Abstract
Objective
The increased incidence of obesity and type 2 diabetes (T2D) among youth has prompted the development of guidelines for healthy cardiorespiratory fitness (CRF) and physical activity (PA) levels in the pediatric population. It is unclear whether youth with T2D meet these guidelines as previous research has not included type 2 diabetics. Therefore, the purpose of this investigation was to examine CRF and PA in youth with T2D and compare these results with recently published normative data for CRF and guidelines for PA in youth.
Methods
Forty adolescents (17 males and 23 females) with T2D were assessed for moderate-to-vigorous PA via the 7-day PA recall. CRF was determined by a progressive cycle ergometer test and indirect calorimetry. PA levels were compared with recently published guidelines for youth of 60 minutes per day, and CRF data were compared with age- and sex-adjusted normative values from the National Health and Nutrition Examination Survey 1999–2002.
Results
Only 17.6% (3/17) of boys and 21.7% (5/23) of girls met PA guidelines, while none of the participants met criteria for healthy CRF. When compared with normative CRF data for US youth, ~93% of boys and 95% of girls scored below the 10th percentile.
Conclusions
These results suggest that youth with T2D exhibit low levels of CRF and the majority do not participate in recommended amounts of PA. Practitioners working with type 2 diabetic youth need to emphasize the importance of regular PA to increase CRF and promote cardiovascular health in an effort to decrease long-term diabetes-related complications.
Keywords: Obesity, insulin resistance, exercise, VO2 peak, children
Introduction
Pediatric obesity has reached epidemic proportions in most developed countries (1). In conjunction with the noted increase in adiposity among youth, type 2 diabetes (T2D) is an emerging clinical manifestation in this population (2). The etiology of obesity in youth is complex and the development of T2D is not well understood; however, it is hypothesized that poor cardiorespiratory fitness (CRF) coupled with a sedentary lifestyle may be contributory (3). As such, the assessment of CRF and physical activity (PA) in various populations of youth has received increased attention (4).
To better ascertain the CRF and PA levels across a representative sample of US youth, these measures were recently included in the National Health and Nutrition Examination Survey (NHANES). Using NHANES 1999–2002 data, Pate et al. (5) published normative values regarding the CRF of contemporary US youth. The authors reported several descriptive characteristics which significantly differentiated fit from unfit youth including: obesity, sex, PA levels, and sedentary behaviors (screen time). Additionally the authors found that approximately one-third of US adolescents aged 12 to 19 years failed to meet criterion referenced standards for acceptable CRF, as defined by the FITNESSGRAM (6). Other investigations have utilized CRF measures from the NHANES dataset to examine associations with T2D risk factors (insulin sensitivity and metabolic syndrome) in youth. Imperatore et al. (7) found CRF to be an independent predictor of insulin sensitivity in adolescents. Whereas, Janssen and Cramp (8) found CRF to be a significant determinant of the metabolic syndrome phenotype in youth. Given the predictive nature of both insulin resistance and the metabolic syndrome for T2D, it is plausible that low CRF may contribute to the development of T2D in youth. Unfortunately, children with T2D were not included in the NHANES analyses of Pate, Imperatore, or Janssen.
Although an increase in the incidence of T2D among youth has been reported, the prevalence remains far lower than that of type 1 diabetes (9). As a result, empirical data from studies of youth with T2D are limited. In fact, most studies examining CRF and/or PA in youth in relation to diabetes risk have not included youth with T2D (10,11). However, in adults, several studies have established a protective effect of both CRF and PA on T2D development (12). Therefore, the purpose of this study was to assess CRF and PA levels in a sample of youth with T2D and compare these findings to age-appropriate normative values for CRF and current pediatric recommendations for PA.
Research design and methods
Participants
Forty adolescent volunteers (age 13–18) with previously diagnosed T2D were invited to participate in this study. Youth were recruited through pediatric diabetes clinics where they were receiving regular care for T2D at the time of study. Participants who developed T2D secondary to treatment for another condition or medication were excluded. In addition, youth were also excluded if they had a known cardiac defect, or had delays in cognitive, psychological, or behavioral functioning. This study was approved by the Institutional Review Board at the University of Illinois at Chicago (UIC). Informed written consent and child assent were obtained from participants and families prior to any testing procedures.
Protocol
Participants were invited to the UIC General Clinical Research Center for collection of study measures. For standardization purposes, all testing occurred in the morning beginning between 08.00–09.00. Height (wall mounted stadiometer) and weight (medical balance beam scale) were measured in light clothing without shoes, and BMI and BMI percentiles were subsequently calculated according to the Centers of Disease Control and Prevention (13). Pubertal stages of maturation were determined during a physical examation (14) by a Pediatric Endocrinologist or Nurse Practitioner (pubic hair in boys and breast development in girls) and T2D duration was determined by medical chart review with parent confirmation. Glycosylated hemoglobin (HbA1c) was determined using the Abbott IMx® assay method (Abbott Park, IL) for quantitative measurement of whole blood (CV < 5%). Upon completion of the clinical and metabolic testing, PA and CRF were subsequently assessed, as described below.
Physical activity
Moderate-to-vigorous PA (MVPA) was assessed by the Seven-Day PA Recall (7 day-PAR) developed by Sallis et al. (15). The 7 day-PAR is a structured interview, which has been shown to be a valid (r = 0.55−0.72) and reliable (r = 0.77) measure of PA in children and adolescents (16). Trained research staff asked participants to recall all activities participants undertook during the preceding 7 days. Activities were divided into light, moderate, hard, and very hard categories with examples for each provided for reference. Duration of activities was recorded in 10-minute blocks. Given the difficulty in quantifying short-bout activities of children, only bouts of at least moderate intensity performed for at least 10 minutes were used in the present analysis. The total amount and duration of MVPA (hard or very hard) was summed for the entire week and expressed as minutes per day of MVPA.
Cardiorespiratory fitness
Peak oxygen consumption (VO2peak) was determined during an all-out progressive exercise test on a cycle ergometer. Expired gasses were collected and analyzed using a Sensormedics® VMAX29 integrated metabolic cart. The metabolic cart was calibrated prior to each testing session using medical grade gas mixtures of known concentrations and a 3.0 L syringe. Prior to testing procedures, participants were familiarized with the exercise equipment and instructed to maintain a cycling cadence of 50–60 revolutions per minute throughout the test. Each participant performed the McMaster cycle test protocol appropriate for adolescents (17). This protocol allows for sex- and height-specific workload adjustments in order to provide steady-state exercise increases in a linear fashion. Exercise testing was terminated when the participant could no longer maintain proper pedaling cadence despite verbal encouragement from research staff. VO2peak was determined at the highest oxygen consumption obtained with a respiratory exchange ratio above 1.0.
Reference data and statistical analysis
PA levels were compared with age-appropriate guidelines and recommendations of at least 60 minutes of MVPA per day (18). CRF data were compared with available normative values for US youth, derived from exercise testing in 3 287 12–19-year-olds participating in NHANES 1999–2002 (5). Additionally, CRF data were compared with criterion-referenced standards of 42 ml/kg/min for males aged 12–19 and 35–37 ml/kg/min for females 12–19 years old (6).
Sex differences in CRF, MVPA, and descriptive characteristics were compared by independent sample t-test. Chi-squared analyses were used to examine sex comparisons with respect to meeting CRF or MVPA guidelines. All data were presented as means ± standard deviation or as percentages. Analyses were performed using SPSS version 15.0 with a type 1 error set at P < 0.05.
Results
Descriptive characteristics of the participants are presented in Table I. Boys were significantly taller and heavier than girls and the sample as a whole was obese. T2D duration was not different between sexes; however, boys tended to exhibited better T2D control than girls (p = 0.06).
Table I.
Physical characteristics of youth with type 2 diabetes (T2D).
| Boys (n = 17) |
Girls (n = 23) |
Total (n = 40) |
|
|---|---|---|---|
| Age (years) | 15.8 ± 1.7 | 15.9 ± 1.8 | 15.8 ± 1.8 |
| Tanner Stage (%) | |||
| 3 | 0 | 13% | 7.5% |
| 4 | 29.4% | 8.7% | 17.5% |
| 5 | 70.6% | 78.3% | 75.0% |
| Ethnicity (%) | |||
| AA | 70.6% | 100% | 87.5% |
| Hisp | 29.4% | 0% | 12.5% |
| T2D Duration (years) | 2.5 ± 1.8 | 2.4 ± 2.2 | 2.5 ± 2.0 |
| HbA1c (%) | 7.7 ± 2.5 | 9.2 ± 2.3 | 8.6 ± 2.5 |
| Height (cm) | 175.7 ± 7.8 | 161.3 ± 6.7* | 167.4 ± 10.1 |
| Weight (kg) | 108.9 ± 39.1 | 86.2 ± 23.2† | 95.8 ± 32.6 |
| BMI (kg/m2) | 34.8 ± 10.9 | 32.9 ± 7.9 | 33.7 ± 9.2 |
| BMI Percentile | 96.2 ± 5.8 | 94.6 ± 5.6 | 95.3 ± 5.7 |
Data presented are means ± standard deviation.
AA: African-American; Hisp: Hispanic; HbA1c: Glycosylated hemoglobin; BMI: Body mass index.
P < 0.05
P < 0.01 for sex comparison.
Physical activity and cardiorespiratory fitness levels
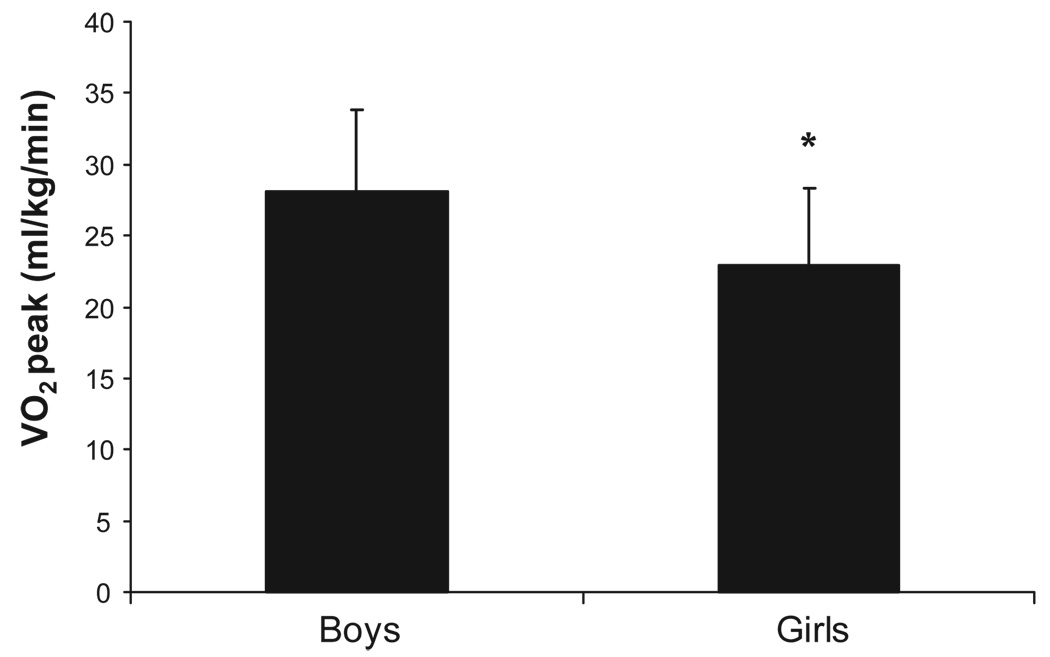
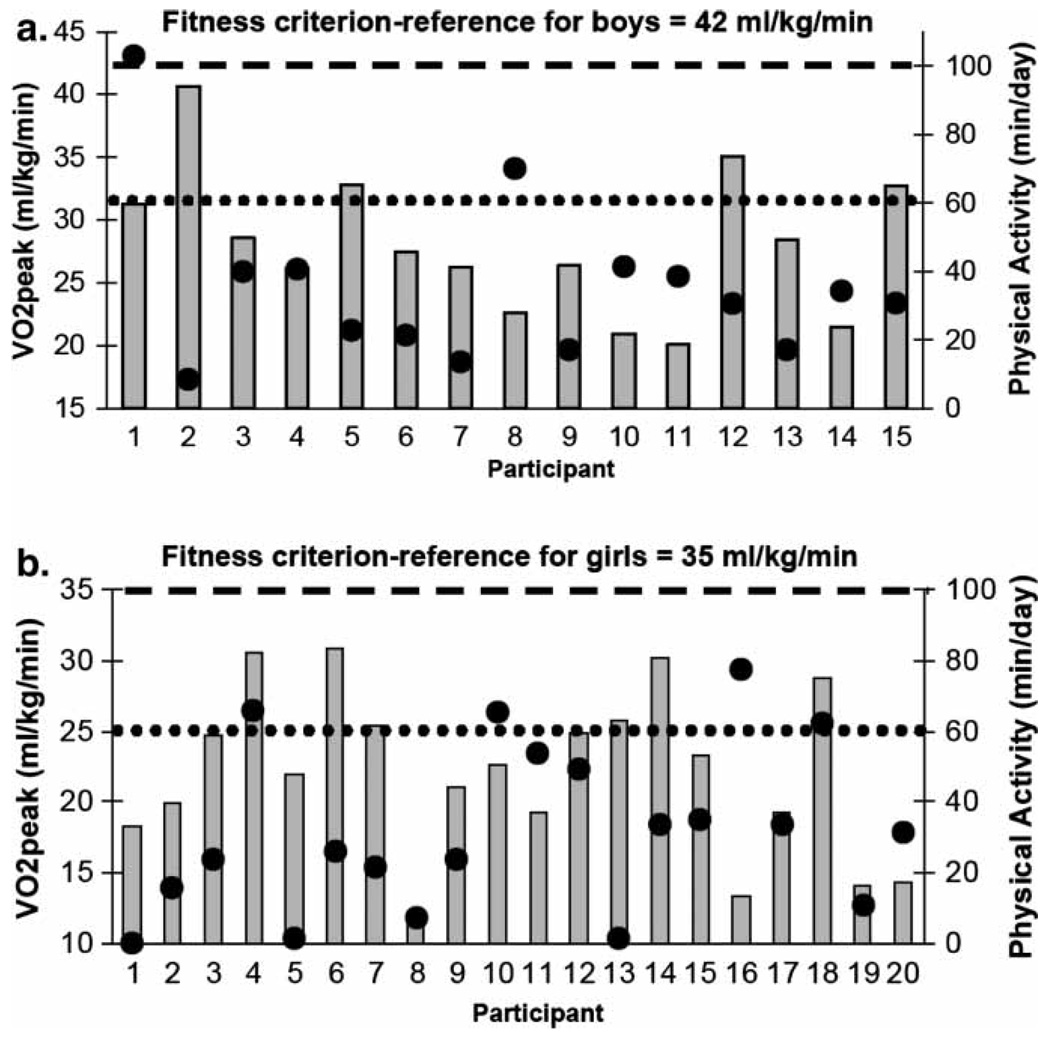
Boys and girls reported performing similar amounts of MVPA (38.8 ± 27.2 vs. 32.3 ± 26.9 minutes/day; p > 0.05). When compared with current recommendations, only 17.6% (3/17) of boys and 21.7% (5/23) of girls reported performing at least 60 minutes of MVPA per day (p > 0.05 for sex comparison). CRF data were not available for five of the 40 participants (3 had blood glucose values > 250 mg/dl and were not tested as per our Data Safety and Monitoring Plan, 1 exceeded the weight limit of 300 pounds for the ergometer, and 1 refused to wear the nose clip and mouthpiece required for exercise testing). No significant MVPA differences were noted between youth who did and did not perform CRF testing (33.3 ± 23.4 vs. 47.1 ± 46.5 minutes/day; p > 0.05). CRF levels (VO2peak) are presented in Figure 1. Boys exhibited ~19% higher CRF levels compared with girls (28.1 ± 5.7 vs. 22.0 ± 5.8 ml/kg/min; p = 0.01). CRF differences were accentuated when oxygen uptake data were expressed in absolute terms and BMI was statistically adjusted for (2.8 ± 1.1 vs. 1.9 ± 1.0 L/min, p < 0.001). Figure 2a and 2b display individual CRF and MVPA levels plotted against age- and sex-reference standards and recommendations for youth. Irrespective of sex, no participant met the criteria for healthy CRF levels. Furthermore, when data were compared with published CRF norms, ~93% of boys and 95% of girls scored below the 10th percentile for US youth (p > 0.05 for sex comparison).
Figure 1.
Cardiorespiratory fitness (VO2peak) in boys and girls with type 2 diabetes. Data are presented as Means ± standard deviation. *P < 0.05.
Figure 2.
Individual cardiorespiratory fitness levels (indicated by gray bars) and physical activity levels (indicated by large black dots) of boys (a) and girls (b). The dashed line represents minimum age and sex criteria for healthy cardiorespiratory fitness and the dotted line represents current pediatric recommendations for physical activity.
Discussion
The incidence of T2D in youth has increased worldwide (2,19). Despite this increase, a dearth of empirical data exists from this population. Our findings suggest that this disorder co-exists with low CRF and low levels of MVPA. These results extend recent findings in healthy youth highlighting CRF and PA as significant determinants of T2D risk (20–22). The inclusion of youth diagnosed with T2D in the present analysis is novel and adds to the growing, but still limited, available data related to T2D in the pediatric population.
Regular PA is essential for promoting healthy growth and development of children (23). The increase in obesity among youth suggests that, as a whole, children are not obtaining sufficient levels of PA to match their caloric intake and thus excess energy is stored in the form of triglycerides (23). Over time, the accumulation of fat and further lack of PA will likely lead to reductions in CRF and increased risk for developing comorbidities, such as T2D (24). In our sample, MVPA was directly related to CRF levels (r = 0.38, p = 0.03), which suggests that youth who participated in greater MVPA had higher levels of CRF. However, the cross-sectional design of our study prohibits our ability to derive any causal relationship from this association. Nonetheless, when compared with contemporary US youth, the participants in the present investigation had profoundly low CRF levels. In addition to low CRF levels, these youth were considerably overweight with a mean BMI percentile for the entire sample consistent with the criteria for obesity. There is debate in the literature as to whether obesity per se impacts CRF levels in youth (25). To be consistent with the NHANES CRF data, we expressed CRF relative to total body mass. Therefore, it is difficult to parcel out whether the comparatively low CRF levels in our sample are the sole function of their high body mass and hence a larger denominator in the oxygen uptake equation or are a reflection of significant impairments in oxygen uptake and utilization. Towards this end, we have recently observed that boys with T2D had significantly lower CRF levels compared with BMI and age-matched non-diabetic controls (26).
It is likely that T2D is the end result of a complex etiology where obesity, CRF, and physical inactivity are key determinants. In terms of prevention, very few successful pediatric obesity treatment programs have been described in the literature (27). Therefore, as children become increasingly more overweight and present with clinical comorbidities, intervention efforts may be best served by focusing on MVPA in order to increase CRF and improve metabolic health rather than focusing on adiposity (28). Clear evidence in adults supports the concept that higher CRF rather than obesity status is the primary determinant of health (29). Furthermore, the protective effect of CRF extends to active overweight adults with T2D who exhibit a lower all-cause mortality risk compared with lean sedentary diabetics (30). These findings suggest that exercise may reduce chronic disease risk independent of obesity and T2D.
Compared with the adult literature, the body of research in the pediatric population examining the associations between PA and T2D is limited. Most studies have focused on healthy or overweight youth at-risk for T2D. A recent study of ~1 700 9- and 15-year-old children from the European Youth Heart Study found that sedentary children were three times more likely to exhibit a clustering of chronic disease risk factors compared with the most active youth (22). Moreover, the weight status of the youth did not impact these odds. Although observational in nature, the findings from this large cohort underscore the importance of PA for preventing obesity-related diseases even in ostensibly healthy youth. In our dataset, we examined whether there was a cross-sectional association between CRF or MVPA and glucose control (HbA1c) as a proxy measure of overall metabolic health. We did not find either CRF (r = −0.20, p = 0.27) or MVPA (r = −0.11, p = 0.49) to be significantly correlated with HbA1c. Perhaps more salient than these cross-sectional findings are smaller scale exercise interventions in overweight youth, which have demonstrated significant improvements in T2D risk factors without changes in body composition (31,32). These studies suggest that PA can improve the metabolic health of overweight youth independent of changes in adiposity. Whether exercise can improve the health status of youth with type 2 diabetes is an important question that remains to be determined.
In addition to obesity and impaired glucose regulation, more than 90% of youth with T2D concurrently exhibit multiple cardiometabolic disease risk factors (33). Given that the leading cause of morbidity and mortality among individuals with T2D is cardiovascular disease (34), it is imperative for these youth to increase their MVPA levels, which may result in higher CRF, as these two constructs have important cardioprotective effects (35). Only 20% (8/40) of the total sample reported performing 60 minutes or more of MVPA per day and none reached the minimum threshold for healthy CRF levels. Our findings coupled with previous research highlighting that nearly 90% of pediatricians do not refer their patients diagnosed with T2D for PA (36) underscores the need to emphasize PA during diabetes education classes for youth.
The cross-sectional nature of our study limits any conclusions related to causality of poor CRF and low PA in the pathogenesis of T2D in our sample. However, the concept of mitochondrial dysfunction and hence impaired oxidative metabolism may be a mechanism underlying the pathogenesis of T2D and a critical precipitating factor, which may explain the low CRF levels in this population (37). This is supported by prospective work in adults, which has established that low CRF at baseline predicts the development of T2D (38). Furthermore, epidemiologic studies, reviewed elsewhere (39), and prospective lifestyle interventions (40) have shown that regular PA can effectively prevent the development of T2D. The protective mechanisms underlying PA may include improvements in peripheral insulin sensitivity (41) as well as improvements in total and regional adiposity (42). Longitudinal studies in children are necessary to examine whether decreases in CRF or PA patterns over time impact future risk of T2D.
We acknowledge several limitations of our study. The relatively small sample size may limit the generalizability of our findings. However, given the lack of available data and the low incidence of this disorder in the general pediatric population we believe that our report addresses a significant challenge, which warrants consideration by both the clinical and research community. Our measure of PA was based upon self-report which is prone to recall error. An objective measure of PA, such as accelerometry would allow for a more accurate assessment of the frequency, intensity, and duration of PA and should be employed in future studies. We assessed CRF using a cycle ergometer whereas NHANES CRF data was measured by a treadmill. VO2peak values are typically lower when assessed by an ergometer compared with treadmill. However, data in obese youth suggest that cycle ergometer and treadmill derived VO2peak values are not significantly different (43). Lastly, we did not have complete data on all of our participants as 5 of the 40 youth did not perform CRF testing.
In conclusion, we have demonstrated that youth with T2D do not meet recommended guidelines for MVPA. Furthermore, the CRF levels of these youth are well below normative and published criterion values. These findings suggest that education programs for youth with T2D should emphasize the importance of a physically active lifestyle to increase CRF levels, support weight management, and improve cardiovascular health.
Acknowledgements
We are grateful to the participants and their families for their involvement, as well as the GCRC staff at UIC. This work was supported by the National Institute of Nursing Research (R01 NR07719) and UIC GCRC (M01-RR-13987). The funding agency had no influence on design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obesity Reviews. 2004;5:4–85. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 2.Haines L, Wan KC, Lynn R, et al. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30:1097–1101. doi: 10.2337/dc06-1813. [DOI] [PubMed] [Google Scholar]
- 3.Malina RM. Physical activity and fitness: Pathways from childhood to adulthood. Am J Hum Biol. 2001;13:162–172. doi: 10.1002/1520-6300(200102/03)13:2<162::AID-AJHB1025>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Steele RM, Brage S, Corder K, et al. Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J Appl Physiol. 2008;105:342–351. doi: 10.1152/japplphysiol.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pate RR, Wang CY, Dowda M, et al. Cardiorespiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999–2002 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2006;160:1005–1012. doi: 10.1001/archpedi.160.10.1005. [DOI] [PubMed] [Google Scholar]
- 6.Cureton KJ, Warren GL. Criterion-referenced standards for youth health-related fitness tests: a tutorial. Res Q Exerc Sport. 1990;61:7–19. doi: 10.1080/02701367.1990.10607473. [DOI] [PubMed] [Google Scholar]
- 7.Imperatore G, Cheng YJ, Williams DE, et al. Physical activity, cardiovascular fitness, and insulin sensitivity among U.S. adolescents: the National Health and Nutrition Examination Survey, 1999–2002. Diabetes Care. 2006;29:1567–1572. doi: 10.2337/dc06-0426. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I, Cramp WC. C ardiorespiratory fitness is strongly related to the metabolic syndrome in adolescents. Diabetes Care. 2007;30:2143–2144. doi: 10.2337/dc07-0734. [DOI] [PubMed] [Google Scholar]
- 9.Duncan GE. Prevalence of Diabetes and Impaired Fasting Glucose Levels Among US Adolescents: National Health and Nutrition Examination Survey, 1999–2002. Arch Pediatr Adolesc Med. 2006;160:523–528. doi: 10.1001/archpedi.160.5.523. [DOI] [PubMed] [Google Scholar]
- 10.Eisenmann JC, DuBose KD, Donnelly JE. F atness, fitness, and insulin sensitivity among 7- to 9-year-old children. Obesity (Silver Spring) 2007;15:2135–2144. doi: 10.1038/oby.2007.254. [DOI] [PubMed] [Google Scholar]
- 11.Ball GD, Shaibi GQ, Cruz ML, et al. Insulin sensitivity, cardiorespiratory fitness, and physical activity in overweight Hispanic youth. Obes Res. 2004;12:77–85. doi: 10.1038/oby.2004.11. [DOI] [PubMed] [Google Scholar]
- 12.LaMonte MJ, Blair SN, Church TS. P hysical activity and diabetes prevention. J Appl Physiol. 2005;99:1205–1213. doi: 10.1152/japplphysiol.00193.2005. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 Growth Charts for the United States: Improvements to the 1977 National Center for Health Statistics Version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Tanner CJ. G rowth at Adolescence. 2nd ed. Oxford: Blackwell Scientific, London; 1962. [Google Scholar]
- 15.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 16.Sallis JF, Buono MJ, Roby JJ, et al. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25:99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Strong WB, Spencer D, Miller MD, et al. The physical working capacity of healthy black children. Am J Dis Child. 1978;132:244–248. doi: 10.1001/archpedi.1978.02120280028006. [DOI] [PubMed] [Google Scholar]
- 18.Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 19.Wei J-N, Sung F-C, Lin C-C, et al. National Surveillance for Type 2 Diabetes Mellitus in Taiwanese Children. JAMA. 2003;290:1345–1350. doi: 10.1001/jama.290.10.1345. [DOI] [PubMed] [Google Scholar]
- 20.Kasa-Vubu JZ, Lee CC, Rosenthal A, et al. Cardiovascular fitness and exercise as determinants of insulin resistance in postpubertal adolescent females. J Clin Endocrinol Metab. 2005;90:849–854. doi: 10.1210/jc.2004-0455. [DOI] [PubMed] [Google Scholar]
- 21.Allen DB, Nemeth BA, Clark RR, et al. Fitness is a stronger predictor of fasting insulin levels than fatness in overweight male middle-school children. J Pediatr. 2007;150:383–387. doi: 10.1016/j.jpeds.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 22.Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 23.Hills AP, King NA, Armstrong TP. T he contribution of physical activity and sedentary behaviours to the growth and development of children and adolescents: implications for overweight and obesity. Sports Med. 2007;37:533–545. doi: 10.2165/00007256-200737060-00006. [DOI] [PubMed] [Google Scholar]
- 24.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 25.Goran M, Fields DA, Hunter GR, et al. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24:841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 26.Shaibi GQ, Faulkner MS, Weigensberg MJ, et al. Cardiorespiratory fitness and physical activity in youth with type 2 diabetes. Pediatr Diabetes. 2008;9:460–463. doi: 10.1111/j.1399-5448.2008.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summerbell CD, Ashton V, Campbell KJ, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews. 2003;(3):CD001872. doi: 10.1002/14651858.CD001872. [DOI] [PubMed] [Google Scholar]
- 28.Cruz ML, Shaibi GQ, Weigensberg MJ, et al. Pediatric Obesity and Insulin Resistance: Chronic Disease Risk and Implications for Treatment and Prevention Beyond Body Weight Modification. Annu Rev of Nutr. 2005;25:435–468. doi: 10.1146/annurev.nutr.25.050304.092625. [DOI] [PubMed] [Google Scholar]
- 29.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 30.Church TS, LaMonte MJ, Barlow CE, et al. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Int Med. 2005;165:2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 31.Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38:1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 32.Bell LM, Watts K, Siafarikas A, et al. Exercise Alone Reduces Insulin Resistance in Obese Children Independently of Changes in Body Composition. J Clin Endocrinol Metab. 2007;92:4230–4235. doi: 10.1210/jc.2007-0779. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2006;29:1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and Cardiovascular Disease: A Statement for Healthcare Professionals From the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 35.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 36.Ditmyer MM, Price JH, Telljohann SK, et al. Pediatricians’ perceptions and practices regarding prevention and treatment of type 2 diabetes mellitus in children and adolescents. Arch Pediatr Adolesc Med. 2003;157:913–918. doi: 10.1001/archpedi.157.9.913. [DOI] [PubMed] [Google Scholar]
- 37.Lowell BB, Shulman GI. Mitochondrial Dysfunction and type 2 Diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 38.Wei M, Gibbons LW, Mitchell TL, et al. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men.[erratum appears in Ann Intern Med 1999;131(5):394] Ann Intern Med. 1999;130:89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- 39.Jeon CY, Lokken RP, Hu FB, et al. Physical Activity of Moderate Intensity and Risk of Type 2 Diabetes: A systematic review. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 40.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houmard JA, Tanner CJ, Slentz CA, et al. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 42.Van Pelt RE, Davy KP, Stevenson ET, et al. Smaller differences in total and regional adiposity with age in women who regularly perform endurance exercise. Am J Physiol Endocrinol Metab. 1998;275:E626–E634. doi: 10.1152/ajpendo.1998.275.4.E626. [DOI] [PubMed] [Google Scholar]
- 43.Loftin M, Sothern MS, Warren B, et al. Comparison of VO2 Peak during treadmill and bicycle ergometry in severely overweight youth. J Sports Sci Med. 2004;3:254–260. [PMC free article] [PubMed] [Google Scholar]