This study reports for the first time the expression of channelopsin-GFP in inner retinal neurons of a nonhuman primate, the common marmoset. The study constitutes an important step in developing a channelrhodopsin-2–based gene therapy for treating blinding retinal degenerative diseases in humans.
Abstract
Purpose.
Converting inner retinal neurons to photosensitive cells by expressing channelrhodopsin-2 (ChR2) offers a novel approach for treating blindness caused by retinal degenerative diseases. In the present study, the recombinant adeno-associated virus serotype 2 (rAAV2)–mediated expression and function of a fusion construct of channelopsin-2 (Chop2) and green fluorescent protein (GFP) (Chop2-GFP) were evaluated in the inner retinal neurons in the common marmoset Callithrix jacchus.
Methods.
rAAV2 vectors carrying ubiquitous promoters were injected into the vitreous chamber. Expression of Chop2-GFP and functional properties of ChR2 were examined by immunocytochemical and electrophysiological methods 3 months after injection.
Results.
The percentage of Chop2-GFP–expressing cells in the ganglion cell layer was found to be retinal region- and animal age-dependent. The highest percentage was observed in the far-peripheral region. Chop2-GFP expression was also found in the foveal and parafoveal region. In the peripheral retina in young animals with high viral concentrations, the expression of Chop2-GFP was observed in all major classes of retinal neurons, including all major types of ganglion cells. The morphologic properties of Chop2-GFP–positive cells were normal for at least 3 months, and ChR2-mediated light responses were demonstrated by electrophysiological recordings.
Conclusions.
The rAAV2-mediated expression of ChR2 was observed in the inner retinal neurons in the marmoset retina through intravitreal delivery. The marmoset could be a valuable nonhuman primate model for developing ChR2-based gene therapy for treating blinding retinal degenerative diseases.
The severe loss of photoreceptors in retinal degenerative diseases such as retinitis pigmentosa could result in partial or complete blindness.1,2 As a new strategy for treating blindness caused by retinal degeneration, we have reported the feasibility of restoring light sensitivity to photoreceptor-deficient retinas in rodents by expressing channelopsin-2 (Chop2) in inner retinal neurons.3 Chop2 is a microbial opsin that, upon binding with retinal chromophore, forms a directly light-gated cation channel termed ChR2 (Chop2 retinalidene).4 Recent studies have demonstrated the long-term stability of the expression of ChR2 in inner retinal neurons5 and the restoration of certain vision-driven behavior in rodents.6,7 Thus, converting inner retinal neurons into photosensitive cells is a promising approach to restoring vision after the death of photoreceptors.
Recombinant adeno-associated virus (rAAV) vectors have become the most promising gene delivery system for gene therapy in many inherited and acquired retinal diseases.8 rAAV-mediated gene delivery through subretinal injection has been successfully used to target transgenes to photoreceptor and retinal pigment epithelial cells both in nonhuman primates (macaque)9–14 and humans.15–17 For restoring retinal light sensitivity after photoreceptor degeneration, it is necessary to target ChR2 to surviving inner retinal neurons. Studies in rodents have shown that the intravitreal injection of rAAV driven by ubiquitous promoters can mediate the robust expression of ChR2 in the inner retinal neurons.3,18,19 There are also clinical benefits of targeting photoreceptors through intravitreal administration.20,21 However, there have been no detailed studies of rAAV-mediated transgene expression in inner retinal neurons in nonhuman primates through intravitreal injection.22 The evaluation of the rAAV-mediated transduction efficiency of Chop2 in the retina of nonhuman primates through intravitreal administration would be an important step in developing ChR2-based gene therapy in humans.
The marmoset is a diurnal New World primate that has been used as a preclinical model for the evaluation of gene transfer methods.23 It has also been used extensively as a nonhuman primate model of the eye and visual system. It possesses diffraction-limited eyes with paraxial optical characteristics similar to those found in human eyes.24 The eyes have foveas,24 and the structure, and developmental sequence of the retina are similar to those of humans.25,26 The anatomy and physiology of the marmoset retina27–32 and central visual system33–39 have also been extensively investigated.
In this study, we examined the efficacy and function of the rAAV-mediated expression of a fusion construct of channelopsin-2 (Chop2) and green fluorescent protein (GFP), Chop2-GFP, with ubiquitous promoters in the retina of the common marmoset, Callithrix jacchus, through intravitreal administration.
Materials and Methods
AAV Vector Injection
The animal protocol and procedures for the use of marmosets were approved by the Institutional Animal Care and Use Committee (IACUC) at the New England College of Optometry, the SUNY College of Optometry, and Wayne State University. The study was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The construction of recombinant adenoassociated virus, serotype 2 (rAAV2), carrying a fusion construct of channelopsin-2 and green fluorescent protein (Chop2-GFP), is described elsewhere.3 Two types of ubiquitous promoters, CAG (a hybrid CMV early enhancer/chicken B-actin promoter) and CMV (human cytomegalovirus immediate-early gene promoter), were tested in Callithrix jacchus marmosets that were 7 (group A) and 25 (group B) months old (Table 1). Viral vectors were packaged and affinity purified by the Gene Therapy Program of the University of Pennsylvania. The animals were anesthetized by intramuscular injection of alfaxalone (2 mg/100 g body weight). The rAAV-2 vectors, diluted in saline (∼30 μL) in a low or high concentration, were injected into the vitreous chamber with a 0.5-mL syringe with a 32-gauge sharp-point needle. The injection site was chosen on the temporal side of the eyeball, 2 mm posterior to the limbus. The animals were given an analgesic NSAID (carprofen, 5–10 mg/kg SC) after surgery. Three months after viral injection, the marmosets were anesthetized with alfaxalone (2 mg/100 g body weight) and euthanatized by intracardiac injection of pentobarbital (10 mg/100 g body weight) and the eyes enucleated.
Table 1.
Details of Animals Used for the Immunocytochemistry Experiments
| Group | Age (mo) | Animals (n) | CAG Promoter |
CMV Promoter High Concentration 1.4 × 1011 GC/mL | |
|---|---|---|---|---|---|
| Low Concentration 1 × 1010 GC/mL | High Concentration 6 × 1012 GC/mL | ||||
| A | 7 | 2 | 1 | 2 | 1 |
| B | 25 | 2 | 1 | 2 | 1 |
Data are expressed as the number of eyes.
Immunocytochemical Staining
After the enucleation, the cornea, lens, and vitreous were removed. The retinas were fixed in 4% paraformaldehyde in phosphate buffer (PB) for 30 minutes. The expression of GFP was examined in retinal wholemounts and in vertical sections.
For cryostat sections, the retinal tissues from the nasal, superior, or inferior sector were cryoprotected in graded sucrose (10%, 20%, and 30% wt/vol, respectively, in PB) and cut at 20 μm. Sections were blocked for 1 hour in a solution containing 5% membrane-blocking agent (Chemiblocker; Chemicon, Temecula, CA), 0.5% Triton X-100 and 0.05% sodium azide (Sigma-Aldrich, St. Louis, MO). Primary antibodies were diluted in the same solution and were applied overnight, followed by incubation (1 hour) with secondary antibodies conjugated to Alexa 594 or Alexa 555 (red fluorescence; Molecular Probes, Eugene, OR) and Alexa 488 (green fluorescence; Molecular Probes) dyes. All steps were performed at room temperature (RT). We used the following antibodies: rabbit anti-GFP (1:2000, cat. no. A21311; Molecular Probes); goat anti-choline acetyltransferase (ChAT, 1:12,000, cat. no. AB144P; Chemicon), mouse anti-calretinin (1:30000, cat. no. MAB1568; Chemicon), mouse anti-protein kinase Cα (PKC, 1:160000, cat. no. sc8393; Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-glycine transporter 1 (Glyt1, 1:20000, cat. no. AB1770; Chemicon,), mouse anti-glutamic acid decarboxylase 65 (GAD65 1:12000, cat. no. MAB351; Chemicon), mouse anti-glutamic acid decarboxylase 67 (GAD67 1:2000, cat. no. MAB5406; Chemicon), and goat anti-Brn3 (1:3000, cat. no. sc6026; Santa Cruz Biotechnology).
We first counted the density of the cells in the ganglion cell layer (GCL) based on DAPI staining. Retinal wholemounts were stained for 20 minutes in 5 μM DAPI. The tissue was rinsed in PB, flat mounted, coverslipped, and viewed under a microscope. Compression of the retina was avoided by intercalating filter papers between the slide and the coverslip. For simplicity, the retinal sectors were divided into six regions (Fig. 1A and 1G, marked by lines) distributed equidistantly every 1.3 mm from the optic nerve head (region 1) to the periphery (region 6). Only the nasal, superior, or inferior sectors of the retina were examined. The temporal sector was excluded from the analysis because of the presence of the injection site and the fovea. The optic nerve head is located approximately 2-mm nasal to the fovea; therefore, we assessed GFP expression starting approximately 2 mm away from the center of the fovea. The maximum ganglion cell density in the marmoset retina is within 2 mm from the fovea, and the decline in the cell density is much shallower farther away from it.32 There was no or very low expression of GFP within 2 mm from the center of the fovea, except for the region within ∼0.4 mm from the center. Within each of these six regions, smaller areas of 0.15 mm2 were randomly chosen for cell counting. The number of the DAPI-labeled cells was averaged from several small areas and converted to cells per square millimeter. There was no significant difference in cell density in the corresponding regions between the two age groups A and B, and the data are therefore combined in Table 2. Similar to other investigators, we did not count the endothelial cells.40 Our results thus represent a mixture of ganglion cells (GCs) and displaced amacrine cells. GFP-positive cells were also counted and averaged in the chosen areas. The percentage of the GFP-positive cells in each of the six regions was calculated and is presented as the mean ± SD.
Figure 1.
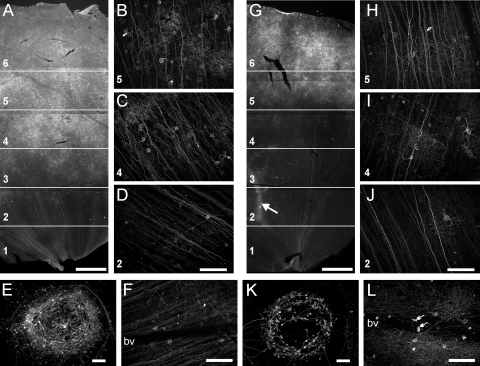
The expression of Chop2-GFP under control of CAG promoter at the concentration of 6 × 1012 GC/mL in group A (A–F) and group B (G–L) marmosets. For analysis, the retinal sectors were divided into six regions (A and G, labeled from the center to periphery with numbers 1–6). The cell densities were counted in characteristic high-magnification images from these regions (B–D and H–J, corresponding regions indicated by numbers). Many GFP-positive neurons were found in the fovea (E, K) and around large blood vessels (G, arrow). Some GFP-positive cells without processes were found inside the blood vessels (L, arrows). bv, blood vessels. Scale bars: (A, G) 1000 μm; (B–F, H–K) 100 μm.
Table 2.
The Percentage of Chop2-GFP–Positive Cells in the GCL in Different Regions of Marmoset Retinas Infected by High Concentrations of rAAV2 Vectors with CAG and CMV Promoters
| Retinal Region | Density of DAPI Labeled Cells (cells/mm2) | GFP-Positive Cells (%) |
|||
|---|---|---|---|---|---|
| CAG Promoter |
CMV Promoter |
||||
| 7 Months (Group A) | 25 Months (Group B) | 7 Months (Group A) | 25 Months (Group B) | ||
| 1 | 7620 ± 554 (7) | 0.2 ± 0.2 (8) | 0.0 ± 0.0 (16) | *0.6 ± 1.1 (9) | 0.0 ± 0.0 (14)* |
| 2 | 6447 ± 698 (7) | 2.5 ± 1.8 (12) | 0.5 ± 0.8 (12) | *1.6 ± 1.9 (8) | 0.3 ± 0.3 (17)* |
| 3 | 5372 ± 573 (7) | 4.1 ± 1.2 (11) | 1.1 ± 1.2 (13) | 4.1 ± 2.0 (7) | 1.3 ± 0.8 (12) |
| 4 | 4535 ± 659 (7) | 4.6 ± 1.4 (8) | 2.2 ± 1.8 (11) | 5.8 ± 0.8 (8) | 3.5 ± 0.9 (11) |
| 5 | 3669 ± 634 (7) | 8.1 ± 2.7 (18) | 3.7 ± 1.3 (9) | 7.3 ± 2.2 (14) | 5.4 ± 1.0 (11) |
| 6 | 3536 ± 105 (7) | 9.6 ± 1.9 (13) | 4.9 ± 2.1 (15) | 7.8 ± 2.4 (6) | 7.2 ± 1.8 (4) |
In six different retinal regions distributed equidistantly every 1.3 mm from the optic nerve head (region 1) to the periphery (region 6, column 1), the cell densities (column 2) and the percentages of GFP-positive cells infected by rAAV2 vectors with CAG (columns 3 and 4) and CMV (columns 5 and 6) promoter for the two age groups (7 months [group A] and 25 months [group B]) were averaged from several small areas (number of small areas is shown in parentheses) and expressed as the mean ± SD.
Data were not significantly different for Group A and B animals for the same promoter (two-tailed t-test).
For the study of individual GCs, retinal wholemounts were blocked for 1 hour at RT. Primary antibodies against GFP and ChAT were then applied for 10 days at 4°C, followed by secondary antibodies overnight. Each wholemount sector was mounted and coverslipped, with filters used as the support. GC analysis was limited to the eccentricities ≥2 mm, and the distances from the fovea were calculated. The level of dendritic stratification was estimated as a percentage of the inner plexiform layer (IPL). Using a ×20 objective, we noted the z-scale positions of OFF-ChAT–positive cell somas (designated 0% IPL) and ON-ChAT–positive cell somas (designated 100% IPL). The border between the ON- and OFF-sublaminae was defined in the middle (50%) of the IPL.41
All images were made with a microscope (Axioplan 2; Carl Zeiss Meditec, Inc., Dublin, CA) equipped with an oscillating grating (Apotome; Carl Zeiss Meditec, Inc.). Image projections were made by collapsing individual z-stacks of optical sections into a single plane. For double labeling, the concentrations of the primary antibodies were adjusted to prevent the “bleeding through” of the fluorescence signal from one channel into another. Brightness and contrast were then adjusted (Photoshop CS4; Adobe Systems, San Jose, CA).
Multielectrode Array Recordings
The multielectrode array recordings are described elsewhere.3 After the eye was enucleated, the retina was perfused in oxygenated extracellular solution at 34°C (in mM): NaCl, 124; KCl, 2.5; CaCl2, 2; MgCl2, 2; NaH2PO4, 1.25; NaHCO3, 26; and glucose, 22 (pH 7.35 with 95% O2 and 5% CO2). The interval between onsets of each light stimulus was 30 seconds. The signals were filtered between 200 Hz (low cutoff) and 20 kHz (high cutoff). The spike raster plot and the averaged spike-rate histogram from individual neurons were analyzed (Offline Sorter software; Plexon, Inc., Dallas, TX). Light stimuli were generated by a scanning monochromator with a 10-nm bandwidth. The light intensity was attenuated by neutral-density filters.
Results
Retinal Region- and Age-Dependent Expression of GFP in Marmosets
Figures 1A–F show the representative expression patterns of GFP viewed in a retinal wholemount from a group A (7 month old) animal injected with CAG-based viral vectors at a high concentration (6 × 1012 GC/mL). Under low magnification (Fig. 1A), GFP fluorescence was observed throughout the retina, but the number of GFP-positive cells was found to be region dependent. The highest number was observed in the far-peripheral regions (5 and 6) followed by the midperipheral regions (3 and 4) and the central region (1 and 2, close to the optic nerve head). Figures 1B–D show fluorescence images taken from the three representative regions 5, 4, and 2, respectively, with the focus plane at the ganglion cell layer (GCL). Individual GFP-labeled cells along with their axons and processes can be seen. For quantitative comparison of the transduction efficiency, we counted the percentage of GFP-positive cells in the GCL in these six regions (Table 2). Clearly, the percentage of GFP-positive cells was the highest in the far-peripheral and the lowest in the central regions. In addition, numerous GFP-labeled cells were observed in the foveal and parafoveal regions of the retina (within ∼0.4 mm of the center of the fovea; Fig. 1E). In the fovea, many GFP-positive cells were found in the inner and outer nuclear layers (INL and ONL). When stained for Brn-3, a known marker of ganglion cells (GCs),42 most of the cells were double-labeled, suggesting that GFP-positive cells are mostly GCs (data not shown).
In group B (25 months old), the overall expression patterns were found to be similar (Fig. 1G). However, the percentage of GFP-positive cells in the GCL was significantly lower than that in group A, especially in the central and midperipheral regions (Figs. 1H–J, Table 2). On the other hand, as in group A, many GFP-positive cells were also observed in the foveal region. The GFP-positive cells formed a circle at the foveal pit (Fig. 1K). In both groups, GFP-positive cells appeared more often around the large blood vessels (Fig. 1G, arrow). In addition, we found many small GFP-labeled cells inside the blood vessels in the retinas in group B (Fig. 1L, arrows). Such GFP-labeled cells were not detected in group A (Fig. 1F).
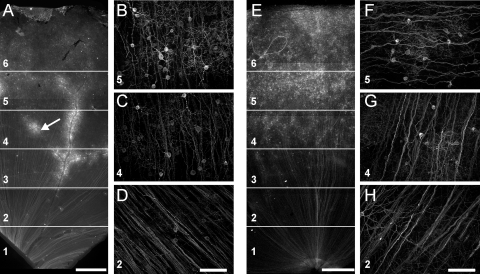
We also examined the rAAV2/2-mediated expression of Chop2-GFP with the CMV promoter (1.4 × 1011 GC/mL, Fig. 2). Similar to the results obtained with the CAG promoter, the expression level was significantly higher in group A (Figs. 2A–D) than in group B (Figs. 2E–H), especially in the central and midperipheral regions of the retina (Table 2). No GFP-labeled cells were observed inside the blood vessels.
Figure 2.
Expression of Chop2-GFP under control of the CMV promoter at the concentration of 1.4 × 1011 GC/mL in group A (A–D) and group B (E–H) marmosets. For analysis, the retinal sectors were divided into six different regions (A, E, labeled with numbers 1–6). The cell densities were counted in characteristic high-magnification images from these regions (B–D, F–H, corresponding regions indicated by numbers). Many GFP-positive neurons were around large blood vessels (A, arrow). bv, blood vessels. Scale bars: (A, E) 1000 μm; (B–D, F–H) 100 μm.
GFP Expression in a Variety of Retinal Neurons
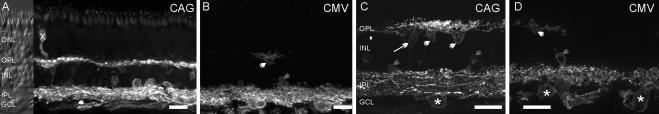
We next examined the GFP expression in retinal vertical sections from the virus-injected group A marmosets. Figures 3A and C show the expression mediated by viral vectors with the CAG promoter at the concentration of 6 × 1012 GC/mL. GFP fluorescence was predominantly observed in the inner retina in ganglion, amacrine, and horizontal cells but not in glial cells. Weakly labeled bipolar cells (Fig. 3C, arrow) were frequently encountered. In the outer retina, photoreceptors were occasionally labeled (Fig. 3A, X). Figures 3B and 3D show the expression mediated by viral vectors with the CMV promoter at a concentration of 1.4 × 1011 GC/mL. GFP fluorescence was predominantly observed in cells located in the GCL, although some horizontal cells and amacrine cells were found in the INL.
Figure 3.
The distribution of Chop2-GFP in retinal vertical sections of group A marmosets shown at low (A, B) and high (C, D) magnifications. (A, C) In the retina infected with a high concentration (6 × 1012 GC/mL) of CAG-based virus, GFP fluorescence was detected in a variety of retinal neurons. (B, D) Retina infected with a high concentration of CMV-based virus exhibited fewer GFP-positive cells. (*) Ganglion cells; gray arrowheads: amacrine cells; white arrow: bipolar cell; white arrowheads: horizontal cells; cross: photoreceptor. Scale bars: 25 μm.
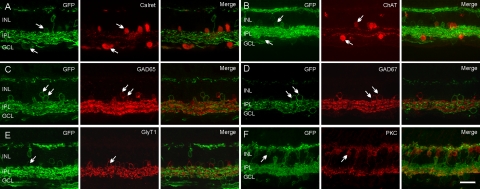
To examine whether rAAV2 could transfect diverse populations of inner retinal neurons, we double labeled the retina of a group A animal for known retinal markers. We did not quantify the percentage of GFP-positive cells in the particular cell types because of topographic variations in virus transduction. Antibodies against calretinin have been reported to label certain amacrine and GCs in the marmoset.43 Some neurons in the INL and GCL labeled for calretinin also expressed GFP (Fig. 4A). Antibodies to choline acetyltransferase (ChAT) are known to immunolabel cholinergic starburst-amacrine cells.44,45 Both regular and displaced starburst-amacrine cells colocalized with GFP-positive cells (Fig. 4B). Approximately half of the amacrine cells in the retina are GABAergic and express glutamic acid decarboxylase isoforms (GAD65 and/or GAD67)46–48 and another half are glycinergic and can be labeled by an antibody against glycine transporter 1 (GlyT1).49–51 All three markers were detected in GFP-positive amacrine cells (Figs. 4C–E). Immunoreactivity for PKC has been reported in rod bipolar cells and type 4 diffuse bipolar cells (DB4) in the macaque and marmoset.52–54 Only a few GFP-positive bipolar cells expressed PKC (Fig. 4F, arrow).
Figure 4.
Identification of Chop2-GFP–positive cells. In the retina, GFP-positive cells (green) were double-labeled (arrows) by antibodies to a variety of cellular markers (red). (A) Calretinin, (B) ChAT, (C) GAD65, (D) GAD67, (E) GlyT1, and (F) PKC. Scale bars, 25 μm.
These results indicate that rAAV2-mediated expression of Chop2-GFP under the control of the CAG promoter occurs in all major types of retinal neurons.
Individual Cells Labeled with GFP in the Retina Infected by Viral Vectors at a Lower Concentration
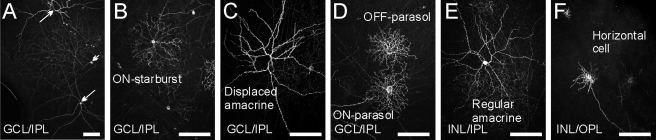
When both marmoset groups were injected with the virus vector at a low concentration (CAG promoter, 2 × 1010 GC/mL), the density of Chop2-GFP–positive cells was very low (Fig. 5). Infected cells were observed in the inner retina, particularly in the GCL, and were morphologically well isolated. Figure 5A is a low-magnification micrograph showing strongly labeled displaced amacrine cells (arrows) and a wide-field GC (arrowhead). In the GCL, we encountered a variety of cells, such as starburst and large, spiny, displaced amacrine cells as well as ON- and OFF-parasol GCs (Figs. 5B–D). In the INL, we found GFP-labeled regular amacrine and horizontal cells (Figs. 5E, 5F).
Figure 5.
Individual cells labeled with GFP in the retina after infection by CAG-based viral vectors at a lower concentration (2 × 1010 GC/mL). (A) GCL in a wholemount retina, shown at low magnification. Two amacrine cells (arrows) and one ganglion cell (arrowhead) were labeled. (B–D) Examples of cells in the GCL: ON-starburst amacrine cell (B), displaced amacrine cell (C), and two parasol ganglion cells with dendrites stratifying in ON- and OFF-sublaminae (D). (E–F) GFP-positive cells in the INL: a regular amacrine cell (E) and a horizontal cell (F). Scale bars, 100 μm.
GFP Expression by Various Types of Ganglion Cells
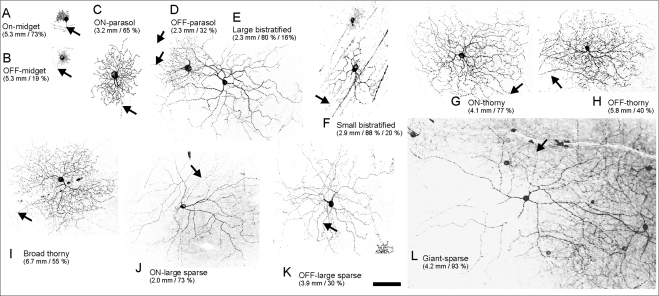
To determine whether different GC types can be targeted, we analyzed 160 GCs according to criteria described in previous studies.28,55 We found that all major GC types were labeled by GFP (Fig. 6).
Figure 6.
Different types of Chop2-GFP–positive ganglion cells. The numbers indicate eccentricity (distance from fovea) and stratification depth (% of IPL). For bistratified cells, the second and third numbers indicate the stratification depth in ON- and OFF-sublaminae, respectively. Arrows: axons. Scale bar, 100 μm.
Midget cells (B-cells) have the smallest dendritic trees and contain a single primary dendrite.28 Based on the stratifications of their dendritic trees relative to the edges of the IPL, we observed both ON- and OFF-midget GCs (Figs. 6A, 6B).
Parasol GCs (A-cells) have large somata and a few stout primary dendrites.28,41 We found that both ON- and OFF-parasol cells (Figs. 6C, 6D) were labeled.
There are several types of wide-field GCs, referring to all nonmidget and nonparasol cells.28,55 Among them are small- and large-field bistratified cells (Figs. 6E, 6F), broadly stratified (Fig. 6I), and monostratified thorny cells (Figs. 6G, 6H), and large, monostratified cells with smooth and sparse dendritic branches (Figs. 6J, 6K). The large bistratified cells have not been previously observed in the marmoset retina but have been found in the macaque56,57 and human retina.58 The dendritic fields of the monostratified or sparse cells were larger than those of parasol cells of the same eccentricity. We also found a limited number of cells with gigantic dendritic fields in the ON-sublamina (Fig. 6L).
Electrophysiological Recordings of ChR2-Mediated Light Responses
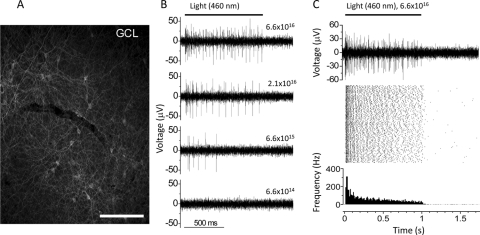
Finally, we examined the ChR2-mediated light responses by using multielectrode array recordings from wholemount retinas. The recordings were made from an 18-month-old marmoset that was injected with virus carrying Chop2-GFP with CAG promoter at the concentration of 2 × 1011 GC/mL. To block photoreceptor-mediated light responses, we performed the recordings in the presence of the AMPA/kinate receptor antagonist CNQX (25 μM), the NMDA receptor antagonist D-APV (25 μM), and the mGluR6 receptor agonist L-APB (5 μM). The recordings were made from the peripheral retina with significant expression of Chop2-GFP (Fig. 7A). Figures 7B and 7C show the representative recordings of the spike firing evoked by light at a wavelength of 460 nm picked up by individual recording electrodes (n = 11). As shown in Figure 7B, the spike firing was light-intensity–dependent with the threshold activity observed at the light intensity of 6.6 × 1015 photons cm−2 s−1, a value close to the expected threshold of ChR2.3 In addition, as shown in Figure 7C from another electrode, the light-mediated spiking activity was remarkably stable. A single raw trace of the spike activity picked up by the electrode and the spike raster plot originated from a single neuron during a 1.5-hour recording session are shown in Figure 7C, top and middle, respectively. The averaged spike rate histogram is shown in the bottom panel. Several pieces of evidence indicate that these were ChR2-mediated response. First, no spike activity was observed when the retina was stimulated with light at the wavelength of 580 nm (data not shown), consistent with the fact that ChR2 is not sensitive to the wavelength of 580 nm. Second, the kinetics of the light-evoked responses was very similar with that recorded in the mouse retinal cells expressing ChR2 channels.3 These light responses, therefore, could not be contributed by melanopsin-expressing GCs because their light responses are slow, especially the OFF rate.59 Finally, no light responses could be recorded from the areas of the same retina that did not show the expression of GFP (data not shown). Together, these results demonstrate the functional expression of ChR2 channels in the marmoset retina.
Figure 7.
Multielectrode array recordings of ChR2-mediated spike activity. (A) The expression of Chop2-GFP in the peripheral retina where the light-mediated spike activity was recorded. Scale bar, 100 μm. (B) The ChR2-mediated spike activities recorded from a single electrode in response to four different light intensities with a wavelength of 460 nm. The light intensities are shown above the traces with the unit of photons cm−2 s−1. (C) A single raw trace of the spike activity recorded from another electrode (top) and the raster plots of 90 consecutive light-elicited spike activities originating from a single neuron (middle); (bottom) averaged spike rate. The duration and interval of the light stimulation were 1 second and 60 seconds, respectively.
Discussion
In this study, we evaluated the rAAV2-mediated expression and function of Chop2-GFP in marmoset retinas after administration into the vitreous chamber. The efficiency and cell specificity of rAAV-mediated transfection are known to be dependent on factors such as the route of injection, the virus serotype, the virus concentration, and promoters.8 We used injection into the vitreous chamber to allow better diffusion and physical accessibility of the viral vectors to the entire inner retina. To further increase the number of the infected cells, we chose the most effective serotype, rAAV2, for inner retinal neurons20 and the ubiquitous promoters CAG and CMV. These two promoters produce slightly different expression patterns when used in the mouse retina (Ivanova E, Pan Z-H, unpublished observations, 2007). Together, they allowed us to assess the limitations of the virus' capability of infecting different cell types (virus tropism). Several interesting properties were observed: First, our result showed a gradient pattern in the transfection efficiency, with the highest in the far-peripheral retina and the lowest in the central retina (except the fovea). This pattern was observed in both 7- and 25-month-old animals, independent of promoters. In addition, the percentage of GFP-positive cells was found to be animal age-dependent. In older animals (25 months), the percentage of GFP-positive cells was found to be markedly reduced. Furthermore, we observed high Chop2-GFP expression in the foveal and parafoveal regions of the retina. Remarkably, all these observed variations in the transfection efficacy appeared to correlate with the properties of the inner limiting membrane (ILM) previously reported in the primate retina.60 The authors in that study described regional variations of the ILM with the thinnest regions around fovea, large blood vessels, and in the periphery. In addition, the ILM was much thinner in younger animals than in older ones. Such patterns of the ILM have also been reported in humans.61,62 Thus, our results in the marmoset suggest that the ILM is a major barrier for the rAAV-mediated gene delivery through intravitreal administration. Further studies for developing effective methods to overcome the barrier of the ILM would be required for retinal gene therapy through intravitreal delivery. A mild digestion of the ILM with a nonspecific protease has been reported to increase the transduction efficiency of several rAAV serotypes in the rat retina through intravitreal injection.21 The removal of the ILM by mechanical peeling may also be a solution.63
Similar to rodents,3,18,19 in the peripheral region of the marmoset retina, GFP expression was found to be predominantly localized in inner retinal neurons, including ganglion, amacrine, and horizontal cells. All major ganglion cell types were transfected. In addition, many bipolar cells, as well as some photoreceptor cells, were transfected with the high concentration of viral vector with CAG promoter. The results thus indicate that rAAV2 vectors are capable of diffusing and reaching the photoreceptor cell layer in the peripheral retina. The relatively low number of infected bipolar cells, especially photoreceptor cells, compared with amacrine and ganglion cells, could be due in part to the physical barriers of the retinal tissue. These barriers appear to render the viral concentration that reaches these more distal retinal layers substantially lower. Consistent with this explanation, as shown in this study, when lower concentrations (CAG, 2 × 1010 GC/mL) of viral vectors were injected, expression of GFP was primarily observed in cells located in the GCL. On the other hand, many cells in the INL and ONL were infected in the fovea.
In this study, we found that rAAV2 vectors with the CAG promoter at high concentrations resulted in the labeling of cells inside the blood vessels. These cells are likely to be the recruited leukocytes caused by the inflammatory response to the viral injection. However, this was not observed in the younger animals (group A) or animals injected with lower viral concentrations. Further study is needed to evaluate the implications of this phenomenon.
To date, most, if not all, ocular gene therapy studies in nonhuman primates have been performed in old-world primates, such as macaques9–14,64,65 but rAAV-mediated gene transfection has not been documented in detail in inner retinal neurons.11,22 Since new- and old-world primates diverged ∼40 million years ago, further studies would have to examine whether differences could exist in the retinal structures between new- and old-world primates that could affect the transduction efficiency of rAAV vectors. Nevertheless, the capability of rAAV-mediated expression in inner retinal cells in marmosets revealed in this study provides a valuable model of the study of the property and function of transgene expression in primates.
The ability to achieve expression of Chop-2-GFP in the marmoset also offers a valuable model for the development of ChR2-based gene therapy. The normal morphology and physiological properties of the Chop2-GFP–expressing cells observed in this study 3 months after the viral-vector injection suggest that the stable expression of functional ChR2 can be achieved in primate retinal neurons. Thus, the use of the marmoset model will facilitate further studies in examining the long-term safety and physiological consequences of Chop2 expression in the primate retina. Targeted expression of Chop2 and other microbial opsins in specific population(s) of inner retinal neurons may be needed, to improve treatment outcomes for the Chop2 and other microbial opsin-based gene therapies.3,6,66 The broad tropism of rAAV2 observed in this study suggests that the use of rAAV2 along with cell-type–specific promoters could allow targeting to specific type(s) of inner retinal neurons in the primate.
In conclusion, we reported the rAAV2-mediated expression of functional ChR2 in the inner retinal neurons in the marmoset through intravitreal delivery. The marmoset could be a valuable nonhuman primate model for evaluating the ChR2-based gene therapy for treating blinding retinal degenerative diseases.
Footnotes
Supported by National Institutes of Health Grants EY17130 (Z-HP) and EY11228 (DT); the Midwest Eye-Banks (EI); and a WSU Medical School Summer Fellowship (G-SH); and Vision Core Grant EY04068 to the Department of Anatomy and Cell Biology at WSU.
Disclosure: E. Ivanova, None; G.-S. Hwang, None; Z.-H. Pan, None; D. Troilo, None
References
- 1. Rolling F. Recombinant AAV-mediated gene transfer to the retina: gene therapy perspectives. Gene Ther. 2004;11(suppl 1):S26–S32 [DOI] [PubMed] [Google Scholar]
- 2. Dinculescu A, Glushakova L, Min SH, et al. Adeno-associated virus-vectored gene therapy for retinal disease. Hum Gene Ther. 2005;16:649–663 [DOI] [PubMed] [Google Scholar]
- 3. Bi A, Cui J, Ma YP, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagel G, Szellas T, Huhn W, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ivanova E, Pan ZH. Evaluation of the adeno-associated virus mediated long-term expression of channelrhodopsin-2 in the mouse retina. Mol Vis. 2009;15:1680–1689 [PMC free article] [PubMed] [Google Scholar]
- 6. Lagali PS, Balya D, Awatramani GB, et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675 [DOI] [PubMed] [Google Scholar]
- 7. Tomita H, Sugano E, Fukazawa Y, et al. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS ONE. 2009;4:e7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353–359 [DOI] [PubMed] [Google Scholar]
- 9. Bennett J, Maguire AM, Cideciyan AV, et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci U S A. 1999;96:9920–9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lotery AJ, Yang GS, Mullins RF, et al. Adeno-associated virus type 5: transduction efficiency and cell-type specificity in the primate retina. Hum Gene Ther. 2003;14:1663–1671 [DOI] [PubMed] [Google Scholar]
- 11. Lebherz C, Maguire AM, Auricchio A, et al. Nonhuman primate models for diabetic ocular neovascularization using AAV2-mediated overexpression of vascular endothelial growth factor. Diabetes. 2005;54:1141–1149 [DOI] [PubMed] [Google Scholar]
- 12. Le Meur G, Weber M, Pereon Y, et al. Postsurgical assessment and long-term safety of recombinant adeno-associated virus-mediated gene transfer into the retinas of dogs and primates. Arch Ophthalmol. 2005;123:500–506 [DOI] [PubMed] [Google Scholar]
- 13. Stieger K, Schroeder J, Provost N, et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther. 2009;17:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weber M, Rabinowitz J, Provost N, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7:774–781 [DOI] [PubMed] [Google Scholar]
- 15. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 18. Farah N, Reutsky I, Shoham S. Patterned optical activation of retinal ganglion cells. Conf Proc IEEE Eng Med Biol Soc. 2007;1:6368–6370 [DOI] [PubMed] [Google Scholar]
- 19. Tomita H, Sugano E, Yawo H, et al. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci. 2007;48:3821–3826 [DOI] [PubMed] [Google Scholar]
- 20. Hellstrom M, Ruitenberg MJ, Pollett MA, et al. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532 [DOI] [PubMed] [Google Scholar]
- 21. Dalkara D, Kolstad KD, Caporale N, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther. 2009;17:2096–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stieger K, Lheriteau E, Moullier P, et al. AAV-mediated gene therapy for retinal disorders in large animal models. ILAR J. 2009;50:206–224 [DOI] [PubMed] [Google Scholar]
- 23. Hibino H, Tani K, Ikebuchi K, et al. The common marmoset as a target preclinical primate model for cytokine and gene therapy studies. Blood. 1999;93:2839–2848 [PubMed] [Google Scholar]
- 24. Troilo D, Howland HC, Judge SJ. Visual optics and retinal cone topography in the common marmoset (Callithrix jacchus). Vision Res. 1993;33:1301–1310 [DOI] [PubMed] [Google Scholar]
- 25. Hendrickson A, Troilo D, Djajadi H, et al. Expression of synaptic and phototransduction markers during photoreceptor development in the marmoset monkey Callithrix jacchus. J Comp Neurol. 2009;512:218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hendrickson A, Troilo D, Possin D, et al. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. J Comp Neurol. 2006;497:270–286 [DOI] [PubMed] [Google Scholar]
- 27. Chan TL, Goodchild AK, Martin PR. The morphology and distribution of horizontal cells in the retina of a New World monkey, the marmoset Callithrix jacchus: a comparison with macaque monkey. Vis Neurosci. 1997;14:125–140 [DOI] [PubMed] [Google Scholar]
- 28. Ghosh KK, Goodchild AK, Sefton AE, et al. Morphology of retinal ganglion cells in a new world monkey, the marmoset Callithrix jacchus. J Comp Neurol. 1996;366:76–92 [DOI] [PubMed] [Google Scholar]
- 29. Ghosh KK, Martin PR, Grunert U. Morphological analysis of the blue cone pathway in the retina of a New World monkey, the marmoset Callithrix jacchus. J Comp Neurol. 1997;379:211–225 [PubMed] [Google Scholar]
- 30. Goodchild AK, Ghosh KK, Martin PR. Comparison of photoreceptor spatial density and ganglion cell morphology in the retina of human, macaque monkey, cat, and the marmoset Callithrix jacchus. J Comp Neurol. 1996;366:55–75 [DOI] [PubMed] [Google Scholar]
- 31. Luo X, Ghosh KK, Martin PR, et al. Analysis of two types of cone bipolar cells in the retina of a New World monkey, the marmoset, Callithrix jacchus. Vis Neurosci. 1999;16:707–719 [DOI] [PubMed] [Google Scholar]
- 32. Wilder HD, Grunert U, Lee BB, et al. Topography of ganglion cells and photoreceptors in the retina of a New World monkey: the marmoset Callithrix jacchus. Vis Neurosci. 1996;13:335–352 [DOI] [PubMed] [Google Scholar]
- 33. Spatz WB. The retino-geniculo-cortical pathway in Callithrix. I. Intraspecific variations in the lamination pattern of the lateral geniculate nucleus. Exp Brain Res. 1978;33:551–563 [DOI] [PubMed] [Google Scholar]
- 34. Spatz WB. The retino-geniculo-cortical pathway in Callithrix. II. The geniculo-cortical projection. Exp Brain Res. 1979;36:401–410 [DOI] [PubMed] [Google Scholar]
- 35. Spatz WB. Loss of ocular dominance columns with maturity in the monkey, Callithrix jacchus. Brain Res. 1989;488:376–380 [DOI] [PubMed] [Google Scholar]
- 36. Sengpiel F, Troilo D, Kind PC, et al. Functional architecture of area 17 in normal and monocularly deprived marmosets (Callithrix jacchus). Vis Neurosci. 1996;13:145–160 [DOI] [PubMed] [Google Scholar]
- 37. Rosa MG, Elston GN. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus): middle temporal area, middle temporal crescent, and surrounding cortex. J Comp Neurol. 1998;393:505–527 [PubMed] [Google Scholar]
- 38. Rosa MG, Tweedale R. Visual areas in lateral and ventral extrastriate cortices of the marmoset monkey. J Comp Neurol. 2000;422:621–651 [DOI] [PubMed] [Google Scholar]
- 39. Rosa MG, Palmer SM, Gamberini M, et al. Resolving the organization of the New World monkey third visual complex: the dorsal extrastriate cortex of the marmoset (Callithrix jacchus). J Comp Neurol. 2005;483:164–191 [DOI] [PubMed] [Google Scholar]
- 40. Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watanabe M, Rodieck RW. Parasol and midget ganglion cells of the primate retina. J Comp Neurol. 1989;289:434–454 [DOI] [PubMed] [Google Scholar]
- 42. Xiang M, Zhou L, Macke JP, et al. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15:4762–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chiquet C, Dkhissi-Benyahya O, Cooper HM. Calcium-binding protein distribution in the retina of strepsirhine and haplorhine primates. Brain Res Bull. 2005;68:185–194 [DOI] [PubMed] [Google Scholar]
- 44. Mariani AP, Hersh LB. Synaptic organization of cholinergic amacrine cells in the rhesus monkey retina. J Comp Neurol. 1988;267:269–280 [DOI] [PubMed] [Google Scholar]
- 45. Rodieck RW, Marshak DW. Spatial density and distribution of choline acetyltransferase immunoreactive cells in human, macaque, and baboon retinas. J Comp Neurol. 1992;321:46–64 [DOI] [PubMed] [Google Scholar]
- 46. Nishimura Y, Schwartz ML, Rakic P. Localization of gamma-aminobutyric acid and glutamic acid decarboxylase in rhesus monkey retina. Brain Res. 1985;359:351–355 [DOI] [PubMed] [Google Scholar]
- 47. Crooks J, Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol. 1992;315:287–302 [DOI] [PubMed] [Google Scholar]
- 48. Vardi N, Auerbach P. Specific cell types in cat retina express different forms of glutamic acid decarboxylase. J Comp Neurol. 1995;351:374–384 [DOI] [PubMed] [Google Scholar]
- 49. Zafra F, Aragon C, Olivares L, et al. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Menger N, Pow DV, Wassle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46 [DOI] [PubMed] [Google Scholar]
- 51. Pow DV, Hendrickson AE. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci. 1999;16:231–239 [DOI] [PubMed] [Google Scholar]
- 52. Martin PR, Grunert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287 [DOI] [PubMed] [Google Scholar]
- 53. Grunert U, Martin PR, Wassle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627 [DOI] [PubMed] [Google Scholar]
- 54. Jusuf PR, Lee SC, Grunert U. Synaptic connectivity of the diffuse bipolar cell type DB6 in the inner plexiform layer of primate retina. J Comp Neurol. 2004;469:494–506 [DOI] [PubMed] [Google Scholar]
- 55. Szmajda BA, Grunert U, Martin PR. Retinal ganglion cell inputs to the koniocellular pathway. J Comp Neurol. 2008;510:251–268 [DOI] [PubMed] [Google Scholar]
- 56. Dacey DM, Peterson BB, Robinson FR, et al. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27 [DOI] [PubMed] [Google Scholar]
- 57. Yamada ES, Bordt AS, Marshak DW. Wide-field ganglion cells in macaque retinas. Vis Neurosci. 2005;22:383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peterson BB, Dacey DM. Morphology of wide-field bistratified and diffuse human retinal ganglion cells. Vis Neurosci. 2000;17:567–578 [DOI] [PubMed] [Google Scholar]
- 59. Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754 [DOI] [PubMed] [Google Scholar]
- 60. Matsumoto B, Blanks JC, Ryan SJ. Topographic variations in the rabbit and primate internal limiting membrane. Invest Ophthalmol Vis Sci. 1984;25:71–82 [PubMed] [Google Scholar]
- 61. Foos RY. Vitreoretinal juncture: topographical variations. Invest Ophthalmol. 1972;11:801–808 [PubMed] [Google Scholar]
- 62. Foos RY. Vitreoretinal juncture over retinal vessels. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977;204:223–234 [DOI] [PubMed] [Google Scholar]
- 63. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000;129:769–777 [DOI] [PubMed] [Google Scholar]
- 64. Stieger K, Le Meur G, Lasne F, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975 [DOI] [PubMed] [Google Scholar]
- 65. Stieger K, Mendes-Madeira A, Le Meur G, et al. Oral administration of doxycycline allows tight control of transgene expression: a key step towards gene therapy of retinal diseases. Gene Ther. 2007;14:1668–1673 [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y, Ivanova E, Bi A, et al. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009;29:9186–9196 [DOI] [PMC free article] [PubMed] [Google Scholar]