Abstract
Rationale
Nonmedical use and abuse of prescription opioids is an increasing public health problem. Intravenous (IV) administration of opioid analgesics intended for oral use is not uncommon, yet little is known about the relative abuse potential of these drugs when administered intravenously to recreational opioid abusers without physical dependence.
Methods
This inpatient study employed a double-blind, randomized, within-subject, placebo-controlled design to examine the relative abuse potential of IV doses of oxycodone, hydrocodone and morphine. Nine healthy adult participants reporting recreational opioid use and histories of IV opioid use completed 11 experimental sessions, including one active-dose practice session. IV doses were infused over 5-min and included three identical doses of each opioid (5, 10 and 20 mg/10 ml) and saline placebo. Physiological, subjective and performance effects were collected before and for 6 h after drug administration.
Results
All three opioids produced prototypical mu agonist effects (e.g., miosis; increased ratings of liking) that were generally dose-related. Pharmacodynamic effects were observed within 5 min of IV administration. Physiological effects were more prolonged than subjective effects for all three drugs. While the magnitude of effects was generally comparable across drugs and qualitatively similar, valid potency assays indicated the following potency relationship: oxycodone > morphine > hydrocodone.
Conclusions
There were modest potency differences between oxycodone, hydrocodone and morphine, but their overall profile of effects was similar, indicating significant abuse potential when administered intravenously.
Keywords: Oxycodone, Hydrocodone, Morphine, Prescription Opioids, Diversion, Abuse Potential
Introduction
Illicit prescription opioid use is now more prevalent in the United States than the use of cocaine, heroin or methamphetamine. Data from the 2008 National Survey on Drug Use and Health indicate that 4.7 million individuals over the age of 12 reported current nonmedical use of prescription opioids, whereas 1.9 million, 200,000 and 314,000 people reported current cocaine, heroin and methamphetamine use, respectively (Substance Abuse and Mental Health Services Administration, 2009). The majority of prescription opioids are formulated for oral use, but they are often taken intranasally or intravenously when misused (Davis and Johnson, 2008; Katz et al., 2008). Recent epidemiological studies have estimated that up to 40% of illicit prescription opioid users, including sporadic users, have experience with intravenous injection of these drugs (Havens et al., 2007; Hays et al., 2003; Katz et al., 2008; Rosenblum et al., 2007). The purpose of this experiment was to determine the abuse potential and relative potencies of intravenous prescription opioids in recreational opioid users with a history of intravenous injection of these drugs, a population that is at increased risk for developing opioid dependence.
While prescription opioid analgesics have been available clinically for decades and the nonmedical use of these drugs is increasingly recognized as a public health problem, only recently has the relative abuse potential of these drugs been examined using controlled laboratory methods in human participants (Comer et al., 2008; 2009; Walsh et al., 2008; Zacny, 2003; Zacny et al., 2005; Zacny and Gutierrez, 2003; 2008; 2009; Zacny and Lichtor, 2008). Many of these studies tested the effects of orally administered opioids (Walsh et al., 2008; Zacny, 2003; Zacny et al., 2005; Zacny and Gutierrez, 2003; 2008; 2009; Zacny and Lichtor, 2008) with results suggesting that oxycodone, hydrocodone and hydromorphone have comparable abuse potential. One study has tested the effects of intravenously administered prescription opioids in opioid-dependent participants (Comer et al., 2008). As with oral dosing, the results of that study demonstrated that intravenous administration of oxycodone and fentanyl produced prototypical opioid-like effects that were qualitatively similar to those of heroin and suggest comparable abuse potential amongst the agents.
The combined findings from previous studies serve to demonstrate that a number of prescription opioids, when administered orally or intravenously, produce qualitatively similar behavioral and physiological effects across three different populations (i.e., non-drug-abusers, recreational opioid users, physically dependent heroin users), indicating significant potential for abuse. However, given that these drugs are taken intravenously by sporadic users (Havens et al., 2007; Katz et al., 2008), it is important to determine the effects of prescription opioids when administered by the intravenous route to a non-dependent population as this is a common route of administration. Hydrocodone and oxycodone were chosen as the test drugs in this study because they are the first and second most commonly prescribed opioids in the United States, respectively (IMS National Prescription Audit Plus™, see Walsh et al., 2008). Moreover, oxycodone and hydrocodone are both full mu opioid receptor agonists that have been identified as specifically contributing to the observed increase in illicit prescription opioid use (Substance Abuse and Mental Health Services Administration, 2007). No study to date has examined the abuse-related effects of intravenous hydrocodone. Oxycodone is available in the United States in combination products (i.e., with aspirin or acetaminophen) and opioid-only formulations and is regulated under Schedule II. Hydrocodone alone is regulated under Schedule II but is presently marketed in the United States only in combination products, which fall under the less tightly regulated Schedule III designation. However, hydrocodone is available as an opioid-only product outside of the United States (see drug procurement information below). Morphine was selected as the reference compound because it is also a full mu opioid receptor agonist and has been extensively studied under controlled laboratory conditions to determine abuse potential and has historically been used in many studies as a positive control test drug (e.g., Comer et al., 2008; Jasinski and Preston, 1986; Teoh et al., 1993; 1994; Walker and Zacny, 1999).
Methods
Participants
Participants were adult recreational opioid users who reported a history of intravenous opioid use and were not physically dependent on opioids at the time of the study (see first section under Results for details). Participants were recruited through local advertisements and were paid for their participation. Individuals who were seeking treatment for their substance abuse or successfully sustaining abstinence in the community were excluded.
All participants were determined to be in good health by medical history and physical examination, an electrocardiogram and laboratory tests. Participants were carefully screened to eliminate those with seizure disorders, asthma or other respiratory disorders, head injury, hypertension, cardiovascular disease or abnormal ECG. All participants reported illicit use of opioids, which was confirmed by urinalysis during screening. This study was approved by the University of Kentucky Institutional Review Board, and participants gave their written informed consent prior to screening and enrollment. The consent document stated that this study would involve intravenous administration of opioid drugs. A Certificate of Confidentiality was obtained from the National Institute on Drug Abuse for the project, and the study was conducted in accordance with the Helsinki guidelines for ethical human research.
Participants enrolled as inpatients for approximately four weeks in this study. Sessions were conducted at least 48 hours apart on weekdays (i.e., Monday, Wednesday and Friday). Prior to each session, urine specimens and breathalyzer tests were obtained and tested for illicit drugs, including methadone, cocaine, THC, benzodiazepines, morphine-derived opioids, amphetamine, barbiturates, methamphetamine, phencyclidine, tricyclic antidepressants (Multi-Drug Screen Test Dip Card 10 panel; American Screen Corp., Shreveport, LA), oxycodone (Oxycodone Dip Card; American Screen Corp., Shreveport, LA), buprenorphine (Buprenorphine Test Card; American Screening Corp., Shreveport, LA) and alcohol (AlcoHawk, Q3 Innovations, Independence, IA) to ensure the absence of recent use. Females were tested for pregnancy (HCG Pregnancy Dip Card; American Screening Corp., Shreveport, LA) during each screening visit and on the morning of each session. Participants could not eat from midnight on the day of a session but could smoke cigarettes up to one hour prior to session; they were not allowed to eat or smoke throughout the duration of the experimental sessions.
Drugs
An existing Investigational New Drug Application from the Food and Drug Administration was modified to support the conduct of this study (#69,214). All study medications were prepared in the Investigational Pharmacy at the University of Kentucky. Commercial suppliers were used to obtain intravenous solutions of oxycodone hydrochloride (10 mg/ml; Napp Pharmaceuticals, Cambridge, United Kingdom), hydrocodone hydrochloride (15 mg/ml; Abbott GmbH & Co, Ludwigshaffen, Germany) and morphine sulfate (25 mg/ml; Amphistar Pharmaceuticals, South El Monte, CA). These solutions were drawn into syringes and diluted with saline to doses of 5, 10 and 20 mg/10 ml for each drug. The 0 mg/10 ml condition (placebo) contained only saline (Hospira, Lake Forest, IL). Each 10 ml dose was infused using a syringe pump (MedFusion® 3500, Smiths Medical, Keene, NH) into a catheter placed into a vein in the participant’s non-dominant arm under the supervision of an anesthesiologist (KWH or his designee) over a period of 5 min. Dose order was random, with the exception that the highest dose of any drug could not be given before a lower dose had been tested.
Study design
A double-blind, within-subject, randomized, placebo-controlled design was used. There were eleven sessions conducted at the University of Kentucky Clinical Research Development and Operations Center (CRDOC), a dedicated inpatient research unit. The first session served as a practice and safety session during which subjects received the intermediate dose of morphine (10 mg/10 ml, IV) under single-blind conditions (data are not reported from this session). Participants then completed ten double-blind, randomized, experimental test sessions in which they received a single test drug. Baseline data were collected for 30 min prior to drug administration at 9:00 a.m. and data collection proceeded for 6 h thereafter.
Experimental sessions
An intravenous catheter was placed into a vein in each participant’s non-dominant arm by CRDOC nursing staff prior to the start of session for dosing and as a precautionary measure to ensure immediate venous access in the event of an emergency. The catheter was kept patent by a saline drip during sessions. Study staff arrived at the UK CRDOC at approximately 8:00 a.m. on session days and completed baseline measures with participants. Participants were seated in a cushioned chair in their hospital room directly in front of a Macintosh Mini, OSX (Apple Computer, Cupertino, CA) that was used to collect the data. The computer was programmed to record physiological measures (except pupil diameter, expired carbon dioxide [CO2] and respiratory rate) and to present questionnaires in the appropriate order; participants entered their responses by using a computer mouse and/or keypad. The research assistant was seated behind the computer to initiate tasks and complete observer-rated measures.
Physiological measures
Oxygen saturation, blood pressure and heart rate were collected every min using an automatic monitoring device (Scholar III model 507ELC2, Criticare Systems INC, Waukesha, WI). Pupil diameter was determined from Polaroid camera photographs (Polaroid Corp., Cambridge, MA) using a two-fold magnification or a pupillometer (PLR-200, NeurOptics, Irvine, CA) in constant room lighting. Pupil photos were used to measure pupil diameter in two participants and, due to a suspension of film manufacturing, the pupillometer was used to measure pupil diameter in seven participants. Expired CO2 (expressed in mm Hg) and respiratory rate (in breaths/minute) were measured using a Capnograph (N85, Nellcor, Boulder, CO).
Subject- and observer-rated measures
Subject-rated measurements included visual analog scales, the Addiction Research Center Inventory (ARCI) short form (Martin et al., 1971) and a 17-item adjective checklist that encompassed both an Agonist Scale and the Fraser Scale (see Walsh et al., 1995 for scale descriptions). An observer-rated opioid adjective rating scale was also used (Walsh et al., 2008). Table 1 outlines when physiological, subject- and observer-rated and performance measures were completed in each session.
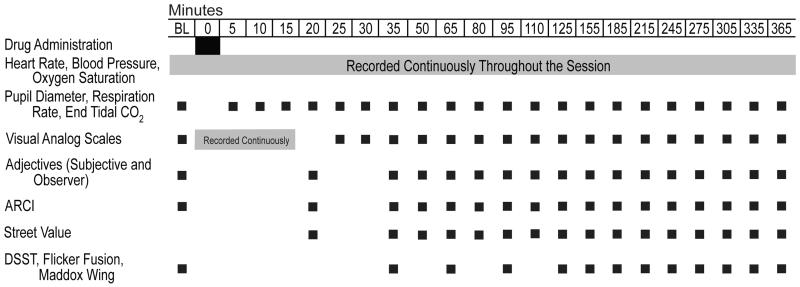
|
Eight visual analog scale items were used throughout each session (six listed in Walsh et al., 2008, plus two additional items: “Does the drug make you have UNPLEASANT THOUGHTS?” and “Does the drug make you have UNPLEASANT BODILY SENSATIONS?”). After the continuous visual analog scale (i.e., 8 items recorded on a min-by-min basis for 20 min following the start of dosing; see Table 1), participants answered four additional questions each time they completed this measure: “Does the drug make you feel IRRITATED?”, “Does the drug make you feel DIZZY?”, “Does the drug make you feel NAUSEATED?” and “Does the drug make it DIFFICULT TO CONCENTRATE?”.
Ocular and performance tasks
Three additional measures were collected as outlined in Table 1, including two ocular tasks sensitive to disruptions of perception and ocular motor control and a computerized Digit Symbol Substitution Task (DSST; McLeod et al., 1982). These tasks were included to measure potential perceptual and performance effects of opioids. The ocular measures are sensitive to perceptual disruptions produced by opioids (see Walsh et al., 2008) and are reported here as nadir CFF 1 and CFF 2 (in Hz) for the Critical Flicker Fusion test and maximum exophoria (in diopters) for the Maddox Wing test.
Statistical Analysis
All measures collected during the experimental sessions were analyzed initially as raw time course data using a two-factor analysis of variance (ANOVA with Proc Mixed to account for any missing data: drug condition x time). The on-line physiological measures, collected on a min-by-min basis during the experimental sessions, were averaged across time to yield intervals ranging from 5 to 30 min, which corresponded to the other physiological data collection. Tukey’s post hoc tests for repeated measures were used to compare scores following active doses to those observed following placebo across time points. When a significant interaction of dose condition and time was observed, significant main effects are not reported. Outcomes are also not reported for measures on which only a significant main effect of time was observed.
In addition to the raw score analyses, peak scores (either nadir or maximum depending upon the direction of effects) for individuals were obtained for repeated measures collected during the experimental sessions; these were analyzed using 1-factor ANOVA with drug condition as the factor. Tukey’s post hoc tests for repeated measures were used to compare peak scores following active doses to those observed following placebo.
Peak effect data from measures where significant main effects of dose were observed were analyzed further comparing oxycodone and hydrocodone to morphine using the Finney (Finney, 1964) method for parallel line bioassays. The analysis of parallel line bioassays is used to determine the relative potency of two compounds. This analysis was used to determine that the dose-response functions (excluding placebo) of oxycodone, hydrocodone and morphine did not differ with respect to shape and slope (i.e., no significant differences in linearity and parallelism, respectively) and showed slopes significantly different from 0 (i.e., significant regression) without differences in magnitude of effect across drugs (i.e., no significant differences in preparation). Six-point bioassays were used for all measures (three doses per drug), log doses were employed and morphine served as the reference compound in the estimates. Data from all measures meeting these criteria were used to calculate relative potency estimates and 95% confidence intervals for those estimates.
Results
Participants
Nineteen participants were screened for the study. Three completed an initial dose ranging pilot study in which the effects of 0, 5, 10 and 15 mg/10 ml of oxycodone, hydrocodone and morphine were tested. After completion of the pilot study, the high dose of each drug was increased to 20 mg/10 ml. Of the remaining sixteen individuals screened, five were lost to follow up and one failed to meet inclusion/exclusion criteria due to opioid physical dependence. Ten participants enrolled into the study; one was withdrawn due to noncompliance and nine completed. Eight of the completers were male and one was female; all were Caucasian (32 ± 2.3 years of age) and all were daily cigarette smokers. Current other drug use in the 30 days prior to screening reported on the Addiction Severity Index and other screening instruments was common, but no participants were physically dependent on any drug. Six participants reported marijuana use (11.0 ± 3.7 days out of the past 30), four reported cocaine use (5.3 ± 2.8 days out of the past 30), five reported use of benzodiazepines (4.1 ± 1.3 days out of the past 30) and amphetamine (2.0 ± 1.0 days out of the past 30) and seven reported alcohol use (7.2 ± 2.4 days out of the past 30). All participants reported recreational use of prescription opioids, most commonly methadone and oxycodone, with an average reported current use of 10 days per month (± 2.3). Three participants had used heroin in the month preceding screening. All participants reported using opioids intravenously (an a priori inclusion criterion) but also reported oral and intranasal recreational opioid use. Two participants reported past histories of treatment for opioid use, but none were seeking treatment at the time of study entry.
Time Course
Physiological Measures
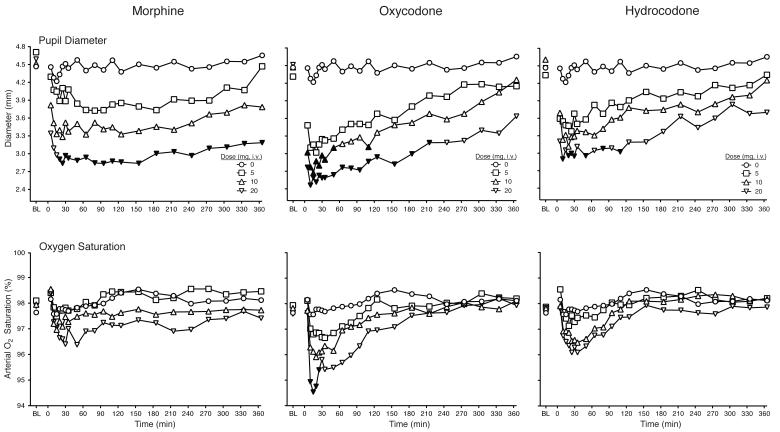
As shown in Figure 1, all three opioids produced dose-dependent decreases in pupil diameter and oxygen saturation. The highest dose of all drugs produced significant decreases in pupil diameter relative to placebo. This effect generally appeared within 5 min of drug administration and was evident for a majority of the 6 h session following oxycodone and morphine dosing. The effects of hydrocodone only lasted for 2 h after dosing. The decreases in oxygen saturation relative to placebo were evident within 5 min of drug administration and persisted approximately 20 min, with post-hoc tests revealing statistical significance after only 20 mg oxycodone administration.
Figure 1.
Data are shown for mean values (n=9) for pupil diameter (top panel) and oxygen saturation (bottom panel) after administration of morphine (left column), oxycodone (middle column) and hydrocodone (right column) as a function of time (X-axis) since drug administration in the 6 h session. Error bars omitted for clarity. Filled symbols indicate a significant difference from the corresponding placebo time point. Time course analysis revealed significant interactions of dose and time condition for pupil diameter and oxygen saturation (F189,1509 = 2.0, p < 0.0001; F189, 1507 = 1.4, p = 0.001, respectively).
All three opioids produced dose- and time-dependent increases in expired CO2 (i.e., interaction of drug condition and time; F189,1512 = 1.2, p = 0.04) and decreases in respiration rate (i.e., main effects of drug condition and time; F9,72 = 2.9, p = 0.006, F21,168 = 3.9, p < 0.001; data not shown). No significant dose-related main effects or interactions were observed on the cardiovascular measures.
Subject- and observer-rated measures
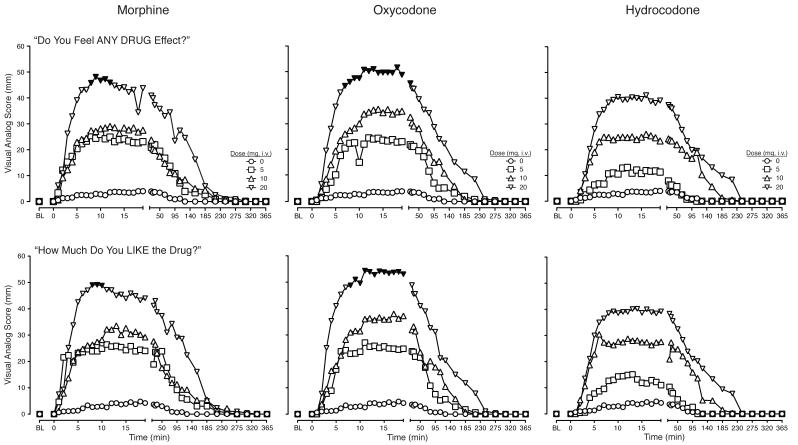
Dose- and time-dependent increases were observed on responses to “Do you feel any DRUG EFFECT?” and “How much do you LIKE the drug?” following the administration of all three opioids, with the exception that the intermediate and low morphine doses produced effects of similar magnitude (Figure 2). The high dose of oxycodone and morphine, but not hydrocodone, increased responses to these items, with statistically significant effects evident approximately 10 min after dosing and dissipating less than 30 min after dosing. For each drug, the effects of lower doses dissipated more quickly than those of higher doses. Similar effects were observed on ratings of “How HIGH are you?” and “Does the drug have any GOOD EFFECTS?” (i.e., interaction of drug condition and time;_F333,2662 values > 1.3, p values < 0.0001; data not shown).
Figure 2.
Data are shown for mean values (n=9) for responses to “Do you feel any DRUG EFFECT?” (top panel) and “How much do you “LIKE” the drug?” (bottom panel) after administration of morphine (left column), oxycodone (middle column) and hydrocodone (right column) as a function of time (X-axis) since drug administration in the 6 h session. The maximum score is 100. Error bars omitted for clarity. Filled symbols indicate a significant difference from the corresponding placebo time point. Time course analysis revealed significant interactions of dose and time condition for “Do you feel any DRUG EFFECT?” and “How much do you “LIKE” the drug?” (F333,2661 = 1.4, p < 0.0001; F333,2662 = 1.3, p = 0.002, respectively).
The low dose of morphine produced transient increases on ratings of “Does the drug have any BAD EFFECTS?” (i.e., main effect of drug condition; F9,72 = 2.1, p = 0.04; data not shown). The high dose of oxycodone produced transient increases for ratings of “Does the drug make it DIFFICULT TO CONCENTRATE?” (i.e., main effects of drug condition and time; F9,72 = 2.4, p = 0.02, F17,136 = 3.9, p < 0.0001; data not shown).
All three opioids also produced dose- and time-dependent increases (i.e., significant interaction effect) on the MBG scale of the ARCI (F108,864 = 1.5, p < 0.001), Subject-Rated Opioid Agonist Adjectives (F144,1151 = 1.5, p < 0.0001), Observer-Rated Opioid Agonist Adjectives (F144,1142 = 1.9, p < 0.0001) and Street Value (F135,1080= 1.7, p < 0.0001; data not shown).
Ocular and performance tasks
No significant dose-related main effects or interactions were observed on the performance measures.
Peak Effects
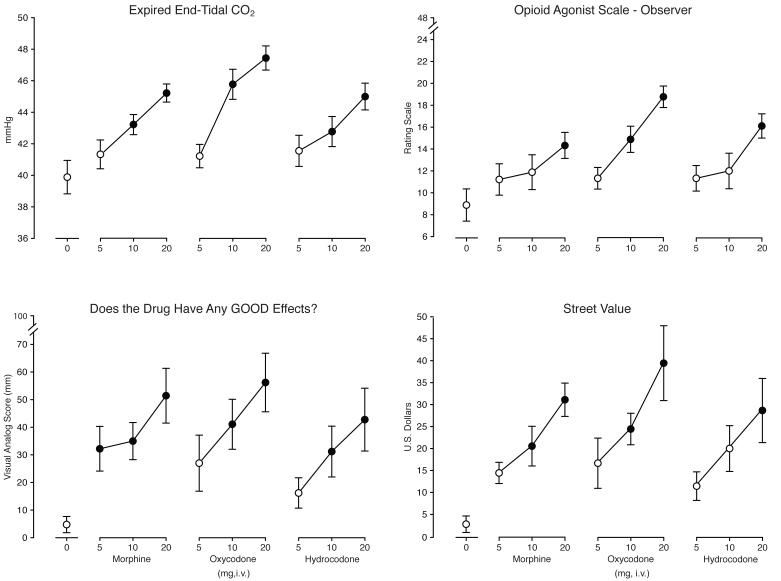
Figure 3 shows outcomes for four representative measures: expired CO2, Observer-Rated Opioid Agonist Adjectives, “Does the drug have any GOOD EFFECTS?” and Street Value. Table 2 shows mean nadir/maximum (depending on the direction of the effect) values from other measures not shown in Figure 3 for which a significant effect of drug condition was observed.
Figure 3.
Data are shown for mean values (n=9) for expired CO2 (top left panel), Observer-Rated Opioid Agonist Adjectives (top right panel), “Does the drug have any GOOD EFFECTS?” (bottom left panel) and Street Value (bottom right panel) as a function of dose (X-axis). Brackets indicate + 1 SEM. A significant effect of Dose Condition was observed for all four measures (F9, 72 values ≥ 7.8, p < 0.0001). Filled symbols are significantly different from placebo (Tukey test; p < 0.05).
Table 2.
Peak values for measures with a significant effect of drug condition. Bolded values are significantly different from placebo.
| Morphine (mg) | Oxycodone (mg) | Hydrocodone (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Measure | Placebo | 5 | 10 | 20 | 5 | 10 | 20 | 5 | 10 | 20 |
| Physiological | ||||||||||
| Pupil Diameter* | 3.9 | 3.4 | 3.0 | 2.6 | 2.8 | 2.6 | 2.4 | 3.2 | 3.0 | 2.8 |
| Oxygen Saturation * | 97.1 | 96.9 | 96.4 | 95.6 | 96.2 | 95.5 | 94.0 | 96.7 | 96.0 | 95.4 |
| Subject-rated | ||||||||||
| Visual analogs | ||||||||||
| High | 4.7 | 27.4 | 30.1 | 49.8 | 26.6 | 37.7 | 55.8 | 12.8 | 27.2 | 40.1 |
| Drug effect | 5.0 | 30.1 | 32.9 | 51.4 | 26.2 | 39.0 | 55.4 | 14.7 | 31.3 | 43.2 |
| Like | 5.3 | 38.7 | 37.2 | 53.6 | 28.0 | 44.2 | 59.3 | 17.3 | 34.7 | 41.7 |
| Bad effect | 1.3 | 22.2 | 10.8 | 10.3 | 2.0 | 0.6 | 4.8 | 0.6 | 2.9 | 12.3 |
| Difficulty concentrating | 0.6 | 1.2 | 0.0 | 9.1 | 0.7 | 8.4 | 15.8 | 0.0 | 5.4 | 2.8 |
| ARCI | ||||||||||
| PCAG | 4.6 | 4.4 | 4.7 | 4.8 | 5.7 | 5.3 | 6.4 | 3.9 | 5.8 | 4.8 |
| AMPH | 2.6 | 2.9 | 3.3 | 4.7 | 2.9 | 3.6 | 3.9 | 2.7 | 2.9 | 3.6 |
| MBG | 1.7 | 4.8 | 5.2 | 9.1 | 4.8 | 6.2 | 8.4 | 3.9 | 5.8 | 6.9 |
| Agonist scale | 9.8 | 14.6 | 14.1 | 18.1 | 14.3 | 17.6 | 19.9 | 13.4 | 14.7 | 16.9 |
| Ocular tasks | ||||||||||
| Maddox wing | 4.1 | 4.3 | 5.0 | 6.3 | 5.2 | 6.3 | 7.3 | 4.8 | 5.8 | 6.6 |
| Flicker/fusion * (#1) | 33.8 | 32.0 | 32.1 | 30.2 | 31.9 | 33.2 | 30.5 | 31.7 | 33.6 | 31.6 |
| Flicker/fusion * (#2) | 33.7 | 31.3 | 31.0 | 29.5 | 32.1 | 32.8 | 30.2 | 31.5 | 33.4 | 31.9 |
Measures were analyzed as peak maximum score unless nadir is indicated (*). Values are mean scores (n = 9) for placebo, morphine, oxycodone and hydrocodone.
Physiological Measures
The 10 and 20 mg doses of all three drugs produced significant increases in expired CO2 (Figure 3). A similar pattern was observed for pupil diameter and oxygen saturation (see Table 2). No significant effects were observed for respiration rate or the cardiovascular measures.
Subject- and observer-rated measures
All three drugs produced significant, dose-dependent increases on the Observer-Rated Opioid Agonist Adjective Scale, the visual analog scale item “Does the drug have any GOOD EFFECTS?” and Street Value estimates (Figure 3). Post hoc tests revealed that the 20 mg dose of each drug produced significant increases on these measures. The intermediate and low doses of the drugs also produced significant increases on some measures.
Table 2 shows the peak/nadir values of the other measures for which a significant effect of drug condition was observed: “Do you feel any DRUG EFFECT?”, “How HIGH are you?”, “How much do you LIKE the drug?”, “Does the drug have any BAD EFFECTS?”, and “Does the drug make it DIFFICULT TO CONCENTRATE?” from the visual analog scale, the MBG, PCAG and AMPH scales of the ARCI and the Subject-Rated Opioid Agonist Adjectives (F9,72 values > 2.4, p values ≤ 0.02).
Two exceptions to the pattern of results observed with subject- and observer-rated measures (see Table 2 and Figure 3) occurred on responses to “Does the drug have any BAD EFFECTS?” and “Does the drug make it DIFFICULT TO CONCENTRATE?”. Only the low dose of morphine significantly increased ratings of “Does the drug have any BAD EFFECTS?” while only the high dose of oxycodone significantly increased ratings of “Does the drug make it DIFFICULT TO CONCENTRATE?”.
Ocular and performance tasks
The high dose of morphine and oxycodone significantly decreased the score on the CFF 2 (F9,72 = 2.7, p = 0.01). The high dose of oxycodone and hydrocodone both increased scores on the Maddox Wing task (F9,72 = 4.2, p < 0.001). No significant effects were observed on the outcome measures for the DSST.
Relative Potencies
Of the eighteen measures for which a significant peak effect was observed, nine valid potency estimates were calculated for oxycodone and six valid potency estimates were calculated for hydrocodone. Table 3 shows these estimates with 95% confidence intervals. The table also includes those measures for which the assay was invalid along with the specific assumptions violated. For the valid oxycodone potency assays, oxycodone was more potent than morphine on seven measures. For the valid hydrocodone potency assays, hydrocodone was less potent than morphine on five measures. Importantly, the potency differences for both drugs were modest (i.e., average potency ratios of 1.03 and 0.92 for morphine relative to oxycodone and hydrocodone, respectively). When comparing across the four measures (ARCI MBG and AMPH, Subject-Rated Opioid Agonist Adjectives and Street Value) that had valid potency estimates for both oxycodone and hydrocodone, the potency relationship was generally oxycodone > morphine > hydrocodone.
Table 3.
Relative potency estimates and 95% confidence intervals based upon Finney’s (1964) bioassay using logarithmic doses.
| Outcome Measure | Morphine relative to Oxycodone |
Confidence intervals |
Assumptions violated |
Morphine relative to Hydrocodone |
Confidence intervals |
Assumptions violated |
|---|---|---|---|---|---|---|
| Physiological | ||||||
| Pupil Diameter * | - | - | 2, 3 | - | - | 2 |
| Oxygen Saturation* | - | - | 3 | - | - | 3 |
| Expired CO2 | - | - | 2, 3 | 0.975 | 0.793-1.188 | |
| Subject-rated | ||||||
| Visual analogs | ||||||
| High | 1.111 | 0.874-1.493 | - | - | 3 | |
| Drug effect | 1.054 | 0.828-1.377 | - | - | 3 | |
| Like | 1.020 | 0.634-1.699 | - | - | 3 | |
| Good effect | 1.051 | 0.785-1.458 | - | - | 3 | |
| Bad effect | - | - | 3, 4 | - | - | 2, 4 |
| Difficulty concentrating | - | - | 3 | - | - | 4 |
| ARCI | ||||||
| PCAG | - | - | 3, 4 | - | - | 4 |
| AMPH | 0.919 | 0.455-1.494 | 0.752 | 0.055-1.222 | ||
| MBG | 1.018 | 0.661-1.613 | 0.862 | 0.352-1.367 | ||
| Agonist Scale | 1.263 | 0.936-2.175 | 0.895 | 0.402-1.455 | ||
| Street value | 1.168 | 0.971-1.472 | 0.928 | 0.666-1.228 | ||
| Ocular tasks | ||||||
| Maddox wing | - | - | 3 | - | - | 3 |
| Flicker/fusion * (#1) | - | - | 4 | - | - | 1, 3, 4 |
| Flicker/fusion * (#2) | 0.667 | 0.017-1.053 | - | - | 3, 4 | |
| Observer-rated adjectives | - | - | 2, 3 | 1.116 | 0.880-1.496 |
All outcome measures, for which significant effects of dose condition were observed in the peak maximum/nadir (denoted by *) were assayed. If an assay was invalid, the assumptions violated are noted by number below (1=Linearity, 2=Parallelism, 3=Preparation, 4=Regression). Morphine is the reference drug. Relative potency is expressed as mg morphine necessary to produce the same effect as 1mg of oxycodone or hydrocodone.
Discussion
Intravenous administration of oxycodone, hydrocodone and morphine to non-physically dependent opioid users produced prototypical mu opioid agonist-like effects (e.g., miosis, increased ratings of drug liking and good effects) and was well tolerated (i.e., no serious adverse events occurred). Abuse potential is a composite of both positive and negative subjective effects and there was little evidence in the present study of negative subjective effects for these three opioids. The time course and peak effects were qualitatively and quantitatively similar across drugs, indicating a comparable potential for abuse of intravenous oxycodone and hydrocodone relative to the positive control, morphine. While potency differences were noted between oxycodone and hydrocodone in comparison to morphine, these differences were very modest (i.e., less than two-fold).
The rapid onset of pharmacodynamic effects (i.e., within 5 min of dosing) following active drug administration is consistent with previously published pharmacokinetic data for oxycodone and morphine (Leow et al., 1992; Stanski et al., 1978). This finding is also in agreement with results of previous human laboratory studies that examined the time course of the pharmacodynamic effects of oxycodone and morphine (Marsch et al., 2001; Tarkilla et al., 1997).
The physiological and subjective effects of hydrocodone dissipated more rapidly than those of oxycodone and morphine. However, comparison of the hydrocodone curves to doses of morphine and oxycodone yielding comparable effects show that the effects of intermediate doses of those drugs also produced effects of shorter duration compared to the highest test doses of oxycodone and morphine. Moreover, although not reported in the Results, time to peak values for corresponding doses of the three drugs did not differ from one another. Overall, the time course data reveal that the three drugs are very similar in time-action profile (with differences dependent upon dose), and this is consistent with their similar reported elimination half-lives (i.e., 2-4 hours; reviewed in Trescot et al., 2008).
The physiological effects of all three drugs generally lasted throughout the 6-h session, while the subjective effects dissipated much earlier; this is also concordant with findings of previous studies that have tested the effects of intravenous opioids (Abreu et al., 2001; Marsch et al., 2001). The differences in time course between physiological and subjective effects are important given that individuals may choose to re-inject an opioid once the subjective effects have subsided and be unaware that potentially dangerous physiological effects (e.g., respiratory depression, decreased oxygen saturation or increased expired CO2) remain that could be potentiated by additional dosing.
The highest dose of all three opioid drugs produced significant effects on peak/nadir physiological and subjective outcomes characteristic of mu opioid agonists (e.g., miosis, increased expired CO2 and ratings of liking), which is also consistent with previous research examining parenteral opioid administration (Comer et al., 2008; 2009; Lamb et al., 1991; Tarkkila et al., 1997). Moreover, effects observed here on ocular tasks (i.e., decreases in CFF threshold and increases in exophoria) are concordant with previous studies (Saarialho-Kere et al., 1989; Walsh et al., 2008; Zacny and Gutierrez, 2003). As in some earlier studies (Comer et al., 2008; Walsh et al., 2008), there were no effects on DSST performance suggesting that doses of these opioids producing robust subjective effects do not reliably impair performance or that this task may be insensitive to the effects of opioid administration.
The peak effects of oxycodone were of greater magnitude, but not significantly different, compared to those of morphine and hydrocodone, which was supported by the relative potency analysis (i.e., valid potency ratios indicated a relationship of oxycodone > morphine > hydrocodone). A number of potency ratios could not be calculated due to violations of the preparation assumption (Table 3). These violations were likely due to the effects of the highest dose of oxycodone and the lowest dose of hydrocodone producing effects outside the upper and lower range, respectively, of those produced by morphine, further supporting the potency relationship obtained with the valid analyses. These data bolster the argument that these widely prescribed opioid analgesics have comparable potential for abuse across routes of administration and extend previous findings to sporadic opioid users.
Our finding that oxycodone was more potent than morphine and hydrocodone is consistent with the results of some previous preclinical and clinical studies (Meert and Vermeirsch, 2005; Zacny and Gutierrez, 2003; 2008; 2009; Zacny and Lichtor, 2008). However, other work has suggested that orally administered oxycodone and hydrocodone are equipotent to one another (Walsh et al., 2008), and that parenteral doses of hydrocodone and oxycodone are equipotent to morphine (Comer et al., 2008; Fraser and Isbell, 1950; Jasinski and Martin, 1967). The reasons for these different potency ratios observed across studies is unknown but could be due to a number of factors including the study population, route of administration and the method used to assess potency.
In closing, these data suggesting comparable abuse potential for intravenous hydrocodone, oxycodone and morphine have implications from both the regulatory and clinical practice perspective. As noted above, oxycodone is available in the United States in both combination and opioid-only formulations and is regulated under Schedule II. Hydrocodone alone is regulated under Schedule II but is presently available in the United States only in combination products, which fall under the less tightly regulated Schedule III designation. Previously published studies suggest that the presence of acetaminophen in opioid combination products does not alter abuse potential (Zacny et al., 2005; Zacny and Gutierrez, 2008), so it may be inferred that parenteral administration of hydrocodone combination products would not result in significantly different effects from those reported here for hydrocodone alone. Thus, it is important that clinician prescribers recognize that the Schedule III designation of these hydrocodone products does not necessarily indicate lower abuse potential and should prescribe with the same degree of caution as with Schedule II opioids.
Acknowledgements
This research was supported by a grant from the National Institute on Drug Abuse (5R01DA016718-05) to Sharon L. Walsh and by the University of Kentucky CRDOC. The authors declare no conflicts of interest relevant to this project. The authors with to thank Lori Craig, Jessica DiCentes and Elizabeth Tammen for technical assistance and Stacy Miller, Todd McCoun, Pieter Steyn and Marie Thompson for medical assistance.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology. 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Sullivan MA, Vosburg SK, Saccone PA, Foltin RW. Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J Opioid Manag. 2009;5:203–212. doi: 10.5055/jom.2009.0022. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WR, Johnson BD. Prescription opioid use, misuse, and diversion among street drug users in New York City. Drug Alcohol Depend. 2008;92:267–276. doi: 10.1016/j.drugalcdep.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Statistical Method in Biological Assay. 2nd edn Hafner; New York: 1964. [Google Scholar]
- Fraser HF, Isbell H. Addiction liabilities of morphinan, 6-methyldihydromorphine and dihydrocodeinone. J Pharmacol Exp Ther. 1950;100:128–135. [PubMed] [Google Scholar]
- Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2007;87:98–102. doi: 10.1016/j.drugalcdep.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Hays L, Kirsh KL, Passik SD. Seeking drug treatment for OxyContin abuse: a chart review of consecutive admissions to a substance abuse treatment facility in Kentucky. J Natl Compr Canc Netw. 2003;3:423–428. doi: 10.6004/jnccn.2003.0035. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Martin WR. Assessment of the dependence producing properties of dihydrocodeinone and codoxime. Clin Pharmacol Ther. 1967;8:266–270. doi: 10.1002/cpt196782266. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Preston KL. Comparison of intravenously administered methadone, morphine and heroin. Drug Alcohol Depend. 1986;17:301–310. doi: 10.1016/0376-8716(86)90079-7. [DOI] [PubMed] [Google Scholar]
- Katz N, Fernandez K, Chang A, Benoit C, Butler SF. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24:528–535. doi: 10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: A dose-response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- Leow KP, Smith MT, Watt JA, Williams BE, Cramond T. Comparative oxycodone pharmacokinetics in humans after intravenous, oral and rectal administration. Ther Drug Monit. 1992;14:479–484. doi: 10.1097/00007691-199212000-00008. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B, Norsten-Höög C. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299:1056–1065. [PubMed] [Google Scholar]
- Martin WR, Sloan BS, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavior Research Methods and Instrumentation. 1982;14:463–466. [Google Scholar]
- Meert TF, Vermeirsch HA. A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol Biochem Behav. 2005;80:309–326. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Magura S, Haddox JD. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U, Mattila MJ, Seppala T. Psychomotor, respiratory and neuroendocrinological effects of a μ-opioid receptor agonist (Oxycodone) in healthy volunteers. Pharmacol Toxicol. 1989;65:252–257. doi: 10.1111/j.1600-0773.1989.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Stanski DR, Greenblatt DJ, Lowenstein E. Kinetics of intravenous and intramuscular morphine. Clin Pharmacol Ther. 1978;24:52–59. doi: 10.1002/cpt197824152. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . The NSDUH Report: Patterns and Trends in Nonmedical Prescription Pain Reliever Use: 2002-2005. Office of Applied Studies; Rockville, MD: 2007. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2009. HHS Publication No. SMA 09-4434. [Google Scholar]
- Tarkilla P, Tuominen M, Lindgren L. Comparison of respiratory effects of tramadol and oxycodone. J Clin Anesth. 1997;9:582–585. doi: 10.1016/s0952-8180(97)00147-5. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Mello NK, Mendelson JH, Kuehnle J, Gastfriend DR, Rhoades E, Sholar W. Buprenorphine effects on morphine- and cocaine-induced subjective responses by drug-dependent men. J Clin Psychopharmacol. 1994;14:15–27. [PubMed] [Google Scholar]
- Teoh SK, Mendelson JH, Mello NK, Kuehnle J, Sintavanarong P, Rhoades EM. Acute interactions of buprenorphine with intravenous cocaine and morphine: an investigational new drug phase I safety evaluation. J Clin Psychopharmacol. 1993;13:87–99. [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–153. [PubMed] [Google Scholar]
- Walker DJ, Zacny JP. Subjective, psychomotor, and physiological effects of cumulative doses of opioid mu agonists in healthy volunteers. J Pharmacol Exp Ther. 1999;289:1454–1464. [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- Zacny JP. Characterizing the subjective, psychomotor, and physiological effects of a hydrocodone combination product (Hycodan) in non-drug-abusing volunteers. Psychopharmacology. 2003;165:146–156. doi: 10.1007/s00213-002-1245-5. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology. 2003;170:242–254. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Subjective, psychomotor, and physiological effects profile of hydrocodone/acetaminophen and oxycodone/acetaminophen combination products. Pain Med. 2008;9:433–443. doi: 10.1111/j.1526-4637.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. Drug Alcohol Depend. 2009;101:107–114. doi: 10.1016/j.drugalcdep.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S, Bolbolan SA. Profiling the subjective, psychomotor, and physiological effects of a hydrocodone/acetaminophen product in recreational drug users. Drug Alcohol Depend. 2005;78:243–252. doi: 10.1016/j.drugalcdep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology. 2008;196:105–116. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]