Abstract
Objective
To compare the in vitro sensitivity/resistance to patupilone versus paclitaxel in uterine serous papillary carcinoma (USPC) with high versus low HER-2/neu expression.
Methods
Six primary USPC cell lines, half of which overexpress HER-2/neu at a 3+ level, were evaluated for growth rate and tested for their in vitro sensitivity/resistance to patupilone versus paclitaxel by MTS assays. Quantitative RT-PCR was used to identify potential mechanisms underlying the differential sensitivity/resistance to patupilone versus paclitaxel in primary USPC cell lines.
Results
Cell lines overexpressing HER-2/neu showed higher proliferation when compared to low HER-2/neu-expressing cell lines. Compared to low-expressing cell lines, high HER-2/neu expressors were significantly more sensitive to patupilone than to paclitaxel (P < 0.0002). In contrast, there was no appreciable difference in sensitivity to patupilone versus paclitaxel in primary USPC cell lines with low HER-2/neu expression. Higher levels of β-tubulin III (TUBB3) and P-glycoprotein (ABCB1) were detected in USPC cell lines with high versus low HER-2/neu expression (P < 0.05).
Conclusions
USPC overexpressing HER-2/neu display greater in vitro sensitivity to patupilone and higher levels of the patupilone molecular target TUBB3 when compared to low HER-2/neu expressors. Due to the adverse prognosis associated with HER-2/neu overexpression in USPC patients, patupilone may represent a promising novel drug to combine to platinum compounds in this subset of aggressive endometrial tumors.
Keywords: Endometrial neoplasms, uterine serous tumors, HER-2/neu, Patupilone, Paclitaxel
Introduction
Endometrial cancer is the most common female genital tract malignancy in the United States, with an incidence of 42,160 new cases and 7,780 deaths in 2009 [1]. Uterine serous papillary carcinoma (USPC) represents the second most common histologic type of endometrial cancer, constituting up to 10% of all endometrial tumors [2-5]. Due to its propensity for early intraabdominal and lymphatic spread and its inborn resistance to chemotherapy, USPC is characterized by a highly aggressive biologic behavior [2-5]. The survival rate is dismal, even when USPC is only a minor component of the histologically more common endometrioid adenocarcinoma, and widespread metastasis and high mortality may occur even in those cases in which tumor is confined to the endometrium or to an endometrial polyp [2-6]. The overall 5-year survival is about 30% for all stages and the recurrence rate after surgery is extremely high (50% to 80%). A better understanding of the molecular basis of the aggressive biologic behavior of USPC as well as the development of novel, more specific and more effective treatment modalities against this variant of endometrial cancer remain a high priority.
Patupilone (epothilone B; EPO906), a macrocyclic polyketide, is a member of the epothilone class, a group of microtubule-stabilizing agents [7]. Although structurally unrelated to the taxanes, patupilone binds to a similar, although not identical, pharmacophore on the B-tubulin subunit of microtubules [7, 8]. Data suggest that patupilone binds to this site with higher affinity than other epothilones or taxanes [9-11] and it is a more potent inducer of tubulin dimerization and is more effective in stabilizing preformed microtubules than paclitaxel [9-12]. This finding has been attributed to patupilone’s ability to competitively inhibit the binding of paclitaxel to microtubules, and in particular, to β-tubulin III [10-12]. Patupilone has been shown to exhibit a 3- to 20-fold higher in vitro cytotoxic potency than paclitaxel, and to have cytotoxic activity in a broad range of paclitaxel-sensitive and resistant cells overexpressing the P-glycoprotein efflux pump [10-12]. Importantly, preclinical data have confirmed that patupilone may retain activity in patients relapsing from or resistant to a taxane-containing chemotherapy [10-12].
Our group has previously reported HER-2/neu overexpression by IHC and amplification of the c-erbB2 gene by FISH in a large percentage of patients harboring USPC [13-15]. These findings, recently confirmed by other groups [16] including the Gynecologic Oncology Group (GOG) in a cooperative multicentric study [17], have identified HER-2/neu overexpression in USPC as an independent variable associated with poor outcome, and one that occurs more frequently in African-American women than in Caucasian women [13-15]. Although HER-2/neu overexpression has been previously associated with resistance to chemotherapy and poor survival in multiple human malignancies including breast [18], ovarian [19] and endometrial carcinoma [20], to our knowledge, no study has evaluated and compared the in vitro chemo-sensitivity/resistance of this highly aggressive variant of endometrial cancer to microtubular targeting agents such as paclitaxel and patupilone. To fill this gap in knowledge, in this study we have carefully analyzed the sensitivity/resistance to patupil one of six primary USPC cell lines, half of which overexpress HER-2/neu at 3+ levels and showed amplification of the c-erbB2 oncogene by FISH [21], and compared such response to that of paclitaxel in vitro. We report the first evidence that primary USPC cell lines overexpressing HER-2/neu display higher levels of the patupilone molecular target B-Tubulin class III (TUBB3) and greater in vitro sensitivity to patupilone when compared to low HER-2/neu expressors. Patupilone may represent a promising novel drug to combine to platinum compounds in patients harboring this rare but highly aggressive variant of endometrial cancer.
Materials and Methods
Drugs
Patupilone – provided by Novartis Pharma, Basel, Switzerland – was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, Mo., USA) as a 10-mM stock solution, divided into 5- μl aliquots, and stored at −20 ° C until use. Paclitaxel was purchased from Bristol-Myers Squibb, Princeton, N.J., USA. Both drugs were diluted in culture medium immediately before addition to cell lines.
Establishment and HER-2/neu expression of USPC Cell Lines
Primary USPC tumor cell lines from six patients with invasive USPC were obtained from fresh tumor biopsies collected at the time of surgery, under approval of the Institutional Review Board. Tumors were staged according to the International Federation of Gynecologists and Obstetricians operative staging system. Six primary USPC cell lines (USPC-ARK-1, USPC-ARK-2, USPC-ARK-3, USPC-ARK-4, USPC-ARK-5, and USPC-ARK-6) were established after sterile processing of the tumor samples from surgical biopsies as described previously [21]. Source-patient characteristics of these six USPC cell lines are described in Table 1. The amplification of the c-erbB2 gene by FISH, expression levels of HER-2/neu receptor by immunohistochemistry (IHC) and mRNA expression levels by quantitative RT-PCR for these primary USPC cell lines have been recently reported [21] and are presented in Table 2.
Table 1.
Patient characteristics from which the six USPC cell lines were established
| Patient | Age (years) | Race* | FIGO^ Stage |
USPC Histopathology |
Year of Diagnosis |
|---|---|---|---|---|---|
| USPC-ARK-1 | 62 | AA | IVA | Pure | 1997 |
| USPC-ARK-2 | 63 | AA | IVB | Pure | 1998 |
| USPC-ARK-3 | 59 | AA | IVB | Mixed | 2006 |
| USPC-ARK-4 | 73 | C | IVB | Pure | 2004 |
| USPC-ARK-5 | 73 | AA | IIIC | Pure | 2006 |
| USPC-ARK-6 | 62 | C | IB | Mixed | 2005 |
AA, African American; C, Caucasian
FIGO, International Federation of Gynecology and Obstetrics
Table 2.
HER-2/neu expression in primary USPC cell lines
| Sample | RT-PCR° | ||
|---|---|---|---|
| IHC* | FISH^ | mRNA Copy Number | |
| Control | 1 | ||
| USPC-ARK-1 | 3+ | 2.5 | 373 |
| USPC-ARK-2 | 3+ | 5.2 | 607 |
| USPC-ARK-3 | 3+ | 4.7 | 677 |
| USPC-ARK-4 | 0 | 1.6 | 7 |
| USPC-ARK-5 | 0 | 1.4 | 13 |
| USPC-ARK-6 | 1+ | 0.9 | 6 |
IHC, Immunohistochemistry
FISH, Fluorescent In-situ Hybridization
RT-PCR, Real-time Polymerase Chain Reaction.
Primary USPC growth rate analysis
The growth rate for each of the USPC cell lines was determined by counting the number of live cells 24, 48, 72 and 96 hours after plating. In brief, cell lines were cultured in RPMI 1640 (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah). When cultures had grown to approximately 80% confluence, cells were harvested from the flask using 0.25% Trypsin EDTA (Invitrogen, Carlsbad, CA), then counted on a hemocytometer chamber and assessed for viability via trypan blue exclusion. Cell density was adjusted to a concentration of 10,000 cells/mL, then cells were seeded into a 6-well microtiter plate (Corning Life Sciences, Lowell, MA) at a density of 20,000 cells per well. Three replicate wells were plated per cell line per time point. Cell plates were incubated at 37°C with 5% CO2 for 24, 48, 72, and 96 hours, at which time they were removed from the incubator, harvested from the wells using 0.25% Trypsin EDTA, counted on a hemocytometer chamber and assessed for viability via trypan blue exclusion .
MTS assay
Cells at log phase of growth were seeded in 96-well culture plates at optimum density and allowed to attach for 24 h. Media were removed after 24 h and replaced with culture medium 200 μl or the same volume of medium containing various concentrations of patupilone and paclitaxel (i.e., from 1 nM to 20,000 nM). After 72-hour incubation, 20 μl of CellTiter 96® AQueous One Solution Reagent containing a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium, inner salt; MTS] (Promega Corporation, Madison, WI) was added, incubated and removed according to manufacturer’s instructions. Absorbance was measured by a 96-well VERSA max plate reader (Molecular Devices, Sunnyvale, CA, USA) at a reference wavelength of 490 nm. Each drug concentration was obtained in 3 replicate wells, and each experiment was repeated a minimum of 3 times. Results are displayed as the average ± standard deviation from 3 to 5 independent experiments.
P-glycoprotein and B-Tubulin class I and III expression by Real-Time PCR
q-RT-PCR was performed with an ABI Prism 7000 Sequence Analyzer using the manufacturer’s recommended protocol (Applied Biosystems, Foster City, CA) to evaluate expression of the P-glycoprotein (ABCB1), B-Tubulin class I (TUBB1) and B-Tubulin class III (TUBB3) genes in samples from all primary cell lines. Each reaction was run in triplicate. The comparative threshold cycle (CT) method (PE Applied Biosystems) was used to determine gene expression in each sample relative to the value observed in the nonmalignant endometrial epithelial cells, using GAPDH (Assay-on-Demand Hs99999905_m1) RNA as an internal control. Briefly, five μg of total RNA from each sample was reverse transcribed using SuperScript III first strand cDNA synthesis (Invitrogen, Carlsbad, CA). Ten μl of reverse transcribed RNA samples (from 500 μl of total volume) were amplified by using the TaqMan Universal PCR Master Mix (Applied Biosystems) to produce PCR products specific for ABCB1, TUBB1 and TUBB3. Primers were obtained from Applied Biosystems: Assay ID: Hs00184491_m1 (ABCB1), Hs00258236_m1 (TUBB1) and Hs00964962_g1 (TUBB3).
Statistical Analysis
All statistical analysis was conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC). For each independent MTS experiment of a given drug on a given cell line, the measures of growth under different dose levels were normalized to the mean of the control group receiving no drug, so that all data were expressed as a proportion of the control. Normalized data then were fit via nonlinear regression to a three-parameter logistic response curve against the base-10 logarithms of dose in nM, and the resulting parameter estimates were used to calculate the value of the IC50 (in log10 units) for that experiment. The collection of log10(IC50)s thereby determined were compared within each cell line for the patupilone-versus-paclitaxel difference using mixed-models ANOVA with drug as the fixed effect and experiment as the random effect. Drug-group means and differences in log10(IC50) were converted by exponentiation to geometric means and ratios of IC50 in nM. Additionally, mixed-models ANOVA, with cell line as a second random effect, was used to examine the impact of HER-2/neu amplification status on drug differences in log10(IC50)s. For qRT-PCR experiments, the relative expressions were converted to log2 units to facilitate hypothesis testing on ratios, and analyzed via mixed-models ANOVA with HER-2/neu amplification status as the fixed effect, and cell line and RT-PCR replication number as the random effects. Differences in all comparisons were considered significant at P values < 0.05.
Results
Growth Curve
The doubling time of all 6 primary USPC cell lines was evaluated by counting the number of live cells 24, 48, 72, and 96 hours after plating as described in the methods section. As shown in Figure 1, population doubling times for USPC-ARK-1, USPC-ARK-2, USPC-ARK-3 (all high HER-2/neu expressors), and USPC-ARK-4 (a low HER-2/neu expressor) were similar, with the USPC cell lines exhibiting doubling times of 22 hours, 21 hrs, 22 hours and 18 hours, respectively. In contrast, a lower growth rate was detected in USPC-ARK-5 and USPC-ARK-6 cells (both low HER-2/neu expressors), with doubling times of 51 hours and 40 hours, respectively (Figure 1).
Figure 1. Growth curves of primary USPC cell lines.
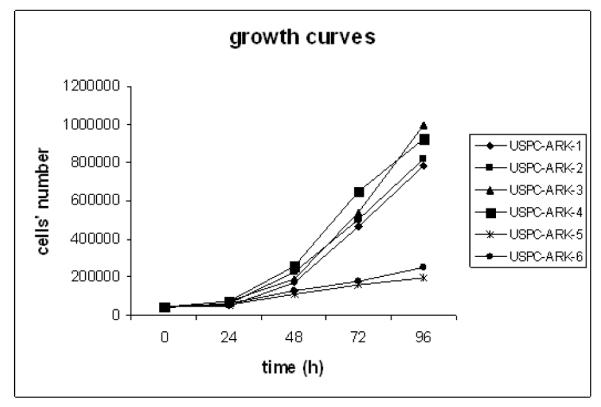
High HER-2/neu expressors (USPC-ARK-1, USPC-ARK-2, USPC-ARK-3) versus Low HER-2/neu expressors (USPC-ARK-4, USPC-ARK-5, USPC-ARK-6).
MTS Chemoresponse assay
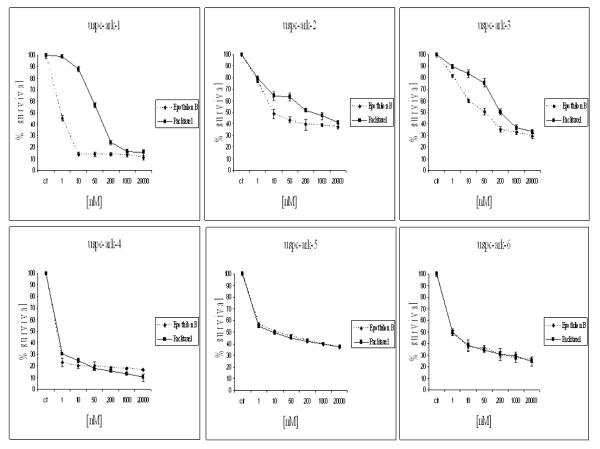
We compared by MTS assays the effects of patupilone versus paclitaxel single-agent chemotherapy against 6 primary USPC cell lines expressing different levels of HER-2/neu using serial dilutions of each anticancer agent in growth medium to create 6 distinct testing concentrations. A consistent and significant increase in sensitivity to patupilone when compared to paclitaxel in the high versus low HER-2/neu expressor USPC cell lines was consistently noted in multiple experiments (Figure 2). As shown in Figure 3 for each individual cell line, with no exception, all USPC cell lines with high HER-2/neu expression, regardless of their sensitivity/resistance to paclitaxel, showed a higher sensitivity to patupilone when compared to paclitaxel. As a group, the three HER-2/neuamplified lines taken together showed significantly lower IC50s for patupilone compared to paclitaxel (Figure 3, P=0.0002), whereas the three unamplified lines taken together did not (P=0.54); moreover, the presence of a significant (P=0.014) HER-2/neu-by-drug interaction shows that the presence/absence of HER-2/neu amplification significantly affects the magnitude of the differential response to patupilone versus paclitaxel (Figure 3). Importantly, the low HER-2/neu expressors, regardless of their fast or slow doubling times, were found to be similarly responsive to patupilone and paclitaxel and in no cell line did the difference in sensitivity detected between the two drugs reach statistical significance (Figure 3). Of interest, although USPC-ARK-1 stood out as the most highly resistant primary USPC cell line to the exposure to paclitaxel, it remained highly sensitive to patupilone exposure (Figure 2 and 3).
Figure 2. Representative dose-response curves following exposure to patupilone versus paclitaxel of USPC primary cell lines with High versus Low HER-2/neu expression.
Survival was assessed by MTS assay as described in the Methods section. Each point on the cell line graph represents the mean of 3 estimations + SE.
Figure 3. IC50s of Patupilone and Paclitaxel in six USPC cell lines.
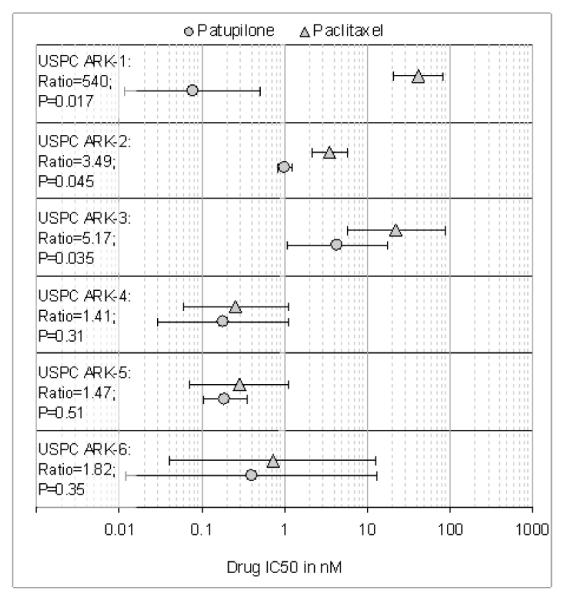
Symbols (error bars) denote means (standard deviations) of IC50s. Text to the left of the symbols denotes the cell line, its paclitaxel/patupilone ratio of IC50s, and the ratio’s P value. Note that the individual ratios are significant (at P<0.05) for the cell lines that show HER-2/neu amplification by FISH, but not significant in the cell lines that don’t. As a class, the three HER-2/neuamplified lines taken together showed significantly lower IC50s for patupilone compared to paclitaxel (P=0.0002), whereas the three unamplified lines taken together did not (P=0.54).
Molecular targets of sensitivity/resistance to Patupilone and Paclitaxel
To elucidate a potential mechanism for USPC chemosensitivity to patupilone, the expression of P-glycoprotein (ABCB1), β-tubulin class I (TUBB1), and β-tubulin class III (TUBB3) was measured and compared by RT-PCR in the high HER-2/neu expressors (USPC-ARK-1, USPC-ARK-2, USPC-ARK-3) versus the low HER-2/neu expressors (USPC-ARK-4, USPC-ARK-5, USPC-ARK-6) cell lines. These three probes were chosen because previous studies have shown major differences in their expression in paclitaxel resistant ovarian cancer cells [22-24]. Experiments were run in triplicate. P-gp (ABCB1) expression was elevated by 44-fold in the high HER2/neu expressors which was a significant difference compared to the low HER-2/neu expressors (Figure 4, P < 0.05). Overexpression of HER-2/neu was also correlated with elevated TUBB3 expression. The difference was significant and equivalent to a 2-fold increase in TUBB3 expression among high compared to low expressors (Figure 4, P < 0.05). There was no appreciable difference observed in the expression of TUBB1 (data not shown).
Figure 4. P-glycoprotein (ABCB1) and β-tubulin class III (TUBB3) expression by RT-PCR in high versus low HER-2/neu expressor cell lines.
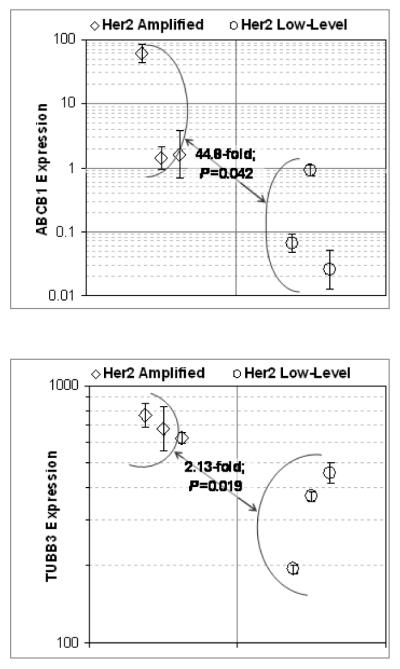
Upper panel: ABCB1; Lower panel: TUBB3. Symbols (error bars) denote the geometric means (SDs) of relative expression in USPC-ARK-1 through ARK-6 ordered from left to right-respectively; symbol shapes and figure brackets denote groupings of cell lines into high and low HER-2/neu expressor groups as indicated by the legends. In each panel, the text between expressor groups reports the geometric mean fold change and P value between high and low expressor groups.
Discussion
Uterine serous carcinoma is a biologically aggressive histologic subtype of endometrial cancer associated with a disproportionate number of deaths. The clinical response of this aggressive tumor subtype to combined cisplatinum-based regimens remain low and of short duration [2-6]. Phase II studies conducted by the Gynecologic Oncology Group (GOG), have shown that 3 drugs (i.e., carboplatin/cisplatin, doxorubicin and paclitaxel) retain the greatest activity against endometrial cancer [25-28], however, many patients treated with the most effective regimen currently available, i.e., paclitaxel/doxorubicin/cisplatin (TAP regimen) still suffer recurrence [28]. To address the need for the development of additional active chemotherapy agents, one ongoing area of investigation involves the relationship between activation of an oncogene like c-erbB2 and resistance to chemotherapy in endometrial cancer patients. Human epidermal growth factor receptor-2 (HER-2/neu; erbB2) has been described as the most potent oncoprotein amongst all four HER family members [29]. When overexpressed, it has been shown to be involved in the mechanism leading to the disruption of normal cellular control and formation of aggressive tumor cells in multiple human tumors including type II endometrial cancer. Consistent with this view, a recent study conducted by the GOG reported HER-2/neu overexpression (either 2+ (moderate) or 3+ (strong)) in 23 of 38 (61%) of the USPC tested [17]. These results are consistent with the HER-2/neu positivity previously reported by our own research group [13] and those of Diaz-Montes et al [16]. While there are reports suggesting that overexpression of the HER-2/neu oncogene in USPC patients may be a marker for intrinsic multidrug resistance as in other human cancers [18-20], to our knowledge, no studies have carefully analyzed and compared the in vitro sensitivity/resistance of primary USPC cell lines to microtubular-targeting agents such as paclitaxel and patupilone and whether overexpression of the HER-2/neu gene in these tumors may directly confer higher chemotherapy resistance.
In this study, we found USPC cell lines overexpressing HER-2/neu to have higher proliferation when compared to low HER-2/neu-expressing cell lines. High HER-2/neu expressors were found to be significantly more sensitive than low HER-2/neu expressors to patupilone exposure when compared to paclitaxel in vitro. In contrast, no significant differences in sensitivity to patupilone versus paclitaxel were detected in USPC cell lines showing low HER-2/neu expression, regardless of their fast or slow growth rate. Taken together, these results exclude the possibility that the different sensitivity to patupilone versus paclitaxel is secondary to a different growth rate in the two groups of USPC cell lines studied, and also suggest that HER-2/neu overexpression results in an increase in cell proliferation in vitro, potentially leading, as previously suggested for breast cancer patients overexpressing HER-2/neu [30], to a more rapid recovery from the cytotoxic effects of chemotherapeutic drugs in vivo.
In agreement with our in vitro results few published reports have demonstrated a clinical response to epothilones in taxane resistant gynecologic malignancies. A phase II study conducted by the GOG reported a 14.3% complete or partial response to ixabepilone in patients with recurrent platinum and taxane-resistant ovarian or primary peritoneal carcinoma [31]. Data published by Smit et al [32] demonstrated that 25% of patients (8 of 32) with refractory/resistant ovarian cancer, who had received prior paclitaxel therapy, had a complete or partial reponse after being treated with patupilone every 3 weeks. Finally, a phase II study to determine the response rate of ixabepilone in patients with persistent or recurrent endometrial cancer who have progressed despite standard therapy has recently been reported [33]. The overall response rate was 12%; one patient achieved a complete remission (2%), and five achieved partial remission (10%). Stable disease for at least 8 weeks was noted in 30 patients (60%).
Multidrug resistance is widely recognized as the principal mechanism by which many cancers develop resistance to chemotherapeutic agents. One of the best-described contributors of this chemoresistance is the overexpression of the MDR-1 gene which codes for the transmembrane efflux pump, P-glycoprotein [34]. This mechanism has been previously attributed to the development of paclitaxel resistance in many cancers and its expression in tumors has been described as a potential marker for predicting chemoresistance and overall poor prognosis [35,36].
The different activity profiles of epothilones and taxanes in solid tumors, however, suggest another mechanism of action. There is evidence to suggest that overexpression of β–tubulin III contributes to taxane resistance and that the antitumor activity of epotholines may be related to its ability to suppress this tubulin subtype. Ferrandina et al [37] investigated the clinical role of β–tubulin III as a potential predictor of response to neoadjuvant platinum/paclitaxel treatment in advanced ovarian cancer patients with unresectable disease. They demonstrated that tumors overexpressing β–tubulin III levels experienced an overall shorter survival compared to cases with relatively lower β–tubulin III content (median overall survival 25 vs. 46 months, P=0.002). Concomitant with these findings, Umezu et al [38] was able to show through immunohistochemical staining, that the expression of β–tubulin III was higher in taxol resistant ovarian cancer cells compared to wild-type. Of 19 patients treated with paclitaxel, 10 patients with high levels of β–tubulin III had no response to chemotherapy compared to 9 patients with low levels of β–tubulin III who had overall response rate of 56% [38]. Taken together, these findings suggest that therapeutic agents that target β–tubulin III may address the problem of paclitaxel resistance.
While the reason for the differences in resistance/sensitivity to the exposure to microtubule-targeting agents between the high and low HER-2/neu expressors remains poorly understood, we hypothesized that USPC with high HER-2/neu expression may differ from their low/negligible HER-2/neu counterparts not only in cell-cycle patterns but also in the expression of genes that are potential targets for patupilone and paclitaxel. To validate/exclude this hypothesis, the expression of P-glycoprotein (P-gp/ABCB1), β-tubulin class I (TUBB1), and β-tubulin class III (TUBB3) were measured and compared by RT-PCR in the different cell lines.
We are the first to report a significant increase in the expression of P-gp and β-tubulin III in UPSC cells that overexpress HER-2/neu. Furthermore, analyzing each individual cell line reveals that the most resistant cell line to paclitaxel, which was also the most sensitive to patipulone (ARK-1), also exhibited the highest expression of both P-gp and β-tubulin III. These findings are consistent with those of Mozzetti et al. [24] who also demonstrated increased levels of P-gp and TUBB3 expression in paclitaxel resistant ovarian cancer cell lines with no appreciable differences found in the expression of the other β-tubulin isotypes. Interestingly, they found that when these cells were selected with patupilone, TUBB3 expression diminished by up to 30-fold suggesting that cells with elevated levels of TUBB3 are more sensitive to patupilone [24]. Similarly, Kavallaris et al. [23] demonstrated that increased expression of β-tubulin III and β-tubulin IVa correlated with high resistance to paclitaxel in ovarian cancer cells. Taken together, these findings combined with our experimental results suggest that the sensitivity to patupilone must be at least partially mediated by its interaction with β-tubulin III. Of note, our UPSC cell lines that were low HER-2/neu expressors did not overexpress β-tubulin III relative to the high HER-2/neu expressors and thus did not show increased sensitivity to patupilone in comparison to its response to paclitaxel. The role, however, of HER-2/neu expression in this molecular pathway remains poorly understood.
In summary, USPC cell lines overexpressing HER-2/neu showed higher proliferation when compared to low HER-2/neu expressing cell lines and demonstrated a significantly higher sensitivity to patupilone when compared to paclitaxel. While a greater understanding of the molecular/genetic differences between USPC with high and low HER-2/neu expression will be necessary before tailoring chemotherapy to improve clinical outcomes, these data strongly suggest that HER-2/neu positive tumors may be significantly more sensitive to patupilone than paclitaxel when used alone or in combination with a platinum compound. The future design and implementation of clinical trials in this regard will ultimately determine the validity of this novel therapeutic combination in patients harboring USPC.
PRECIS.
USPC primary cell lines with high HER-2/neu expression display greater in vitro chemosensitivity to patupilone versus paclitaxel.
Acknowledgments
Supported in part by grants from the Angelo Nocivelli, the Berlucchi and the Camillo Golgi Foundation, Brescia, Italy, NIH R01 CA122728-01A2 to AS, and grants 501/A3/3 and 0027557 from the Italian Institute of Health (ISS) to AS. This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute.
Footnotes
Conflict of interest statement The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thun M, Xu J, Hao Y, Ward E, Siegel R, Jemal A. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Levenback C, Burke TW, Silva E, et al. Uterine papillary serous carcinoma (USPC) treated with cisplatin, doxorubicin, and cyclophosphamide (PAC) Gynecol Oncol. 1992;46:317–321. doi: 10.1016/0090-8258(92)90224-7. [DOI] [PubMed] [Google Scholar]
- 5.Nicklin L, Copeland LJ. Endometrial papillary serous carcinoma: pattern of spread and treatment. Clin Obstet Gynecol. 1996;39:686–695. doi: 10.1097/00003081-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PE. The management of serous papillary uterine cancer. Schwartz PE. Curr Opin Oncol. 2006;18(5):494–9. doi: 10.1097/01.cco.0000239890.36408.75. [DOI] [PubMed] [Google Scholar]
- 7.Lee JJ, Swain SM. The Epothilones: Translating from the Laboratory to the Clinic. Clin Cancer Res. 2008;14(6):1618–24. doi: 10.1158/1078-0432.CCR-07-2201. [DOI] [PubMed] [Google Scholar]
- 8.Höfle G, Bedorf N, Steinmetz H, Schumberg D, Gerth K, Reichenbach H. Epothilones A and B-novel 16-membered macrolides with cytotoxic activity: isolation, crystal structure, and conformation in solution. Angew Chem Int Ed Engl. 1996;35:1567–1569. [Google Scholar]
- 9.Lee FY, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC, Kramer RA. BMS-247550: A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–37. [PubMed] [Google Scholar]
- 10.Kowalski RJ, Giannakakou P, Hamel E. Activities of the Microtubule-stabilizing agents Epothilones A and B with purified tubulin and in cells resistant to Paclitaxel. J Biol Chem. 1997;272:2534–41. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 11.Giannakakou P, Gassio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T. A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci. 1999;97:2094–2909. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forli S, Manetti F, Altmann K, Botta M. Evaluation of Novel Epothilone Analogues by means of a Common Pharmacophore and a QSAR Pseudoreceptor Model for Taxanes and Epothilone. ChemMedChem. 2010;5:35–40. doi: 10.1002/cmdc.200900303. [DOI] [PubMed] [Google Scholar]
- 13.Santin AD, Bellone S, Van Stedum S, Bushen W, De Las Casas LE, Korourian S, Tian E, Roman JJ, Burnett A, Pecorelli S. Determination of HER-2/neu Status in Uterine Serous Papillary Carcinoma: Comparative Analysis of Immunohistochemistry and Fluorescence in situ Hybridization. Gynecol Oncol. 2005;98:24–30. doi: 10.1016/j.ygyno.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, De Las Casas LE, Roman JJ, Burnett A, Pecorelli S. Amplification of c-erbB2 Oncogene: a Major Prognostic Indicator in Uterine Serous Papillary Carcinoma. Cancer. 2005;104(7):1391–7. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 15.Santin AD, Bellone S, Siegel ER, Palmieri M, Thomas M, Cannon MJ, Kay HH, Roman JJ, Burnett A, Pecorelli S. Racial differences in overexpression of epidermal growth factor type II receptor (HER2/neu): a major prognostic indicator in Uterine Serous Papillary Cancer. Am J Obstet Gynecol. 2005;192:813–818. doi: 10.1016/j.ajog.2004.10.605. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Montes TP, Ji H, Smith Sehdev AE, Zahurak ML, Kurman RJ, Armstrong DK, Bristow RE. Clinical significance of HER-2/neu overexpression in uterine serous carcinoma. Gynecol Oncol. 2006;100:139–44. doi: 10.1016/j.ygyno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Grushko TA, Filiaci VL, Mundt AJ, Ridderstrale K, Olopade OI, Fleming GF. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology Group. Gynecol Oncol. 2008;108(1):3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright C, Angus B, Nicholson S, Sainsbury JR, Cairns J, Gullick WJ, Kelly P, Harris AL, Horne CH. Expression of c-erbB-2 oncoprotein: a prognostic indicator in human breast cancer. Cancer Res. 1989;49:2087–2090. [PubMed] [Google Scholar]
- 19.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, McKenzie S, Yin S, Bast RC., Jr Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 20.Hetzel DJ, Wilson TO, Keeney GL, Roche PC, Cha SS, Podratz KC. HER-2/neu expression: a major prognostic factor in endometrial cancer. Gynecol Oncol. 1992;47:179–85. doi: 10.1016/0090-8258(92)90103-p. [DOI] [PubMed] [Google Scholar]
- 21.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, Abu-Khalaf M, Buza N, Tavassoli FA, Hui P, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. In vitro Activity of Pertuzumab in Combination with Trastuzumab in Uterine Serous Papillary Adenocarcinoma. Br J Cancer. 2010;102(1):134–43. doi: 10.1038/sj.bjc.6605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, Poruchynsky MS. Paclitaxel-resistant human ovarian cancer cells have mutant β-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 23.Kavallaris M, Kuo D, Burkhart C, Regl DL, Norris MD, Haber M, Horwitz SB. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific β-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozzetti S, Iantomasi R, De Maria I, Prislei S, Mariani M, Camperchioli A, Bartollino S, Gallo D, Scambia G, Ferlini C. Molecular Mechanisms of Patupilone Resistance. Cancer Res. 2008;68(24):10197–10204. doi: 10.1158/0008-5472.CAN-08-2091. [DOI] [PubMed] [Google Scholar]
- 25.Thigpen JT, Buchsbaum HJ, Mangan C, Blessing JA. Phase II trial of adriamycin in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Cancer Treat Rep. 1979;63:21–7. [PubMed] [Google Scholar]
- 26.Thigpen JT, Blessing JA, Homesley H, Creasman WT, Sutton G. Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 1989;33:68–70. doi: 10.1016/0090-8258(89)90605-7. [DOI] [PubMed] [Google Scholar]
- 27.Ball HG, Blessing JA, Lentz SS, Mutch DG. A phase II trial of paclitaxel in patients with advanced or recurrent adenocarcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;62:278–81. doi: 10.1006/gyno.1996.0227. [DOI] [PubMed] [Google Scholar]
- 28.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, Kline R, Burger RA, Goodman A, Burks RT. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 29.Yarden Y, Sliwkowski MX. Untangling the erbB signaling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 30.Pegram MD, Finn RS, Arzoo K, Beryt M, Pietras RJ, Slamon DJ. The effect of HER-2/neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene. 1997;15:537–547. doi: 10.1038/sj.onc.1201222. [DOI] [PubMed] [Google Scholar]
- 31.Chen T, Molina A, Moore S, Goel S, Desai K, Hamilton A, Griffin T, Colevas AD, Mani S, Muggia F. Epothilone B analog (BMS-247550) at the recommended phase II dose (RPTD) in patients with gyneologic and breast cancers. J Clin Oncol. 2004;22(Suppl):155s. (Abstr 2115) [Google Scholar]
- 32.De Geest K, Blessing JA, Morris RT, Yamada SD, Monk BJ, Zweizig SL, Matei D, Muller CY, Richards WE. Phase II clinical trial of ixabepilone in patients with recurrent or persistent platinum- and taxane-resistant ovarian or primary peritoneal cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2009;28:149–153. doi: 10.1200/JCO.2009.24.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dizon DS, Blessing JA, McMeekin DS, Sharma SK, Disilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. Journal of Clinical Oncology. 2009;27(19):3104–8. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottesman M. Mechanisms of Cancer Drug Resistance. Annu Rev Med. 2002;53:613–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 35.Baekelandt MM, Holm R, Nesland JM, Trope CG, Kristensen GB. P-glycoprotein expression is a marker for chemotherapy resistance and prognosis in advanced ovarian cancer. Anticancer Res. 2000;20:1061–7. [PubMed] [Google Scholar]
- 36.Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF, Goodman A, Seiden MV. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004;93:98–106. doi: 10.1016/j.ygyno.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 37.Ferrandina G, Zannoni G, Martinelli E, Paglia A, Gallotta V, Mozzetti S, Scambia G, Ferlini C. Class III Tubulin Overexpression is a Marker of Poor Clinical Outcome in Advanced Ovarianc Cancer Patients. Clin Cancer Res. 2006;12(9) doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 38.Umezu T, Shibata K, Kajiyama H, Terauchi M, Ino K, Nawa A, Kikkawa F. Taxol Resistance Among the Different Histological Subtypes of Ovarian Cancer may be Associated with the Expression of Class III β–ubulin. Int J of Gynecological Pathology. 2008;27:207–12. doi: 10.1097/PGP.0b013e318156c838. [DOI] [PubMed] [Google Scholar]