Abstract
Background
Mild cognitive deficits have been reported in essential tremor (ET); however, these cognitive deficits have been assessed in cross-sectional rather than longitudinal analyses.
Objective
To determine whether decline in cognitive test scores occurs at a faster rate in ET cases than controls.
Methods
In a population-based study of older people (≥65 years) in central Spain (Neurological Disorders in Central Spain, NEDICES), non-demented ET cases and controls were followed prospectively. Participants with baseline or incident Parkinson’s disease or dementia were excluded, as were participants who developed incident ET. At baseline (1994–1995) and at follow-up (1997–1998), a 37-item version of the Mini-Mental State Examination (37-MMSE) was administered.
Results
2,319 participants (72.4 ± 5.8 years) included 135 prevalent ET cases and 2,184 controls. At baseline, the mean 37-MMSE in cases was 28.8 ± 5.8 vs. 30.2 ± 4.8 in controls (p = 0.02). During the three year follow-up period, the 37-MMSE declined by 0.70 ± 3.2 points in cases vs. 0.11 ± 3.8 points in controls (p = 0.03). In analyses that adjusted for age, education and other potential confounders, the case-control difference remained robust.
Discussion
In this population-based, prospective study of non-demented elders, baseline cognitive test scores were lower in ET cases than controls; moreover, during the three-year follow-up period, these scores declined at a rate that was seven-times faster in ET cases. This study provides evidence that cognitive deficits in ET are not static and they appear to be progressing at a faster rate than in elders without this disease.
Keywords: Essential tremor, epidemiology, cognitive, dementia
Introduction
Non-motor manifestations are an increasingly recognized feature of essential tremor (ET) [1–4]. For example, mild cognitive deficits have been reported in ET cases in a series of cross-sectional studies [5–8]. The mechanistic basis for these deficits as well their clinical significance have yet to be fully explored. Cognitive deficits in ET have only been studied cross-sectionally, with cases evaluated at one point in time. To our knowledge, there have been no longitudinal studies documenting changes in these test scores over time, comparing ET cases and their counterparts without this disease. We hypothesized that the cognitive deficits in ET would not be static, but rather, cognitive test scores in ET cases would worsen, and that this worsening would occur at a rate above and beyond that observed in similarly-aged controls. To address this question, we utilized data from a population-based study in Spain in which ET cases and controls were prospectively evaluated at two times points separated by three years [9–13].
Methods
Study Population
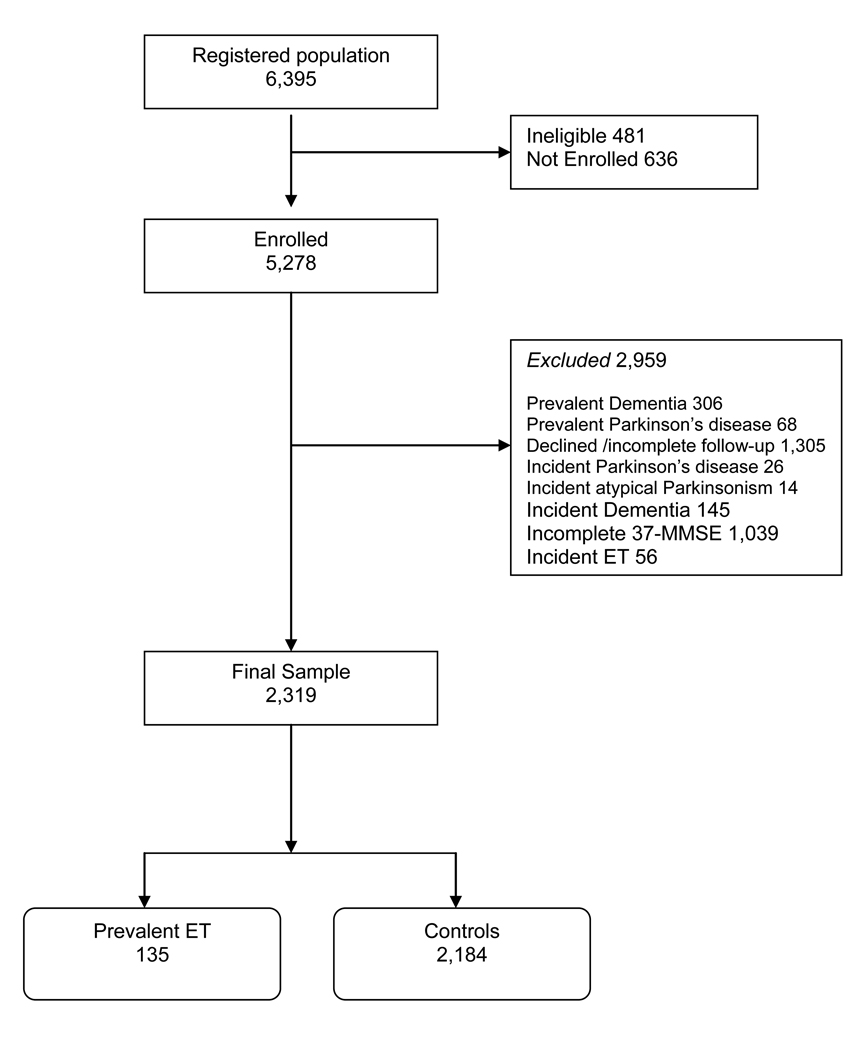
Data for these analyses were derived from the Neurological Disorders in Central Spain (NEDICES) study, a longitudinal, population-based survey of the prevalence, incidence, and determinants of major age-associated conditions of the elderly, including Parkinson’s disease, ET, and dementia [9–13]. Detailed accounts of the study population and sampling methods have been published [9–13]. The study population was composed of elderly subjects, ≥65 years old, living in three communities in central Spain (Las Margaritas, Lista and Arévalo). The registered study population was 6,395, but 481 individuals were ineligible (census issues, address errors or death), leaving 5,914 eligible subjects, of whom 5,278 were enrolled (Figure). Institutional ethics approval was obtained and written informed consent was obtained from participants at the time of enrollment.
Figure 1.
Flowchart of Study Participants.
Study Evaluation
Detailed accounts of the study assessments have been published [9–13]. Face-to-face evaluations were performed at baseline (1994 – 1995) and then at follow-up (1997–1998). A questionnaire was mailed to participants who were unavailable for these face-to-face interviews.
The face-to-face interview included data collection on demographics, current medications, medical conditions (e.g., diabetes mellitus, hypertension, heart disease), lifestyle habits, and the presence of depressive symptoms (the question, “do you suffer from depression? ”). As described [5, 9], a neurological examination was performed, comprised of a general neurological examination, a tremor examination, and the motor portion of the Unified Parkinson's Disease Rating Scale (UPDRS) [14]. In addition, a 37-item Mini-Mental State Examination [37-MMSE] was administered [15]. This was a Spanish adaptation of the standard MMSE [16]. More specifically, the 37-MMSE was adopted to Chilean, Maltese and Spanish socio-cultural backgrounds and then validated against clinical diagnoses of dementia [15]. The 37-MMSE included all of the standard MMSE[16] items as well as three additional items: (1) an attention task, i.e., “say 1, 3, 5, 7, 9 backwards”, (2) a visual order, i.e., a man raising his arms, and (3) a simple construction task, i.e., copying two overlapping circles [13]. Ten percent of our sample was illiterate, although only a small proportion was completely illiterate and many could read or write a simple phrase. If the participant was completely illiterate, then the one 37-MMSE reading item and the one 37-MMSE writing item were assigned the value 0.
Diagnostic criteria for Parkinson’s disease [10], ET [12] and dementia [13] have been described elsewhere.
Final Selection of Participants
Of the 5,278 participants, we excluded 306 with prevalent dementia (Figure). This was because our aim was to assess changes in cognitive scores among non-demented persons. We further excluded 68 participants with prevalent Parkinson’s disease, a disorder known to be associated with cognitive changes and dementia [17]. A further 1,305 participants who were evaluated at baseline were excluded because they declined a follow-up assessment or had incomplete follow-up assessments, had died or were unreachable. We also excluded 26 participants with incident Parkinson’s disease and 14 with incident atypical Parkinsonism, as these may be associated with cognitive changes or dementia [17]. The 145 participants who developed incident dementia were excluded because our goal was to assess non-demented persons. Furthermore, the conversion rate to incident dementia has been reported previously in NEDICES and is higher in ET cases than controls [13]. The 1,039 participants without complete 37-MMSE at both assessments were excluded. These were primarily the participants who were unavailable for face-to-face interviews (i.e., evaluated by mailed questionnaire). The 56 controls who developed incident ET were also excluded, as ET is associated with cognitive impairment [5, 9]. The final sample, 2,319 participants, included 135 with prevalent ET and 2,184 controls (Figure). The final sample of 2,319 was similar to the base sample of 5,278 participants in terms of gender (1,331 [57.4%] vs. 3,040 [57.6%] women, chi-square = 0.03, p = 0.87), education (338 [14.6%] vs. 708 [13.4%] with secondary studies, chi-square = 1.83, p = 0.18) and, on average, 1.9 years younger (72.4 ± 5.8 vs. 74.3 ± 7.0 years, t = 1.46, p < 0.001).
Statistical Analyses
Analyses were performed in SPSS (version 17.0). Baseline characteristics of ET cases and controls were compared using Student’s t tests and chi square tests. The change in 37-MMSE score = baseline score – follow-up score. The 37-MMSE scores (baseline, follow-up, and change in MMSE) were not normally distributed, even after log-transformation. Therefore, scores were compared using a non-parametric approach (Mann-Whitney test). Linear regression analyses were not possible because the change in 37-MMSE was not normally distributed. Therefore, to initially assess the effects of possible confounders (age, education, depressive symptoms), stratified analyses were performed. Due to the loss of power in these stratified analyses, p values were not reported; rather, the aim of these analyses was to determine whether the magnitude of the case-control difference persisted after stratification. In additional analyses, we divided change in 37-MMSE into tertiles (lower tertile ≥ 2 point improvement in score, upper tertile ≥ 1 point decline in score), comparing cases and controls with respect to their distribution within these tertiles (chi-square test). In addition, logistic regression analyses were performed, thereby allowing us to assess, for a second time, the possible confounding effects of age, education and depressive symptoms. In these models, the dependent variable was the upper tertile of 37-MMSE change (reference = lower tertile) and the independent variable was case-control status.
Results
The 2,319 participants (mean ± standard deviation age = 72.4 ± 5.8 years), included 135 prevalent ET cases and 2,184 controls. The mean follow-up was 3.4 ± 0.5 years in both cases and controls. Cases were, on average, 1.2 years older than controls (Table 1). A higher proportion of cases was illiterate and had depressive symptoms, but in other respects, cases and controls were similar (Table 1). At baseline, the mean 37-MMSE in cases was 28.8 ± 5.8 vs. 30.2 ± 4.8 in controls (Mann Whitney, p = 0.02). During the three year follow-up period, the 37-MMSE declined by 0.70 ± 3.21 points in ET cases (median = 1 point) vs. 0.11 ± 3.81 points in controls (median = 0 points) (Mann-Whitney, p = 0.03) (Table 2).
Table 1.
Demographic and Clinical Characteristics of ET Cases and Controls
| ET Cases (N = 135) | Controls (N = 2,184) | Significance | |
|---|---|---|---|
| Age (years) | 73.6 ± 6.3 | 72.4 ± 5.8 | 0.03a |
| Gender (female) | 79 (58.5) | 1,252 (57.3) | 0.79b |
| Educational level | 0.005b | ||
| Illiterate | 24 (17.8) | 210 (9.6) | |
| Can read and write | 61 (45.2) | 911 (41.7) | |
| Primary Studies | 37 (27.4) | 738 (33.8) | |
| Secondary Studies | 13 (9.6) | 325 (14.9) | |
| Current Smoker | 16 (11.9) | 256 (11.7) | 0.94b |
| Current Ethanol Consumption |
47 (34.8) | 794 (36.4) | 0.82b |
| Diabetes mellitus* | 25 (18.7) | 349 (16.1) | 0.43b |
| Hypertension* | 71 (52.6) | 1,082 (49.6) | 0.50 |
| Heart disease* | 12 (8.9) | 204 (9.4) | 0.86b |
| Depressive symptoms* | 55 (40.7) | 491 (22.6) | <0.001b |
Student’s t test
Chi-square test
Data on some participants were missing.
Mean ± standard deviation and frequency (%) are reported.
Table 2.
Decline in 37-MMSE Score During Follow-up
| Decline in 37-MMSE Score During Follow-up 135 ET Cases |
Decline in 37-MMSE Score During Follow-up 2,184 Controls |
|
|---|---|---|
| All Participants | 0.70 ± 3.21 | 0.11 ± 3.81 |
| Age Strata | ||
| Tertile 1 (≤ 68 years) | 0.74 ± 3.10 (66.5 ± 0.9) | 0.01 ± 3.90 (66.6 ± 1.1) |
| Tertile 2 (69 – 75 years) | 0.50 ± 2.80 (71.6 ± 1.9) | 0.10 ± 3.53 (71.6 ± 2.0) |
| Tertile 3 (≥ 76 years) | 0.88 ± 3.72 (80.9 ± 3.4) | 0.25 ± 4.14 (80.5 ± 3.8) |
| Educational Level Strata | ||
| Illiterate | −0.58 ± 3.12 | −0.34 ± 4.37 |
| Can read and write | 1.13 ± 3.48 | 0.04 ± 3.80 |
| Primary Studies | 0.78 ± 2.89 | 0.17 ± 3.48 |
| Secondary Studies | 0.77 ± 2.52 | 0.46 ± 4.13 |
| Depressive Symptoms Strata | ||
| Yes | 0.73 ± 3.40 | −0.02 ± 3.73 |
| No | 0.67 ± 3.25 | 0.15 ± 3.83 |
| Community | ||
| Las Margaritas | 0.85 ± 3.28 | 0.01 ± 3.80 |
| Lista | 0.30 ± 2.98 | −0.06 ± 3.71 |
| Arévalo | 0.77 ± 3.30 | 0.33 ± 3.89 |
Mean ± standard deviation are reported. Negative values indicate that the baseline 37-MMSE score was lower than the 37-MMSE score at follow-up (i.e., improvement in score). All positive values indicate a decline in score (i.e., baseline 37-MMSE > follow-up 37-MMSE).
In each age stratum, the numbers in parentheses indicate the mean ± SD age of participants in that stratum; these values demonstrate that cases and controls had similar ages within the three age strata.
In analyses that stratified by age, education and depressive symptoms, each of which could have confounded the observed association between case status and cognitive decline, the case-control difference remained robust. In nearly all strata, the decline in 37-MMSE score in ET cases was several fold higher than the decline in scores in controls (e.g., in the youngest age tertile, the 37-MMSE declined by 0.74 ± 3.10 points in ET cases vs. 0.01 ± 3.90 points in controls) (Table 2). In analyses that stratified by community (Las Margaritas, Lista, Arévalo), the results were similar (i.e., in each community, the decline in 37-MMSE score in ET cases was several fold higher than the decline in scores in controls), indicating that the case-control difference occurred across communities.
In 23 (1.0%) participants, the 37-MMSE changed by greater than 10 points; when these outliers were excluded, the results were similar (the 37-MMSE declined by 0.78 ± 3.05 points in ET cases vs. 0.06 ± 3.43 points in controls, Mann-Whitney, p = 0.018).
Change in 37-MMSE was stratified into tertiles; 73 (54.1%) cases vs. 949 (43.5%) controls were in the upper tertile and 30 (22.2%) cases vs. 685 (31.4%) controls were in the lower tertile (chi-square for tertile comparison = 6.81, p = 0.03). In a logistic regression model, cases were 1.76 times more likely than controls to have a change in 37-MMSE in the upper vs. lower tertile (OR = 1.76, 95% CI = 1.14 – 2.72, p = 0.01). To further assess the possible confounding effects of age, education and depressive symptoms, we adjusted for these in a logistic regression model, and cases were 1.86 times more likely than controls to have a change in 37-MMSE in the upper vs. lower tertile (OR = 1.86, 95% CI = 1.19 – 2.89, p = 0.006).
At baseline, 347 participants (40 [29.6%] ET cases vs. 307 [14.1%] controls, p < 0.001) were taking medication with central nervous system effects (anticonvulsants like primidone, anxiolytics, antihistamines, etc). At follow-up, 489 participants (53 [39.3%] ET cases vs. 436 [20.0%] controls, p < 0.001) were taking these medications. In a regression model that adjusted for age, education, depressive symptoms and use of these medications (taking medication vs. not taking medication) during baseline or follow-up, cases were 1.78 times more likely than controls to have a change in 37-MMSE in the upper vs. lower tertile (OR = 1.78, 95% CI = 1.14 – 2.78, p = 0.01).
The 37-MMSE was divided into sub-scores, and changes in 37-MMSE subscores were compared in cases and controls (Table 3). The greatest differences were in the areas of attention and calculation, language, and construction/copying, where decline was several-fold higher in cases than controls (Table 3). Given the small point ranges of the subscores, none of the subscore differences reached statistical significance, although there were several trends (for attention and calculation, p = 0.09; for language, p = 0.20; and for construction/copying, p = 0.14). When these three sub-scores were combined, the case-control difference reached significance (0.53 ± 2.81 vs. −0.13 ± 3.12 point change, Mann-Whitney, p = 0.03).
Table 3.
Decline in 37-MMSE Sub-Scores During Follow-up
| Decline in 37-MMSE Sub-Scores During Follow-up 135 ET Cases |
Decline in 37-MMSE Sub-Scores During Follow-up 2,184 Controls |
|
|---|---|---|
| Orientation (10 points) | 0.17 ± 1.48 | 0.21 ± 1.21 |
| Immediate Recall (3 points) | −0.02 ± 0.26 | −0.01 ± 0.36 |
| Attention and Calculation (10 points) |
0.23 ± 2.28 | −0.17 ± 2.57 |
| Recall (3 points) | 0.03 ± 1.30 | 0.05 ± 1.21 |
| Language (9 points) | 0.12 ± 1.21 | −0.01 ± 1.24 |
| Copying (2 points) | 0.17 ± 0.82 | 0.04 ± 0.73 |
Mean ± standard deviation are reported. Negative values indicate that the baseline score was lower than the score at follow-up (i.e., improvement in score). All positive values indicate a decline in score (i.e., baseline score > follow-up score).
Age of onset of ET ranged from 15 – 85 years (mean = 63.5 ± 13.9 years). The change in 37-MMSE did not differ based on age of onset. The change in 37-MMSE was 0.75 ± 3.28 among the 52 (38.5%) cases with age of ET onset ≥65 years and 0.62 ± 3.13 among the 83 (61.5%) cases with age of ET onset <65 years (Mann-Whitney, p = 0.85). Similarly, the change in 37-MMSE did not differ with regards to family history of ET. The change in 37-MMSE was 0.20 ± 3.51 among the 54 (40.0%) cases with a family history of ET (≥1 reportedly affected first- or second-degree relative) and 1.03 ± 2.97 among the 81 (60.0%) cases without a family history (Mann-Whitney, p = 0.28). The change in 37-MMSE was 1.78 ± 3.51 among the 19 ET cases with both arm and cranial tremor (neck or voice) vs. 0.52 ± 3.14 among the 116 ET cases with only arm tremor, a difference that likely did not reach significance due to the modest sample size (Mann-Whitney, p = 0.22).
ET cases had lower 37-MMSE scores than controls at baseline, suggesting that some of them may already have had mild cognitive impairment. In an additional analysis, we excluded all ET cases with baseline 37-MMSE scores that were below the baseline 37-MMSE score of controls (i.e., scores < 31). In these analyses, the 37-MMSE declined by 1.32 ± 2.85 points in the 65 remaining ET cases (median baseline 37-MMSE = 33) vs. 0.11 ± 3.81 points in controls (median baseline 37-MMSE = 31)(Mann-Whitney, p = 0.003). In another analyses, we included rather than excluded all participants with prevalent and incident dementia. In these analyses, the 37-MMSE declined by 0.38 ± 3.99 points, including 0.95 ± 3.48 points in the 147 ET cases vs. 0.34 ± 4.02 points in 2,299 controls (Mann-Whitney, p = 0.03).
Discussion
In this prospective study of non-demented, community-dwelling elders, baseline cognitive test scores were lower in ET cases than controls; moreover, during the three year follow-up period, these scores declined at a rate which was seven-times faster in ET cases than controls. Furthermore, this case-control difference was independently observed in each of the three communities under study (Las Margaritas, Lista, and Arévalo).
This study provides evidence that the cognitive deficits in ET are not static and they seem to be progressing at a faster rate than in elders without this disease. Indeed, two recent population-based studies in Spain [13] and New York [18] reported that baseline ET was a risk factor for incident dementia. Most of those cases with new-onset dementia were clinically diagnosed with incident Alzheimer’s disease [13, 18]. One postmortem study reported an increase in Alzheimer’s-type pathologies in ET compared to control brains [19]. The progressive cognitive changes in ET as well as the pathophysiological mechanisms which underlie these changes both require further study.
ET cases on average experienced a 0.7 point reduction in the 37-MMSE over 3 years. Although this reduction was seven-times greater than that seen in controls, in absolute terms, it was a modest change. We excluded participants who developed incident dementia. These excluded participants experienced, on average, a 5.11 point reduction in the 37-MMSE over 3 years, a change that is far larger than observed in our non-demented cases.
Although all of our ET cases were age 65 and older, the age of tremor onset was as low as 15 years in some cases and, in approximately one-third of our cases, onset preceded age 65. The accelerated decline in cognitive test scores, however, occurred in ET cases with younger as well as older onset ET, indicating that age of onset was not an important predictor or modifier of the rate of cognitive decline in ET.
The 37-MMSE uses two simple copying tasks. Participants are scored on cognitive issues (e.g., their ability to reproduce the proper spatial relationships between lines) rather than the presence or absence of tremor. Nonetheless, it is possible that tremor could have impeded the ability to properly reproduce these cognitively-based features of the drawings. It is important to note, however, that the case-control difference was not solely in the copying subscore of the 37-MMSE, but was observed in several other domains (attention and calculation and language), indicating that tremor alone could not have explained our results.
The rate of cognitive decline seen observed in the current sample, inclusive of prevalent and incident dementia cases, was 0.38 ± 3.99 points. The annual rate of change was 0.13/ 37 points (0.34%). This annual rate of change is similar to that reported in other cohorts of population-dwelling elders (e.g., 0.07/30 points = 0.24%) [20].
This study had limitations. First, the 37-MMSE is a relatively abbreviated screening tool for dementia. The use of more detailed neuropsychological test batteries would enable future investigators to study these changes in greater detail. Nevertheless, even with this relatively simple, abbreviated tool, we were able to establish clear case-control differences. Second, the 37-MMSE was administered at two time points; use of additional time points would allow one to assess the extent to which the case-control difference continued beyond the three-year time window. This study also had several strengths. First, cases were compared to a large sample size of several thousand controls. Second, the assessments were conducted prospectively in a standardized manner. Third, we were able to adjust for the potential confounding effects of a number of important factors. Finally, the study was population based, allowing us to assess a group of patients with relatively mild ET unselected for medical treatment or surgery.
In summary, using a prospective, population-based design, we demonstrated that cognitive test scores in ET cases declined at an accelerated rate compared to controls. This study provides further evidence that cognitive deficits in ET are not static. Further studies are needed of the mechanistic basis which underlies both the cognitive deficits and frank dementia that seem to accompany this disorder.
Acknowledgements
We gratefully acknowledge the vital help of the other members of the NEDICES Study Group, who are listed in the Appendix. We also wish to express our sincere thanks to J. de Pedro-Cuesta, M.J. Medrano, and J. Almazán, the municipal authorities, family doctors, nurses, and the populations of Getafe, Lista, and Arévalo County. The NEDICES was supported by the Spanish Health Research Agency (FIS 93/0773 and 96/1993) and the Spanish Office of Science and Technology. Dr. Louis was supported by R01 NS042859 and R01 NS039422 from the National Institutes of Health, Bethesda, Maryland.
Appendix
The members of the Neurological Disorders in Central Spain (NEDICES) Study Group are as follows: J.M. Morales, R. Gabriel, A. Portera-Sánchez, A. Berbel, A. Martínez-Salio, J. Díaz-Guzmán, J. Olazarán, J. Pardo, J. Porta-Etessam, F. Pérez del Molino, J. Rivera-Navarro, M. Alonso, C. Gómez, C. Saiz, G. Fernández, P. Rodríguez, and F. Sánchez-Sánchez.
Footnotes
Statistical Analyses: The statistical analyses were conducted by Dr. Louis.
References
- 1.Tan EK, Fook-Chong S, Lum SY, et al. Non-motor manifestations in essential tremor: use of a validated instrument to evaluate a wide spectrum of symptoms. Parkinsonism Relat Disord. 2005;11:375–380. doi: 10.1016/j.parkreldis.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Troster AI, Woods SP, Fields JA, et al. Neuropsychological deficits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? Eur J Neurol. 2002;9:143–151. doi: 10.1046/j.1468-1331.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 3.Findley LJ. Expanding clinical dimensions of essential tremor. J Neurol Neurosurg Psychiatry. 2004;75:948–949. doi: 10.1136/jnnp.2004.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galvin JE. When a tremor is not just a tremor: cognitive and functional decline in essential tremor, a more complex disorder than we thought. J Am Med Dir Assoc. 2009;10:218–220. doi: 10.1016/j.jamda.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 6.Gasparini M, Bonifati V, Fabrizio E, et al. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. 2001;248:399–402. doi: 10.1007/s004150170181. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- 8.Sahin HA, Terzi M, Ucak S, Yapici O, Basoglu T, Onar M. Frontal functions in young patients with essential tremor: a case comparison study. J Neuropsychiatry Clin Neurosci. 2006;18:64–72. doi: 10.1176/jnp.18.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Benito-Leon J, Louis ED, Bermejo-Pareja F. Elderly-onset essential tremor is associated with dementia. Neurology. 2006;66:1500–1505. doi: 10.1212/01.wnl.0000216134.88617.de. [DOI] [PubMed] [Google Scholar]
- 10.Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62:734–741. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- 11.Morales JM, Bermejo FP, Benito-Leon J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public Health. 2004;118:426–433. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 13.Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: A population-based study. Mov Disord. 2007;22:1573–1580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 14.Fahn SER. Members of the UPDRS Development Committee. In: Fahn S, Marsden C, Goldstein M, Calne DB, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 15.Baldereschi MMF, Quiroga P, et al. Cognitive versus functional screening for dementia across different countries: cross-cultural validation of the Mini-Mental State Examination (MMSE) and the Pfeffer activities questionnaire (PFAQ) against the standardised clinical diagnosis of dementia. Neurology. 1994;44:A365. [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Stern Y, Marder K, Tang MX, Mayeux R. Antecedent clinical features associated with dementia in Parkinson's disease. Neurology. 1993;43:1690–1692. doi: 10.1212/wnl.43.9.1690. [DOI] [PubMed] [Google Scholar]
- 18.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73:621–625. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 20.Eslinger PJ, Swan GE, Carmelli D. Changes in Mini-Mental State Exam in community-dwelling older persons over 6 years: relationship to health and neuropsychological measures. Neuroepidemiology. 2003;22:23–30. doi: 10.1159/000067113. [DOI] [PubMed] [Google Scholar]