity in vivo (Goldstein et al., 2008) (Figure 3, mouse). Alternative approaches to enrich for basal cells with tissue‐regenerative capacity include purification based on aldehyde dehydrogenase (ALDH) enzymatic activity (Burger et al., 2009).
Figure 3.
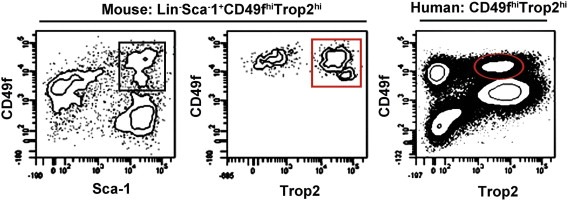
Stem cell‐enriched populations in the human and mouse prostate. The most enriched murine prostate stem cell fraction is defined by Lin−Sca‐1+CD49fhiTrop2hi. Left plot shows Lin(CD31/CD45/Ter119)‐ cells stained for CD49f and Sca‐1. The basal population (Sca‐1+CD49fhi, black box) can be further sub‐divided based on expression of Trop2, with the Trop2hi basal fraction (red box) containing the majority of prostate‐regenerating cells. Human prostate stem/progenitor cells are contained within the CD49fhiTrop2hi fraction (red oval).
In the human prostate, we and others have purified stem‐like cells from the basal compartment. Collins et al. (2001) demonstrated that basal cells can generate luminal cells in vitro (Robinson et al., 1998), and that a subset of basal cells expressing integrin alpha2/beta1 (and CD133) show the ability to form prostate‐like acini in vivo (Richardson et al., 2004). Using markers first identified in the mouse prostate, we have purified human prostate basal cells expressing high levels of CD49f and Trop2 with sphere‐forming activity (Goldstein et al., 2008) (Figure 3, human). We have recently found that cells from the basal fraction demonstrate robust tissue‐regenerative activity when transplanted into immunodeficient mice (Goldstein et al., 2010). Regenerated prostatic tubules contain distinct p63+/K5+ basal and AR+/K8+ luminal layers that strongly resemble benign human prostatic ducts. Dissociated cells from primary outgrowths demonstrate the cellular heterogeneity present in original specimens when analyzed by flow cytometry (Goldstein et al., 2010).
2.5. Evidence for a luminal cell with stem/progenitor activity
Although evidence supports a basal location for stem cells in the prostate, it does not necessarily imply that all luminal secretory cells are terminally‐differentiated. If progenitor cells reside within the prostate luminal compartment, it is important to determine whether these progenitors are luminal‐restricted, as has been demonstrated in the human and mouse mammary gland (Asselin‐Labat et al., 2007; Lim et al., 2009), or if they can generate basal cells as well. Several groups have investigated the possibility that luminal cells with stem/progenitor characteristics exist in the rodent prostate. Isaacs showed that while basal cells preferentially survive androgen ablation, a subset of luminal cells demonstrate castration‐resistance as well (English et al., 1987), and Tsujimura et al. (2002) found that small clusters of luminal cells retain BrdU label. These studies suggest the existence of progenitors in the luminal compartment, but functional studies are necessary to demonstrate that luminal cells have proliferative potential.
Our group demonstrated that a fraction of luminal cells can generate luminal (Keratin 5− Keratin 8+) colonies in vitro and in vivo, but do not have tubule‐forming activity (Lawson et al., 2010), suggesting the existence of luminal‐restricted progenitor cells in the normal mouse prostate. Wang et al. (2009) demonstrated that in the castrated mouse prostate, a population of castration‐resistant luminal cells expressing the homeobox transcription factor Nkx3‐1 (termed CARNs) can generate prostatic tissue with basal, luminal and neuroendocrine cells. This study shows that the prostate hierarchy is more complex than it previously appeared, and suggests that androgen ablation can give tissue‐regenerative activity to a luminal cell. It is not clear if CARNs, or any luminal cell with tissue‐regenerative activity, exists in the non‐castrated murine prostate. Since human patients are not castrated prior to the onset of advanced disease, a parallel human CARN population cannot be identified directly from benign patient tissue. Regardless of their relevance to the human disease, CARNs may prove useful in understanding mechanisms of castration‐resistance and tissue‐regeneration.
2.6. Proposed epithelial hierarchy of the normal prostate
Evidence from our group shows that a subset of cells contained within the basal fraction of the murine and human prostate can regenerate prostatic tubules (Goldstein et al., 2008; Goldstein et al., 2010). Based on this data, we would place stem cells with a basal phenotype at the top of the prostate epithelial hierarchy. When murine prostate epithelial cells are plated in a two‐dimensional assay in vitro, only two types of colonies are observed based on keratin staining: K5+/K8+ colonies, and K5−/K8+ colonies (Lawson et al., 2010). The outgrowths suggest that these colonies arise from a bipotent (K5+/K8+) progenitor and a luminal‐restricted (K5−/K8+) progenitor. Since K5+/K8− colonies are not observed, evidence does not support a basal‐restricted progenitor, in contrast to observations from the mammary gland (Stingl et al., 1998, 2001).
If luminal colonies are derived from luminal‐restricted progenitors, one might ask if all bipotent colonies arise from stem cells. Although colony‐forming cells and prostate‐regenerating cells are both highly enriched within the Trop2hi basal fraction (Goldstein et al., 2008), we observe a much greater number of these cells capable of generating bipotent colonies than generating tubules. This data suggests that a bipotent progenitor with colony‐forming but not tubule‐forming capacity also resides within the basal compartment. In addition, not all basal cells form tubules, colonies or spheres, suggesting that a fraction of basal cells are mature and do not generate other cell types. The majority of luminal cells do not generate any structures, suggesting a mature status.
Neuroendocrine (NE) cells represent a rare third cell type in prostate glands, and are named for their morphology, with neurite‐like extensions, and their secretion of neuropeptides (Abrahamsson, 1999). Despite the fact that NE cells are routinely found in prostate cancer, with increased expression in late stage metastatic disease (Huang et al., 2006; Abrahamsson et al., 1989; Bohrer and Schmoll, 1993; Ahlgren et al., 2000; Jiborn et al., 1998), their precise function is unknown. NE cells are localized to the epithelial glands, but their origin has been debated in the literature. Immunohistochemical stains have led some to propose that NE cells originate from the neural crest (Aumuller et al., 1999) and are not derived from an epithelial source. Studies demonstrating that purified stem/progenitor cells can generate tubules with NE cells upon transplantation into immunodeficient mice (Goldstein et al., 2008; Leong et al., 2008) suggest that NE cells have an epithelial origin. However, neural crest‐derived NE cells from recipient mice could have migrated into regenerated tubules. To exclude this possibility, we purified fluorescently‐labeled Trop2hi basal cells from transgenic mice expressing DsRed under the β‐actin promoter and transplanted into non‐labeled immunodeficient mice. Fluorescently‐labeled NE cells were detectable in regenerated tubules, demonstrating that NE cells are donor derived and not host‐derived, and suggesting that a common progenitor can generate NE, basal and luminal cells (Goldstein et al., 2008).
Our combined evidence suggests that a stem cell within the basal layer of the normal non‐castrated prostate that exhibits tubule‐forming capacity can give rise to multi‐potent progenitor cells within the basal layer. This progenitor likely gives rise to neuroendocrine cells, mature basal cells and luminal‐restricted progenitors that generate mature luminal cells. Many intermediate cell types have been proposed based on immunohistochemical staining (van Leenders et al., 2000; Verhagen et al., 1992). However, our functional evidence leads us to this proposed epithelial hierarchy in the normal, non‐castrated prostate (Figure 4). Future interrogation of intermediate cells using functional criteria should lead to a more complex model.
Figure 4.
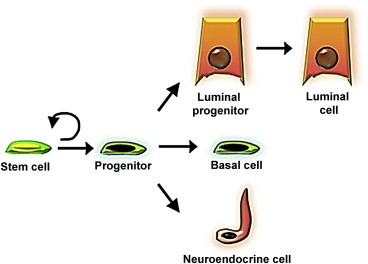
Proposed model of the prostate epithelial hierarchy. Stem cells within the basal layer likely give rise to multi‐potent progenitor or intermediate cells that generate all three epithelial cell types. Evidence supports the existence of a luminal‐restricted progenitor that can give rise to mature luminal cells.
Leong et al. described the identification of prostate‐regenerating cells based on expression of several markers, most notably c‐kit (CD117), however it is unclear what cell type is isolated by this approach and how these cells relate to basal and luminal stem and progenitor cells. We and others find no expression of c‐kit in the basal layer of the normal prostate [Goldstein et al., unpublished results and E.L. Wilson, personal communication] and Wang et al. (2009) reported no luminal c‐kit expression in the castrated prostate. Therefore, future studies are necessary to determine where c‐kit‐expressing prostate cells fall into the hierarchy and if they have an epithelial origin.
3. The role of tissue stem cells in cancer‐initiation
An important issue in the field currently under debate is the cell‐of‐origin for prostate cancer (Huang and Witte, 2010). Much evidence in the general cancer literature supports primitive cells as efficient targets for transformation (Barker et al., 2009; Zhu et al., 2009; So et al., 2003; Passegue et al., 2004), although evidence also supports a role for cells with a more mature status in oncogenesis (Huntly et al., 2004; Krivtsov et al., 2006; Cozzio et al., 2003). Due to the observed expansion of malignant luminal cells and the relative loss of basal cells in human prostate tumors, the traditional view in the field is that luminal cells serve as the likely target population (Okada et al., 1992). However our finding that Trop2hi basal cells have the capacity for tri‐lineage differentiation (Goldstein et al., 2008) suggests that prostate cancer, comprised primarily of luminal and neuroendocrine cells (Okada et al., 1992; di Sant'Agnese, 1992), likely originates from a progenitor cell with multi‐lineage differentiation potential or a mature cell that acquires this property. That age is a major risk factor for prostate cancer suggests that long‐lived, self‐renewing stem cells are best suited to acquire enough mutations to initiate the disease. Are primitive cells in the prostate the preferred targets for transformation, and do they represent the only cell or one of multiple cells‐of‐origin?
3.1. Evidence for primitive prostate cells as targets for transformation
Two general approaches have been taken to determine the target cells for oncogenic transformation. One approach utilizes cell‐type specific promoters to drive the expression of an oncogene or deletion of a tumor‐suppressor to initiate the disease. Although this approach is utilized effectively in genetically engineered mouse strains, it does not allow for parallel studies in the human system. Our group have taken an alternative approach, analogous to studies in the hematopoietic system, where genetic alterations are introduced into purified cell populations and transplanted into mice. We found that the Sca‐1+ fraction, enriched in prostate‐regenerating cells, was capable of initiating prostatic intraepithelial neoplasia (PIN) lesions upon activation of the PI3K pathway via over‐expression of myristoylated AKT (Xin et al., 2005).
Utilizing both approaches, our group and others have shown that the basal compartment is susceptible to transformation. In the Pten‐null prostate cancer model, Wang et al. (2006a) demonstrated an expansion of basal stem/progenitor cells, and Mulholland et al. (2009) recently found that cells expressing the basal cell‐specific marker p63 could initiate prostate cancer after deletion of Pten. Our group went on to show that the basal fraction is an efficient target population for prostate cancer‐initiation in response to multiple oncogenic events including activation of the PI3K pathway, enhanced AR signaling, and increased expression of the ETS family transcription factor ERG (Lawson et al., 2010).
Although stem‐like basal cells are a likely target population, other studies have shown that prostate cancer can arise from luminal cells. Using a human PSA‐promoter that is active primarily in murine prostate luminal cells, Ma et al. (2005) were able to demonstrate prostate cancer‐initiation after loss of Pten. Korsten et al. (2009) went on to show that the genetic alterations are first seen in a subset of luminal cells expressing the progenitor markers Trop2 (TACSTD2) and Sca‐1. Wang et al. showed that CARNs can serve as a cell‐of‐origin for prostate cancer after loss of PTEN (Wang et al., 2009), proving that PI3K pathway activation in multiple target cells is sufficient for cancer‐initiation.
To address the potential cell‐of‐origin for human prostate cancer, we have lentivirally transduced prospectively‐isolated basal and luminal fractions with oncogenes that are both implicated in human prostate cancer and have been demonstrated to transform murine prostate epithelium. We have found that cells in the CD49fhiTrop2hi human prostate basal fraction can serve as target cells for transformation upon transplantation into immunodeficient mice (Goldstein et al., 2010).
Results from the mouse suggest the co‐existence of multiple target cells in the context of murine prostate cancer, and early studies in the human system implicate basal cells as one cell‐of‐origin for human prostate cancer (summarized in Figure 5). As has been demonstrated in the hematopoietic and mammary systems (Lim et al., 2009; So et al., 2003; Passegue et al., 2004; Huntly et al., 2004; Krivtsov et al., 2006; Cozzio et al., 2003; Prat and Perou, 2009), different genetic alterations may have the capacity to transform different target cells. Alternatively, different clinical sub‐types of cancer may arise from different cell types. While the majority of human prostate tumors are acinar‐type adenocarcinomas, rare variant forms such as small cell carcinoma and ductal carcinoma have been described (Huang and Witte, 2010; Pinthus et al., 2000; Epstein, 2010). Understanding how different target cells self‐renew and initiate the disease may be useful to not only sub‐divide patients with these properties but also to identify therapies to specifically attack one or another target cell.
Figure 5.
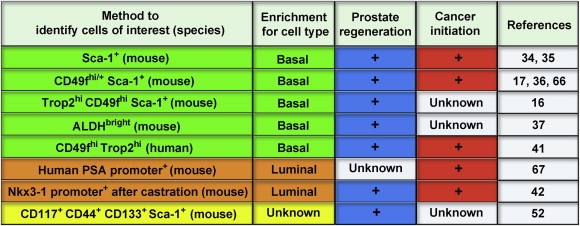
Summary of studies demonstrating prostate‐regeneration and/or prostate cancer‐initiation. Numerous approaches have been taken to identify prostate‐regenerating cells (blue) and prostate cancer‐initiating cells (red) from the mouse prostate. These methods are known to enrich for cells with a basal (green), luminal (orange) or unknown (yellow) location in the gland. We have recently extended these studies to the human prostate system to demonstrate that cells with a basal phenotype can generate human prostatic tubules and human prostate cancer.
4. Do prostate tumors contain tumor‐propagating (cancer stem) cells?
The cellular heterogeneity within tumors has only recently been probed to identify cellular components with greater tumorigenicity that may serve as unique targets for therapy. The cancer stem cell (CSC) hypothesis proposes that a subset of cells within a tumor are responsible for both self‐renewing to make more of themselves and producing the remaining cellular heterogeneity represented in the tumor (Reya et al., 2001). The gold standard assay to demonstrate this concept involves transplanting primary tumor cells into immunodeficient mice. It has been shown in hematologic cancers (Bonnet and Dick, 1997; Lapidot et al., 1994) and in several solid tumors (Al‐Hajj et al., 2003; O'Brien et al., 2007; Singh et al., 2004) that a sub‐fraction of cancer cells can propagate the tumor in mice, while the remaining fraction is depleted for this activity. However, this view needs to be critically examined in each experimental system, as multiple groups have found notable exceptions where the majority of primary cancer cells have tumor‐propagating potential (Quintana et al., 2008; Kelly et al., 2007; Williams et al., 2007).
4.1. Propagation of prostate tumors in mice
Over the last decade, a significant amount of work has been done to establish xenografts of primary human prostate cancers. Numerous groups have demonstrated that small bits of cancerous prostate tissue isolated from both primary and metastatic sites can be propagated and serially passaged in mice (Pinthus et al., 2003, 1996, 1997, 2000, 1993, 2000, 1994). Some tumors can maintain their growth and differentiation properties for up to 76 passages (Corey et al., 2003), demonstrating long‐term proliferative potential and self‐renewal of prostate cancer cells. Several groups have used cell lines and xenografts as models to investigate potential prostate cancer stem cells (Hurt et al., 2008, 2006, 2007, 2008). It is important to note that these models have likely undergone in vitro and/or in vivo selection processes that may not accurately represent the cellular heterogeneity of primary prostate tumors. The most convincing evidence for cancer stem cells in prostate cancer would be the demonstration that dissociated cells from primary prostate tumors can propagate a tumor representing the heterogeneity of the original tumor.
4.2. Lack of evidence for cancer stem cells in primary prostate tumors
While tissue fragments from primary prostate tumors can be propagated in mice (Pinthus et al., 2003, 1996, 1997, 2000, 1993, 2000, 1994), the routine demonstration that dissociated cells can generate such tumors has yet to be reported. Collins et al. (2005) reported the isolation of a subset of cells from primary tumors, termed prostate cancer stem cells, with proliferative potential in vitro. Although prostate cancer is defined by an absence of basal cells, the putative prostate cancer stem cells exhibited basal cell properties (Collins et al., 2005). Interestingly, these cells were purified based on their expression of CD44, a marker of basal cells in benign prostate that is primarily expressed on chromogranin A‐expressing neuroendocrine cells and tumor‐infiltrating lymphocytes in prostate tumors (Palapattu et al., 2009). The gold standard, tumor‐propagation upon transplantation into mice, has yet to be met, and therefore it remains unclear if primary prostate tumors contain cancer stem cells.
4.3. Are prostate tumors devoid of stem‐like cells?
Despite the lack of evidence for tumor‐propagating cells, such a cell may still exist in the context of human prostate cancer. Morrison and colleagues have demonstrated that optimization of assay conditions can vastly alter the number of cells capable of tumor formation in mice (Quintana et al., 2008). While only 1 melanoma cell per million can generate tumors in NOD/SCID mice, 1 in 4 has the capacity for tumor formation when injected into NOD/SCID IL2Rγnull mice with Matrigel (Quintana et al., 2008). Perhaps similar optimization of transplantation techniques may elucidate a population of dissociated primary prostate tumor cells capable of tumor formation in mice. Alternatively, prostate tumors may be devoid of primitive cells. Strong evidence, as presented in this review, supports the existence of progenitor‐type cells in the basal compartment of the mouse and human prostate. During prostate cancer progression, the luminal compartment expands and basal cells are lost (Grisanzio and Signoretti, 2008). Perhaps this loss of basal cells corresponds to a loss of progenitor‐type cells during cancer progression. Late stage disease, accompanied by metastasis and castration‐resistant prostate cancer, enriches for basal cell genes (Bui and Reiter, 1998) and may also enrich for stem‐like cells. A third possibility is that neuroendocrine cells, rare in normal tissue with increasing numbers during disease progression, represent a progenitor cell in prostate cancer (Palapattu et al., 2009). Neuroendocrine cells are quiescent, castration‐resistant, and do not express detectable levels of androgen receptor (AR) or prostate‐specific antigen (PSA) (Huang et al., 1993, 2006, 2007, 1993, 2009). Finally, different sub‐types of prostate cancer, such as small cell carcinoma, may have different propensities to contain stem‐like components. Future studies will be necessary to determine if a subset of prostate cancer cells are responsible for tumorigenic activity and can be uniquely targeted, or if therapies should focus on eradicating the bulk tumor.
5. How can studying stem cells be useful for understanding and treating prostate cancer?
To date, researchers have taken two central approaches to apply the study of tissue stem cells to cancer. In the first approach, the antigenic profile of stem and progenitor cell populations are used to prospectively isolate cells from primary cancers with tumorigenic activity. Dick and colleagues first demonstrated that cells with the same profile as normal human hematopoietic progenitors (CD34+ CD38−) were the only cells capable of transplanting leukemia into mice (Bonnet and Dick, 1997; Lapidot et al., 1994) and this approach has been useful for the purification of tumorigenic cells from solid tumors as well (Ginestier et al., 2007). Using a different approach that does not rely on expression of surrogate markers for primitive cell populations, researchers are asking whether mechanisms that regulate stem cell survival and function will also regulate cancer cells. Such an approach has led to the identification of new targets for solid tumors, such as Bmi‐1 in lung cancer (Dovey et al., 2008) and the Wnt pathway in chronic myelogenous leukemia (Zhao et al., 2007).
5.1. Properties shared by primitive prostate cells and castration‐resistant prostate cancer cells
Since the antigenic profile of normal human prostate stem cells (enriched in the CD49fhiTrop2hi basal fraction) is extremely low or not represented in prostate tumors and is unlikely to be useful for purifying cancer stem/progenitor cells, we should focus our attention on identifying critical regulators of stem cell function that may modulate prostate cancer cells. Primitive cells of the prostate likely share three properties with castration‐resistant prostate cancer cells: castration‐resistance, tissue‐regeneration, and self‐renewal. Although Trop2hi basal cells and CARNs have different molecular properties and cellular localization, both populations contain prostate‐regenerating cells that exhibit castration‐resistance and self‐renewal. Transcriptional profiling of these distinct populations may be useful to identify common mechanisms that regulate castration‐resistance and self‐renewal activity.
5.2. Self‐renewal pathways in stem cells and prostate cancer
Several candidate pathways have been implicated in modulating stem‐like cells and are commonly dysregulated in prostate cancer, suggesting that they may be targetable. The PI3K pathway, shown to promote self‐renewal of murine prostate basal stem/progenitor cells (Mulholland et al., 2009), is activated in the majority of prostate tumors (Yoshimoto et al., 2006). The Ets transcription factor ERG, a regulator of hematopoietic myeloid progenitor self‐renewal (Pereira et al., 1998) and normal prostate differentiation (Zong et al., 2009; Klezovitch et al., 2008), is frequently involved in translocations resulting in increased protein expression in prostate cancer (Tomlins et al., 2007). Other genes and pathways that regulate stem cell self‐renewal and similar developmental processes, such as the Wnt (Chen et al., 2004; Bierie et al., 2003; Mulholland et al., 2002; Ontiveros et al., 2008; Yang et al., 2002) and Notch pathways (Belandia et al., 2005, 2005, 2008, 2001, 2006, 2010, 2006), c‐Myc (Gurel et al., 2008; Ellwood‐Yen et al., 2003), Bcl‐2 (Catz and Johnson, 2003; McDonnell et al., 1992) and Bmi‐1 (Berezovska et al., 2006; Glinsky et al., 2005; van Leenders et al., 2007), have altered expression in prostate cancer and may be involved in cancer cell survival and self‐renewal. Recent work from our group has established Bmi‐1 as a regulator of both normal murine prostate stem cell self‐renewal and murine prostate cancer‐initiation (R.U. Lukacs, Unpublished Results). Defining the dominant self‐renewal pathways that regulate primitive prostate cells may elucidate critical targets for the treatment of prostate cancer.
6. Conclusions
In this review, we have evaluated studies that link primitive cells to prostate cancer, highlighting the functional studies. Strong evidence supports the existence of tissue stem cells in the prostate with the properties of castration‐resistance, tissue‐regeneration and self‐renewal. In addition to the numerous groups that have isolated stem cells from the normal prostate basal layer, a population of luminal cells in the castrated prostate can serve as stem cells. We also present evidence that primitive cells can serve as targets for prostate cancer‐initiation. The fact that prostate cancer is so heavily linked to age strongly suggests that long‐lived cells are the only cells capable of acquiring enough mutations to initiate cancer, supporting the experimental evidence of immature cells with cancer‐initiating activity. The epithelial hierarchy of the prostate needs to be further interrogated to identify intermediate progenitor‐type cells, so that their susceptibility to oncogenesis can be assayed as well. Surprisingly little evidence exists to support a stem‐like cell in primary prostate tumors, however there may be a rare subset of cells in advanced disease with tumorigenic activity. These experimental findings have important implications for treatment and need to be resolved.
We have determined that the link between stem cells and prostate cancer involves three functional properties. Castration‐resistance, tissue‐regeneration and self‐renewal are likely shared by primitive prostate cells and castration‐resistant prostate cancer cells that can mediate tumor formation in the absence of androgen. Studying stem cells and their properties will hopefully lead both to a better understanding of cell survival and proliferation in androgen‐depleted conditions and to the development of new targets for advanced disease.
Acknowledgements
We thank Barbara Anderson for manuscript preparation and members of the Witte lab for helpful discussion. A.S.G. is supported by an institutional Ruth L. Kirschstein National Research Service Award. O.N.W. is an Investigator of the Howard Hughes Medical Institute and is supported by a challenge award from the Prostate Cancer Foundation.
Goldstein Andrew S., Stoyanova Tanya, Witte Owen N., (2010), Primitive origins of prostate cancer: In vivo evidence for prostate‐regenerating cells and prostate cancer‐initiating cells, Molecular Oncology, 4, doi: 10.1016/j.molonc.2010.06.009.
References
- Abrahamsson, P.A. , 1999. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 39, 135–148. [DOI] [PubMed] [Google Scholar]
- Abrahamsson, P.A. , Falkmer, S. , Falt, K. , Grimelius, L. , 1989. The course of neuroendocrine differentiation in prostatic carcinomas. An immunohistochemical study testing chromogranin A as an “endocrine marker”. Pathol. Res. Pract. 185, 373–380. [DOI] [PubMed] [Google Scholar]
- Ahlgren, G. , Pedersen, K. , Lundberg, S. , Aus, G. , Hugosson, J. , Abrahamsson, P.A. , 2000. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate. 42, 274–279. [DOI] [PubMed] [Google Scholar]
- Akashi, K. , Traver, D. , Miyamoto, T. , Weissman, I.L. , 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 404, 193–197. [DOI] [PubMed] [Google Scholar]
- Al-Hajj, M. , Wicha, M.S. , Benito-Hernandez, A. , Morrison, S.J. , Clarke, M.F. , 2003. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 100, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society, 2009. What are the key statistics about prostate cancer?. http://www.cancer.org/Cancer/ProstateCancer/DetailedGuide/prostate-cancer-key-statistics [Google Scholar]
- Asselin-Labat, M.L. , Sutherland, K.D. , Barker, H. , Thomas, R. , Shackleton, M. , Forrest, N.C. , Hartley, L. , Robb, L. , Grosveld, F.G. , van der Wees, J. , 2007. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 9, 201–209. [DOI] [PubMed] [Google Scholar]
- Aumuller, G. , Leonhardt, M. , Janssen, M. , Konrad, L. , Bjartell, A. , Abrahamsson, P.A. , 1999. Neurogenic origin of human prostate endocrine cells. Urology. 53, 1041–1048. [DOI] [PubMed] [Google Scholar]
- Barker, N. , van Es, J.H. , Kuipers, J. , Kujala, P. , van den Born, M. , Cozijnsen, M. , Haegebarth, A. , Korving, J. , Begthel, H. , Peters, P.J. , Clevers, H. , 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Barker, N. , Ridgway, R.A. , van Es, J.H. , van de Wetering, M. , Begthel, H. , van den Born, M. , Danenberg, E. , Clarke, A.R. , Sansom, O.J. , Clevers, H. , 2009. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 457, 608–611. [DOI] [PubMed] [Google Scholar]
- Belandia, B. , Powell, S.M. , Garcia-Pedrero, J.M. , Walker, M.M. , Bevan, C.L. , Parker, M.G. , 2005. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol. Cell Biol. 25, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovska, O.P. , Glinskii, A.B. , Yang, Z. , Li, X.M. , Hoffman, R.M. , Glinsky, G.V. , 2006. Essential role for activation of the Polycomb group (PcG) protein chromatin silencing pathway in metastatic prostate cancer. Cell Cycle. 5, 1886–1901. [DOI] [PubMed] [Google Scholar]
- Bierie, B. , Nozawa, M. , Renou, J.P. , Shillingford, J.M. , Morgan, F. , Oka, T. , Taketo, M.M. , Cardiff, R.D. , Miyoshi, K. , Wagner, K.U. , 2003. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 22, 3875–3887. [DOI] [PubMed] [Google Scholar]
- Blanpain, C. , Lowry, W.E. , Geoghegan, A. , Polak, L. , Fuchs, E. , 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 118, 635–648. [DOI] [PubMed] [Google Scholar]
- Bohrer, M.H. , Schmoll, J. , 1993. Immunohistochemical and morphometric studies on neuroendocrine differentiation of prostate carcinomas. Verh Dtsch Ges Pathol. 77, 107–110. [PubMed] [Google Scholar]
- Bonkhoff, H. , Stein, U. , Remberger, K. , 1993. Androgen receptor status in endocrine-paracrine cell types of the normal, hyperplastic, and neoplastic human prostate. Virchows Arch. A Pathol. Anat. Histopathol. 423, 291–294. [DOI] [PubMed] [Google Scholar]
- Bonnet, D. , Dick, J.E. , 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737. [DOI] [PubMed] [Google Scholar]
- Bui, M. , Reiter, R.E. , 1998. Stem cell genes in androgen-independent prostate cancer. Cancer Metastasis Rev. 17, 391–399. [DOI] [PubMed] [Google Scholar]
- Burger, P.E. , Xiong, X. , Coetzee, S. , Salm, S.N. , Moscatelli, D. , Goto, K. , Wilson, E.L. , 2005. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc. Natl. Acad. Sci. USA. 102, 7180–7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, P.E. , Gupta, R. , Xiong, X. , Ontiveros, C.S. , Salm, S.N. , Moscatelli, D. , Wilson, E.L. , 2009. High aldehyde dehydrogenase activity: a novel functional marker of murine prostate stem/progenitor cells. Stem Cells. 27, 2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz, S.D. , Johnson, J.L. , 2003. BCL-2 in prostate cancer: a minireview. Apoptosis. 8, 29–37. [DOI] [PubMed] [Google Scholar]
- Chan, K.S. , Espinosa, I. , Chao, M. , Wong, D. , Ailles, L. , Diehn, M. , Gill, H. , Presti, J. , Chang, H.Y. , van de Rijn, M. , 2009. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Shukeir, N. , Potti, A. , Sircar, K. , Aprikian, A. , Goltzman, D. , Rabbani, S.A. , 2004. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 101, 1345–1356. [DOI] [PubMed] [Google Scholar]
- Collins, A.T. , Habib, F.K. , Maitland, N.J. , Neal, D.E. , 2001. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J. Cell Sci. 114, 3865–3872. [DOI] [PubMed] [Google Scholar]
- Collins, A.T. , Berry, P.A. , Hyde, C. , Stower, M.J. , Maitland, N.J. , 2005. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951. [DOI] [PubMed] [Google Scholar]
- Corey, E. , Quinn, J.E. , Buhler, K.R. , Nelson, P.S. , Macoska, J.A. , True, L.D. , Vessella, R.L. , 2003. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate. 55, 239–246. [DOI] [PubMed] [Google Scholar]
- Cotsarelis, G. , Cheng, S.Z. , Dong, G. , Sun, T.T. , Lavker, R.M. , 1989. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 57, 201–209. [DOI] [PubMed] [Google Scholar]
- Cozzio, A. , Passegue, E. , Ayton, P.M. , Karsunky, H. , Cleary, M.L. , Weissman, I.L. , 2003. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 17, 3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha, G.R. , Lung, B. , 1978. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J. Exp. Zool. 205, 181–193. [DOI] [PubMed] [Google Scholar]
- Dalrymple, S. , Antony, L. , Xu, Y. , Uzgare, A.R. , Arnold, J.T. , Savaugeot, J. , Sokoll, L.J. , De Marzo, A.M. , Isaacs, J.T. , 2005. Role of notch-1 and E-cadherin in the differential response to calcium in culturing normal versus malignant prostate cells. Cancer Res. 65, 9269–9279. [DOI] [PubMed] [Google Scholar]
- Dontu, G. , Abdallah, W.M. , Foley, J.M. , Jackson, K.W. , Clarke, M.F. , Kawamura, M.J. , Wicha, M.S. , 2003. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey, J.S. , Zacharek, S.J. , Kim, C.F. , Lees, J.A. , 2008. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc. Natl. Acad. Sci. USA. 105, 11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirew, P. , Stingl, J. , Raouf, A. , Turashvili, G. , Aparicio, S. , Emerman, J.T. , Eaves, C.J. , 2008. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat. Med. 14, 1384–1389. [DOI] [PubMed] [Google Scholar]
- Ellis, W.J. , Vessella, R.L. , Buhler, K.R. , Bladou, F. , True, L.D. , Bigler, S.A. , Curtis, D. , Lange, P.H. , 1996. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin. Cancer Res. 2, 1039–1048. [PubMed] [Google Scholar]
- Ellwood-Yen, K. , Graeber, T.G. , Wongvipat, J. , Iruela-Arispe, M.L. , Zhang, J. , Matusik, R. , Thomas, G.V. , Sawyers, C.L. , 2003. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 4, 223–238. [DOI] [PubMed] [Google Scholar]
- English, H.F. , Santen, R.J. , Isaacs, J.T. , 1987. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 11, 229–242. [DOI] [PubMed] [Google Scholar]
- Epstein, J.I. , 2010. Prostatic ductal adenocarcinoma: a mini review. Med. Princ Pract. 19, 82–85. [DOI] [PubMed] [Google Scholar]
- Garraway, I.P. , Sun, W. , Tran, C.P. , Perner, S. , Zhang, B. , Goldstein, A.S. , Hahm, S.A. , Haider, M. , Head, C.S. , Reiter, R.E. , 2009. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier, C. , Hur, M.H. , Charafe-Jauffret, E. , Monville, F. , Dutcher, J. , Brown, M. , Jacquemier, J. , Viens, P. , Kleer, C.G. , Liu, S. , 2007. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 1, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky, G.V. , Berezovska, O. , Glinskii, A.B. , 2005. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J. Clin. Invest. 115, 1503–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A.S. , Lawson, D.A. , Cheng, D. , Sun, W. , Garraway, I.P. , Witte, O.N. , 2008. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. USA. 105, 20882–20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A.S. , Huang, J. , Guo, C. , Garraway, I.P. , Witte, O.N. , 2010. Identification of a cell-of-origin for human prostate cancer. Science. 329, 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee, R.T. , Murray, T. , Bolden, S. , Wingo, P.A. , 2000. Cancer statistics. CA Cancer J. Clin. 2000, (50) 7–33. [DOI] [PubMed] [Google Scholar]
- Grisanzio, C. , Signoretti, S. , 2008. p63 in prostate biology and pathology. J. Cell Biochem. 103, 1354–1368. [DOI] [PubMed] [Google Scholar]
- Gurel, B. , Iwata, T. , Koh, C.M. , Jenkins, R.B. , Lan, F. , Van Dang, C. , Hicks, J.L. , Morgan, J. , Cornish, T.C. , Sutcliffe, S. , 2008. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod. Pathol. 21, 1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, K.U. , Reynolds, S.D. , Watkins, S. , Fuchs, E. , Stripp, B.R. , 2004. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 164, 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Yao, J.L. , di Sant'Agnese, P.A. , Yang, Q. , Bourne, P.A. , Na, Y. , 2006. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate. 66, 1399–1406. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Wu, C. , di Sant'Agnese, P.A. , Yao, J.L. , Cheng, L. , Na, Y. , 2007. Function and molecular mechanisms of neuroendocrine cells in prostate cancer. Anal. Quant Cytol. Histol. 29, 128–138. [PubMed] [Google Scholar]
- Huang, J. , Witte, O.N. , 2010. A seminal finding for prostate cancer?. N. Engl. J. Med. 362, 175–176. [DOI] [PubMed] [Google Scholar]
- Huggins, C. , 1943. Endocrine control of prostatic cancer. Science. 97, 541–544. [DOI] [PubMed] [Google Scholar]
- Huggins, C. , Hodges, C.V. , 1941. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297. [DOI] [PubMed] [Google Scholar]
- Huntly, B.J. , Shigematsu, H. , Deguchi, K. , Lee, B.H. , Mizuno, S. , Duclos, N. , Rowan, R. , Amaral, S. , Curley, D. , Williams, I.R. , 2004. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 6, 587–596. [DOI] [PubMed] [Google Scholar]
- Hurt, E.M. , Kawasaki, B.T. , Klarmann, G.J. , Thomas, S.B. , Farrar, W.L. , 2008. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br. J. Cancer. 98, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs, J.T. , 1987. Control of cell proliferation and cell death in the normal and neoplastic prostate: a stem cell model. In Rodgers C.H., Benign Prostatic Hyperplasia. National Institutes of Health; Bethesda, Maryland: 85–94. [Google Scholar]
- Iwai, N. , Zhou, Z. , Roop, D.R. , Behringer, R.R. , 2008. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 26, 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiborn, T. , Bjartell, A. , Abrahamsson, P.A. , 1998. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 51, 585–589. [DOI] [PubMed] [Google Scholar]
- Kelly, P.N. , Dakic, A. , Adams, J.M. , Nutt, S.L. , Strasser, A. , 2007. Tumor growth need not be driven by rare cancer stem cells. Science. 317, 337 [DOI] [PubMed] [Google Scholar]
- Kiel, M.J. , Yilmaz, O.H. , Iwashita, T. , Yilmaz, O.H. , Terhorst, C. , Morrison, S.J. , 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 121, 1109–1121. [DOI] [PubMed] [Google Scholar]
- Klein, K.A. , Reiter, R.E. , Redula, J. , Moradi, H. , Zhu, X.L. , Brothman, A.R. , Lamb, D.J. , Marcelli, M. , Belldegrun, A. , Witte, O.N. , Sawyers, C.L. , 1997. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat. Med. 3, 402–408. [DOI] [PubMed] [Google Scholar]
- Klezovitch, O. , Risk, M. , Coleman, I. , Lucas, J.M. , Null, M. , True, L.D. , Nelson, P.S. , Vasioukhin, V. , 2008. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc. Natl. Acad. Sci. USA. 105, 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsten, H. , Ziel-van der Made, A. , Ma, X. , van der Kwast, T. , Trapman, J. , 2009. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS One. 4, e5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnen, J.L. , Janssen, P.J. , Ruizeveld de Winter, J.A. , van Krimpen, H. , Schroder, F.H. , van der Kwast, T.H. , 1993. Do neuroendocrine cells in human prostate cancer express androgen receptor?. Histochemistry. 100, 393–398. [DOI] [PubMed] [Google Scholar]
- Krivtsov, A.V. , Twomey, D. , Feng, Z. , Stubbs, M.C. , Wang, Y. , Faber, J. , Levine, J.E. , Wang, J. , Hahn, W.C. , Gilliland, D.G. , 2006. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 442, 818–822. [DOI] [PubMed] [Google Scholar]
- Lapidot, T. , Sirard, C. , Vormoor, J. , Murdoch, B. , Hoang, T. , Caceres-Cortes, J. , Minden, M. , Paterson, B. , Caligiuri, M.A. , Dick, J.E. , 1994. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 367, 645–648. [DOI] [PubMed] [Google Scholar]
- Lawson, D.A. , Zong, Y. , Memarzadeh, S. , Xin, L. , Huang, J. , Witte, O.N. , 2010. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc. Natl. Acad. Sci. USA. 107, 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, D.A. , Xin, L. , Lukacs, R.U. , Cheng, D. , Witte, O.N. , 2007. Isolation and functional characterization of murine prostate stem cells. Proc. Natl. Acad. Sci. USA. 104, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leenders, G. , Dijkman, H. , Hulsbergen-van de Kaa, C. , Ruiter, D. , Schalken, J. , 2000. Demonstration of intermediate cells during human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy. Lab. Invest. 80, 1251–1258. [DOI] [PubMed] [Google Scholar]
- van Leenders, G.J. , Dukers, D. , Hessels, D. , van den Kieboom, S.W. , Hulsbergen, C.A. , Witjes, J.A. , Otte, A.P. , Meijer, C.J. , Raaphorst, F.M. , 2007. Polycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features. Eur. Urol. 52, 455–463. [DOI] [PubMed] [Google Scholar]
- Leong, K.G. , Wang, B.E. , Johnson, L. , Gao, W.Q. , 2008. Generation of a prostate from a single adult stem cell. Nature. 456, 804–808. [DOI] [PubMed] [Google Scholar]
- Leong, K.G. , Gao, W.Q. , 2008. The Notch pathway in prostate development and cancer. Differentiation. 76, 699–716. [DOI] [PubMed] [Google Scholar]
- Lim, E. , Vaillant, F. , Wu, D. , Forrest, N.C. , Pal, B. , Hart, A.H. , Asselin-Labat, M.L. , Gyorki, D.E. , Ward, T. , Partanen, A. , 2009. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 15, 907–913. [DOI] [PubMed] [Google Scholar]
- Lukacs, R.U. , Lawson, D.A. , Xin, L. , Zong, Y. , Garraway, I. , Goldstein, A.S. , Memarzadeh, S. , Witte, O.N. , 2008. Epithelial stem cells of the prostate and their role in cancer progression. Cold Spring Harb Symp. Quant Biol. 73, 491–502. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Ziel-van der Made, A.C. , Autar, B. , van der Korput, H.A. , Vermeij, M. , van Duijn, P. , Cleutjens, K.B. , de Krijger, R. , Krimpenfort, P. , Berns, A. , 2005. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 65, 5730–5739. [DOI] [PubMed] [Google Scholar]
- McDonnell, T.J. , Troncoso, P. , Brisbay, S.M. , Logothetis, C. , Chung, L.W. , Hsieh, J.T. , Tu, S.M. , Campbell, M.L. , 1992. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 52, 6940–6944. [PubMed] [Google Scholar]
- Morrison, S.J. , Uchida, N. , Weissman, I.L. , 1995. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11, 35–71. [DOI] [PubMed] [Google Scholar]
- Morrison, S.J. , Weissman, I.L. , 1994. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1, 661–673. [DOI] [PubMed] [Google Scholar]
- Mulholland, D.J. , Cheng, H. , Reid, K. , Rennie, P.S. , Nelson, C.C. , 2002. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J. Biol. Chem. 277, 17933–17943. [DOI] [PubMed] [Google Scholar]
- Mulholland, D.J. , Xin, L. , Morim, A. , Lawson, D. , Witte, O. , Wu, H. , 2009. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 69, 8555–8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, C.A. , Pollett, A. , Gallinger, S. , Dick, J.E. , 2007. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 445, 106–110. [DOI] [PubMed] [Google Scholar]
- Okada, H. , Tsubura, A. , Okamura, A. , Senzaki, H. , Naka, Y. , Komatz, Y. , Morii, S. , 1992. Keratin profiles in normal/hyperplastic prostates and prostate carcinoma. Virchows Arch. A Pathol. Anat. Histopathol. 421, 157–161. [DOI] [PubMed] [Google Scholar]
- Ontiveros, C.S. , Salm, S.N. , Wilson, E.L. , 2008. Axin2 expression identifies progenitor cells in the murine prostate. Prostate. 68, 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palapattu, G.S. , Wu, C. , Silvers, C.R. , Martin, H.B. , Williams, K. , Salamone, L. , Bushnell, T. , Huang, L.S. , Yang, Q. , Huang, J. , 2009. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 69, 787–798. [DOI] [PubMed] [Google Scholar]
- Passegue, E. , Wagner, E.F. , Weissman, I.L. , 2004. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 119, 431–443. [DOI] [PubMed] [Google Scholar]
- Patrawala, L. , Calhoun, T. , Schneider-Broussard, R. , Li, H. , Bhatia, B. , Tang, S. , Reilly, J.G. , Chandra, D. , Zhou, J. , Claypool, K. , 2006. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 25, 1696–1708. [DOI] [PubMed] [Google Scholar]
- Patrawala, L. , Calhoun-Davis, T. , Schneider-Broussard, R. , Tang, D.G. , 2007. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 67, 6796–6805. [DOI] [PubMed] [Google Scholar]
- Pereira, D.S. , Dorrell, C. , Ito, C.Y. , Gan, O.I. , Murdoch, B. , Rao, V.N. , Zou, J.P. , Reddy, E.S. , Dick, J.E. , 1998. Retroviral transduction of TLS-ERG initiates a leukemogenic program in normal human hematopoietic cells. Proc. Natl. Acad. Sci. USA. 95, 8239–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinthus, J.H. , Waks, T. , Schindler, D.G. , Harmelin, A. , Said, J.W. , Belldegrun, A. , Ramon, J. , Eshhar, Z. , 2000. WISH-PC2: a unique xenograft model of human prostatic small cell carcinoma. Cancer Res. 60, 6563–6567. [PubMed] [Google Scholar]
- Prat, A. , Perou, C.M. , 2009. Mammary development meets cancer genomics. Nat. Med. 15, 842–844. [DOI] [PubMed] [Google Scholar]
- Pretlow, T.G. , Wolman, S.R. , Micale, M.A. , Pelley, R.J. , Kursh, E.D. , Resnick, M.I. , Bodner, D.R. , Jacobberger, J.W. , Delmoro, C.M. , Giaconia, J.M. , 1993. Xenografts of primary human prostatic carcinoma. J. Natl. Cancer Inst. 85, 394–398. [DOI] [PubMed] [Google Scholar]
- Pretlow, T.G. , Schwartz, S. , Giaconia, J.M. , Wright, A.L. , Grimm, H.A. , Edgehouse, N.L. , Murphy, J.R. , Markowitz, S.D. , Jamison, J.M. , Summers, J.L. , 2000. Prostate cancer and other xenografts from cells in peripheral blood of patients. Cancer Res. 60, 4033–4036. [PubMed] [Google Scholar]
- Quintana, E. , Shackleton, M. , Sabel, M.S. , Fullen, D.R. , Johnson, T.M. , Morrison, S.J. , 2008. Efficient tumour formation by single human melanoma cells. Nature. 456, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya, T. , Morrison, S.J. , Clarke, M.F. , Weissman, I.L. , 2001. Stem cells, cancer, and cancer stem cells. Nature. 414, 105–111. [DOI] [PubMed] [Google Scholar]
- Reynolds, B.A. , Weiss, S. , 1996. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 175, 1–13. [DOI] [PubMed] [Google Scholar]
- Richardson, G.D. , Robson, C.N. , Lang, S.H. , Neal, D.E. , Maitland, N.J. , Collins, A.T. , 2004. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 117, 3539–3545. [DOI] [PubMed] [Google Scholar]
- Robinson, E.J. , Neal, D.E. , Collins, A.T. , 1998. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 37, 149–160. [DOI] [PubMed] [Google Scholar]
- Rock, J.R. , Onaitis, M.W. , Rawlins, E.L. , Lu, Y. , Clark, C.P. , Xue, Y. , Randell, S.H. , Hogan, B.L. , 2009. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA. 106, 12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Sant'Agnese, P.A. , 1992. Neuroendocrine differentiation in human prostatic carcinoma. Hum. Pathol. 23, 287–296. [DOI] [PubMed] [Google Scholar]
- Scher, H.I. , Sawyers, C.L. , 2005. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 23, 8253–8261. [DOI] [PubMed] [Google Scholar]
- Shackleton, M. , Vaillant, F. , Simpson, K.J. , Stingl, J. , Smyth, G.K. , Asselin-Labat, M.L. , Wu, L. , Lindeman, G.J. , Visvader, J.E. , 2006. Generation of a functional mammary gland from a single stem cell. Nature. 439, 84–88. [DOI] [PubMed] [Google Scholar]
- Shi, X. , Gipp, J. , Bushman, W. , 2007. Anchorage-independent culture maintains prostate stem cells. Dev. Biol. 312, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou, J. , Ross, S. , Koeppen, H. , de Sauvage, F.J. , Gao, W.Q. , 2001. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res. 61, 7291–7297. [PubMed] [Google Scholar]
- Singh, S.K. , Hawkins, C. , Clarke, I.D. , Squire, J.A. , Bayani, J. , Hide, T. , Henkelman, R.M. , Cusimano, M.D. , Dirks, P.B. , 2004. Identification of human brain tumour initiating cells. Nature. 432, 396–401. [DOI] [PubMed] [Google Scholar]
- So, C.W. , Karsunky, H. , Passegue, E. , Cozzio, A. , Weissman, I.L. , Cleary, M.L. , 2003. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell. 3, 161–171. [DOI] [PubMed] [Google Scholar]
- Stingl, J. , Eaves, C.J. , Kuusk, U. , Emerman, J.T. , 1998. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation. 63, 201–213. [DOI] [PubMed] [Google Scholar]
- Stingl, J. , Eaves, C.J. , Zandieh, I. , Emerman, J.T. , 2001. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat. 67, 93–109. [DOI] [PubMed] [Google Scholar]
- Stingl, J. , Eirew, P. , Ricketson, I. , Shackleton, M. , Vaillant, F. , Choi, D. , Li, H.I. , Eaves, C.J. , 2006. Purification and unique properties of mammary epithelial stem cells. Nature. 439, 993–997. [DOI] [PubMed] [Google Scholar]
- Tomlins, S.A. , Laxman, B. , Dhanasekaran, S.M. , Helgeson, B.E. , Cao, X. , Morris, D.S. , Menon, A. , Jing, X. , Cao, Q. , Han, B. , 2007. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 448, 595–599. [DOI] [PubMed] [Google Scholar]
- Tsujimura, A. , Koikawa, Y. , Salm, S. , Takao, T. , Coetzee, S. , Moscatelli, D. , Shapiro, E. , Lepor, H. , Sun, T.T. , Wilson, E.L. , 2002. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J. Cell Biol. 157, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Griend, D.J. , Karthaus, W.L. , Dalrymple, S. , Meeker, A. , DeMarzo, A.M. , Isaacs, J.T. , 2008. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 68, 9703–9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen, A.P. , Ramaekers, F.C. , Aalders, T.W. , Schaafsma, H.E. , Debruyne, F.M. , Schalken, J.A. , 1992. Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res. 52, 6182–6187. [PubMed] [Google Scholar]
- Wainstein, M.A. , He, F. , Robinson, D. , Kung, H.J. , Schwartz, S. , Giaconia, J.M. , Edgehouse, N.L. , Pretlow, T.P. , Bodner, D.R. , Kursh, E.D. , 1994. CWR22: androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer Res. 54, 6049–6052. [PubMed] [Google Scholar]
- Wang, S. , Garcia, A.J. , Wu, M. , Lawson, D.A. , Witte, O.N. , Wu, H. , 2006. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl. Acad. Sci. USA. 103, 1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.D. , Leow, C.C. , Zha, J. , Tang, Z. , Modrusan, Z. , Radtke, F. , Aguet, M. , de Sauvage, F.J. , Gao, W.Q. , 2006. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev. Biol. 290, 66–80. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Kruithof-de Julio, M. , Economides, K.D. , Walker, D. , Yu, H. , Halili, M.V. , Hu, Y.P. , Price, S.M. , Abate-Shen, C. , Shen, M.M. , 2009. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 461, 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Li, Y. , Banerjee, S. , Kong, D. , Ahmad, A. , Nogueira, V. , Hay, N. , Sarkar, F.H. , 2010. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J. Cell Biochem. [DOI] [PubMed] [Google Scholar]
- Williams, R.T. , den Besten, W. , Sherr, C.J. , 2007. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev. 21, 2283–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, L. , Ide, H. , Kim, Y. , Dubey, P. , Witte, O.N. , 2003. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc. Natl. Acad. Sci. USA. 100, (Suppl. 1) 11896–11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, L. , Lawson, D.A. , Witte, O.N. , 2005. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc. Natl. Acad. Sci. USA. 102, 6942–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, L. , Lukacs, R.U. , Lawson, D.A. , Cheng, D. , Witte, O.N. , 2007. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 25, 2760–2769. [DOI] [PubMed] [Google Scholar]
- Yang, F. , Li, X. , Sharma, M. , Sasaki, C.Y. , Longo, D.L. , Lim, B. , Sun, Z. , 2002. Linking beta-catenin to androgen-signaling pathway. J. Biol. Chem. 277, 11336–11344. [DOI] [PubMed] [Google Scholar]
- Yoshimoto, M. , Cutz, J.C. , Nuin, P.A. , Joshua, A.M. , Bayani, J. , Evans, A.J. , Zielenska, M. , Squire, J.A. , 2006. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet. Cytogenet. 169, 128–137. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, Z. , Ahmed, F. , Banerjee, S. , Li, Y. , Sarkar, F.H. , 2006. Down-regulation of Jagged-1 induces cell growth inhibition and S phase arrest in prostate cancer cells. Int. J. Cancer. 119, 2071–2077. [DOI] [PubMed] [Google Scholar]
- Zhao, C. , Blum, J. , Chen, A. , Kwon, H.Y. , Jung, S.H. , Cook, J.M. , Lagoo, A. , Reya, T. , 2007. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 12, 528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , Gibson, P. , Currle, D.S. , Tong, Y. , Richardson, R.J. , Bayazitov, I.T. , Poppleton, H. , Zakharenko, S. , Ellison, D.W. , Gilbertson, R.J. , 2009. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 457, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong, Y. , Xin, L. , Goldstein, A.S. , Lawson, D.A. , Teitell, M.A. , Witte, O.N. , 2009. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc. Natl. Acad. Sci. USA. 106, 12465–12470. [DOI] [PMC free article] [PubMed] [Google Scholar]


