Abstract
CART (Cocaine- and amphetamine-regulated transcript) peptide has been implicated in playing a modulatory role in reward and reinforcement. Previously, our laboratory demonstrated that injections of CART peptide (CART 55–102) into the nucleus accumbens (NAc) attenuated both cocaine- and dopamine-induced increases in locomotor activity (LMA), and attenuated cocaine reward as well. In this study, the effects of CART peptide on LMA induced by dopamine receptor agonists were evaluated after intra-accumbal injections in male, Sprague-Dawley rats. Effects of the D1 receptor agonist, SKF-81297, saline, CART 55–102, or CART 55–102 and SKF-81297 together, were compared. The SKF-81297-induced increase in LMA was potentiated by co-administration of CART, while injection of CART alone had no significant effect. Injection of the D2 agonist, 7-OH-DPAT, had no effect on LMA, and the combination of both 7-OH-DPAT and CART peptide also had no effect. Quinelorane, a D3 receptor agonist, did not alter LMA, nor did the combination of CART peptide and quinelorane. The next experiment examined the effects of CART peptide on LMA induced by co-injection of both the D1 agonist, SKF-81297, and the D2 agonist, 7-OH-DPAT. The combination of SKF-81297 and 7-OH-DPAT induced greater LMA than SKF-81297 alone. Co-administration of CART peptide along with the D1 and D2 agonists reduced LMA. These results strongly suggest that CART peptide reduces the effects of psychostimulants by modulating the simultaneous activation of both D1 and D2 dopamine receptors, rather than by affecting the action of any individual dopamine receptor.
Keywords: CART peptide, Nucleus accumbens, dopamine, dopamine receptors, D1-D2 synergy
INTRODUCTION
CART (Cocaine- and amphetamine-regulated transcript) peptide is a neurotransmitter believed to play a homeostatic role in psychostimulant reward and reinforcement as well as in other processes (Jaworski and Jones, 2006; Rogge et al., 2008). CART mRNA and CART peptide are found abundantly in the mesolimbic pathway, including the ventral tegmental area (VTA) and the nucleus accumbens (NAc) (Douglass et al., 1995; Koylu et al 1998). CART peptide (CART 55–102) has been demonstrated to have minor psychostimulant-like properties when injected into the VTA, inducing locomotor activity (LMA) and producing a slight conditioned place preference (Kimmel et al., 2000; Kimmel et al., 2002). However, Jaworski et al. (2007), using isobolographic analysis, demonstrated that the LMA-inducing effects of combined administration of intra-VTA CART peptide and systemic cocaine are sub-additive; intra-VTA administration of CART peptide actually attenuated cocaine-induced LMA. Interestingly, injection of CART peptide into the NAc alone had no effect on LMA; however intra-accumbal CART administration attenuated systemic cocaine- or amphetamine-induced LMA (Jaworski et al., 2003; Kim et al., 2003). Psychostimulants increase LMA by increasing the synaptic concentration of dopamine primarily by acting at the dopamine transporter. Intra-accumbal injection of dopamine produced a dose-dependent increase in LMA which was also attenuated by administration of CART peptide into the NAc (Jaworski et al., 2003). It was recently found that intra-accumbal CART peptide attenuated cocaine reward in a self-administration paradigm (Jaworski et al., 2008). These studies support the hypothesis that CART peptide modulates the behavioral effects of psychostimulants acting through the dopaminergic system.
Numerous studies have been conducted on the effects of dopamine agonists and antagonists on behaviors associated with the effects of psychostimulants. D1 receptor agonists, including SKF-81297, stimulate LMA when injected in the NAc (Meyer, 1993; Meyer et al., 1993; Choi et al., 2000; David et al., 2004; Bachtell et al., 2005) whereas intra-accumbal injections of D2 receptor agonists have little to no effect (Meyer, 1996; Bachtell et al., 2005) or suppress (Choi et al., 2000; David et al., 2004) LMA. There is also some evidence supporting a dose-dependent biphasic effect on LMA (initial hypolocomotion followed by a period of hyperlocomotion) by D2 agonists administered both peripherally (Ireland et al., 2005) and infused directly into the NAc (Meyer, 1996). Because CART peptide can reduce the effects of intra-accumbal dopamine and systemic cocaine and amphetamine, this study was undertaken to identify the specific dopamine receptors affected by CART peptide. Therefore, the effects of intra-accumbal CART peptide on LMA following intra-accumbal administration of D1, D2 or D3 agonists alone or in combination, have been investigated. These receptors are known to interact with psychostimulants or CART peptide (Hunter et al, 2006)
METHODS
Animals
Male Sprague-Dawley rats (Charles River Laboratories Inc., Wilmington, MA) weighing approximately 300 g at the start of the experiment were singly housed in static cages in a temperature- and humidity-controlled animal care facility and maintained on a 12-h light/dark schedule (lights on at 7:00 AM). Food and water were available ad libitum. Under anesthesia induced by a combination of ketamine (70 mg/kg i.p.; Fort Dodge Animal Health, Fort Dodge, IA) and medetomidine (0.5 mg/kg, i.p.; Pfizer Animal Health, Exton, PA) the rats were implanted with a 22-gauge stainless steel bilateral cannula guide (Plastics One, Roanoke, VA) placed above the NAc (AP +1.7, ML +1.7, DV −5.7; relative to bregma; Paxinos and Watson, 1986). After the surgery, the rats were given atipamezole (0.2 mg/kg, s.c.) to reverse the sedative effects of medetomidine and meloxicam (1mg/kg, s.c.). The rats were given 5–7 days to recover from surgery prior to the start of experiments. All procedures were approved by the Emory Animal Care and Use Committee and in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication 85–23).
Intra-Accumbal Injections and Measurement of Locomotor Activity (LMA)
LMA was measured in a 40 X 40 X 20 cm Plexiglass cage (Omnitech Electronics, Columbus Ohio) equipped with 16 photobeams front to back and 16 photobeams side to side spaced 2.4 cm apart. The activity cages were equipped with an exhaust fan and a 10-W light. The experimental data were collected and analyzed on a PC-compatible computer using Digipro software (Omnitech Electronics). The data was collected in bins of 15 minutes during habituation and testing.
Approximately five days after surgery the rats were placed in the activity cages then given a sham injection to habituate the rats to both the experimental chamber and handling. Prior to injections the rats were placed in the experimental chambers for a 30 minute habituation period. The drugs were injected in combination in a volume of 1.0 or 1.5 μl/side (0.6 μl/minute) using a 23-gauge injector cannula extending 1mm beyond the guide cannula. Following injections the rats were returned to the activity cages for the 180 min.
Drugs
SKF-81297 (R-(+)-6-Chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide); 7-OH-DPAT [(±)-2-Dipropylamino-7-hydroxy-1,2,3,4-tetrahydronaphthalene hydrobromide] and quinelorane were obtained from Sigma-Aldrich, Inc. (St. Louis, MO) and dissolved in distilled water. The drugs were made fresh daily from stock solutions and diluted using bacteriostatic 0.9% NaCl saline. CART 55–102, often referred to as “CART peptide” in the text, was purchased from Bachem (King of Prussia, PA).
Histology
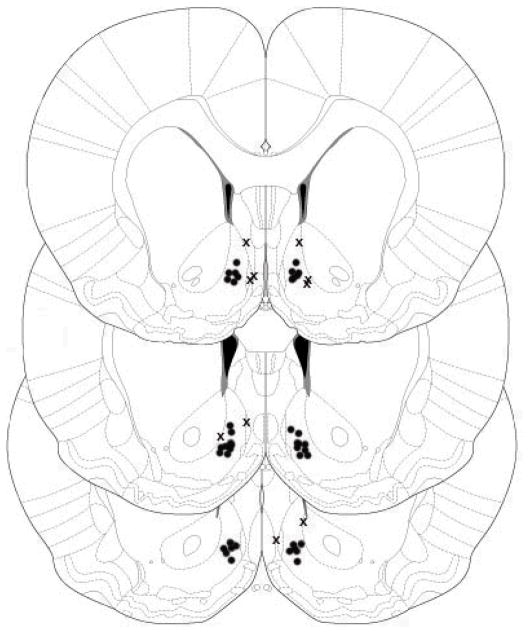
The rats were decapitated, the brains removed and rapidly frozen for sectioning. Coronal slices (20 μm thick) were examined under light microscopy to confirm cannula placement (Figure 1). Most injections were in the area of the shell, but injections of dye revealed that the injectate spread to the core as well. Five animals were removed from the study due to misplaced cannulae.
Figure 1.
Cannula placement in the nucleus accumbens (NAc) of rats included (●) and excluded (X) in the data analysis.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism v 4.0 for Windows (GraphPad Software, Inc., San Diego, CA). Comparisons were made using a one-way ANOVA. Post hoc comparisons were made using Newman-Keuls Multiple Comparison test. Statistical significance is stated as p < 0.05.
RESULTS
Effects of CART peptide on D1 receptor agonist-induced LMA
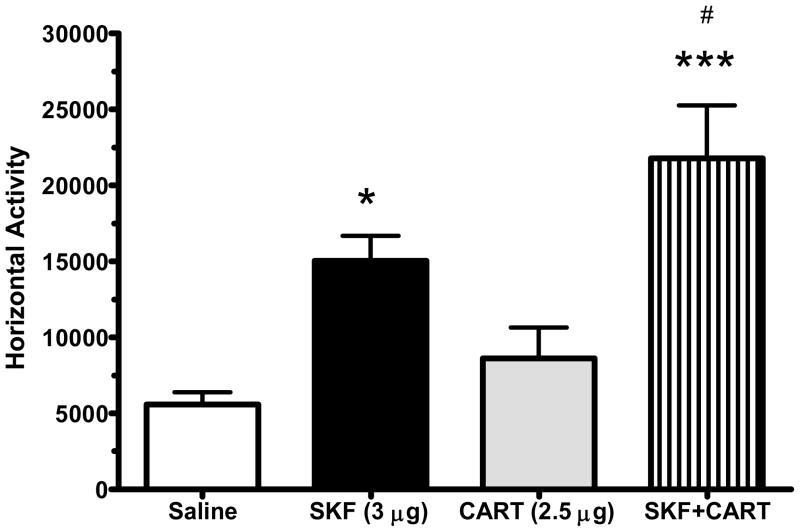
There was a main effect of treatment on horizontal locomotor activity following intra-accumbal infusions of SKF-81927, saline or CART [F(3,31) = 10.69; p = 0.0002]. Intra-accumbal infusions of SKF-81297 (3 μg/side, n=8) increased horizontal activity and distance traveled compared to saline treated animals (n=8) (p < 0.05) Figure 2). As shown previously (Jaworski et al., 2003), infusions of CART peptide (2.5 μg/side, n=8) directly in to the NAc had no effect on LMA. The co-administration of CART peptide (2.5 μg/side) and SKF-81297 did not attenuate the activity induced by SKF (n=8) alone, and in fact increased LMA compared to SKF alone (p < 0.05). This is somewhat surprising since CART peptide reduces the LMA effects of cocaine, amphetamine, or dopamine. This dose of CART peptide has been used in many previous studies (Jaworski et al., 2003, 2007, 2008). The dose of SKF-81297 was determined in preliminary studies and is similar to doses used in the literature (Bachtell et al., 2005).
Figure 2.
The D1 receptor agonist, SKF-81297 (3 μg/side) significantly increased horizontal LMA compared to saline. CART (2.5 μg/side) had no effect on horizontal activity alone but potentiated the effects of SKF-81297 (3ug/side).
* p < 0.05 versus saline, *** p < 0.001 versus saline
# p < 0.05 versus SKF-81297 alone.
Effects of CART peptide on the action of D2 or D3 receptor agonists
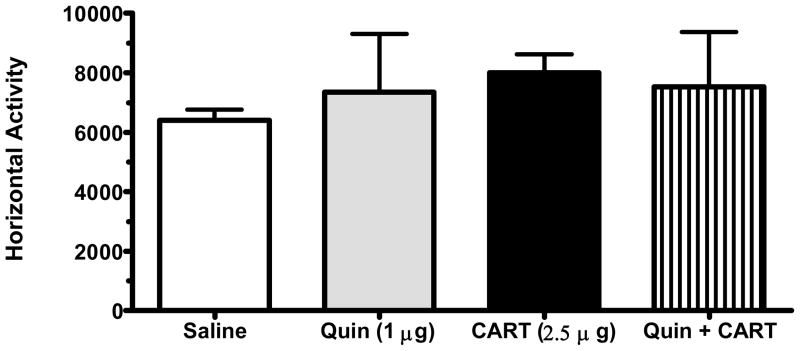
LMA, following administration of 7-OH-DPAT (5 μg/side) alone (n=8) or in combination (n=8) with CART peptide (2.5 μg/side), did not differ from LMA following saline administration (n=8) (Figure 3). The dose of 7-OH-DPAT is similar to that used in other studies involving the NAc (Bachtell et al., 2005). Similarly, the D3 agonist, quinelorane, failed to affect LMA when used alone (n=4) or in combination (n=6) with CART peptide (2.5 μg/side) (Figure 4). Preliminary data using a range of quinelorane doses (1 – 10 μg/side), as well as the combined administration of quinelorane (1 μg/side) and SKF-81297 (1.5 μg/side), showed no effect on LMA compared to saline (data not shown).
Figure 3.

7-OH-DPAT (5 μg/side) alone or in combination with CART had no effect on horizontal activity compared to that for saline.
Figure 4.
Quinelorane (1 μg/side), a D3 receptor agonist, did not increase LMA alone or in combination with CART (2.5 μg/side).
Effects of CART peptide on LMA induced by the combined administration of D1 and D2 receptor agonists
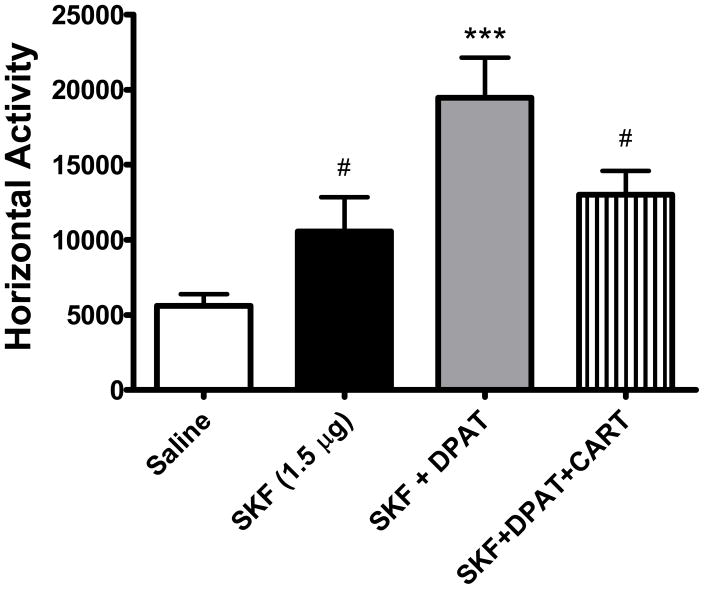
The next experiment (Figure 5) examined the effect of CART peptide on LMA induced by the combination of the D1 receptor agonist, SKF-81297 and the D2 receptor agonist, 7-OH-DPAT. These experiments were undertaken because of the literature indicating that simultaneous activation of both D1 and D2 receptors resulted in synergistic motor effects, producing greater locomotion than by activation of D1 receptors alone (David et al., 2004). Infusion of the SKF drug at 1.5 ug/side (n=8) produced an increase in LMA as expected (Figure 5). The combined administration of SKF-81297 (1.5 μg/side) and 7-OH-DPAT (5 μg/side) (n=14) produced a robust increase in LMA [F (3, 45) = 6.844, p = 0.0007] that was significantly greater than saline (n=8) (p < 0.001) or SKF-81297 alone (n=8) (p < 0.05) as expected (Figure 5). Addition of CART peptide attenuated the LMA induced by the combined co-administration of the D1 and D2 agonists (n=15) (p < 0.05).
Figure 5.
When administered into the NAc in combination, SKF-81297 (1.5 μg/side) and 7-OH-DPAT (5 μg/side) induced greater LMA than SKF-81297 alone. Co-administration of CART peptide (2.5 ug/side) with both dopamine agonists attenuated the heightened LMA induced by simultaneously stimulating D1 and D2 receptors.
*** p < 0.001 versus saline
# p < 0.05 versus SKF + DPAT
DISCUSSION
The relationship between CART peptide and psychostimulants has been the subject of many studies since the initial characterization of CART mRNA as an mRNA elevated following acute amphetamine and cocaine administration (Douglass et al., 1995). While some laboratories could not reproduce these results (Vrang et al., 2002; Marie-Claire et al., 2003; Hunter et al., 2005), Hubert and Kuhar (2008) recently showed that the percentage of Fos positive CART cells in the NAc increased after cocaine, supporting the idea the psychostimulant drugs activate CART neurons in the NAc.
Several studies of the physiological effects of CART peptide indicated that intra-accumbal injection of CART peptide blunted the locomotor (LMA) effects of psychostimulants (Jaworski et al., 2003; Kim et al., 2003; Kim et al., 2007; Yoon et al., 2007). Although intra-accumbal administration of CART peptide alone had no effect on LMA, CART attenuated the LMA induced by systemic cocaine (Jaworski et al., 2003) or amphetamine (Kim et al., 2003). CART also blocked the expression of cocaine- (Yoon et al., 2007) and amphetamine- induced behavioral sensitization (Kim et al., 2007). LMA induced by psychostimulants is a result of increases in synaptic dopamine which in turn acts on postsynaptic receptors. CART has also been demonstrated to attenuate LMA induced by direct intra-accumbal injections of dopamine (Jaworski et al., 2003). More recently, CART peptide has been shown to reduce the apparent reward value of cocaine in a self-administration paradigm (Jaworski et al., 2008). These findings has lead to the hypothesis that a role of CART peptide in the NAc is to homeostatically regulate the activity of the dopamine system (Rogge et al 2008).
This study was carried out to identify which dopamine receptors were associated with the blunting effect of the peptide. It was found that the locomotor effects due to relatively selective activation of D1, D2 or D3 receptors were not blunted by CART peptide. In fact, intra-accumbal CART peptide seemed to potentiate the effects of intra-accumbal SKF81297, a D1 agonist. Thus, none of these individual receptors could simply and solely be associated with the blunting effects of CART peptide on dopamine or psychostimulants. But CART peptide did reduce the effects of simultaneous activation of D1 and D2 receptors.
Literature reports over many years have suggested that simultaneous activation of D1 and D2 receptors results in a significant enhancement of LMA compared to that observed by activation of D1 receptors alone. Robertson and Robertson (1986) and Braun and Chase (1986) first noted the synergistic effects of D1 and D2 agonists on dopamine-related behavior in rats. In addition to the synergistic effects of D1 and D2 agonists on locomotor behavior, stimulation of both receptors in the NAc mediates reward. Infusion of the D1 agonist, SKF 38393, not the D2/D3 agonist, quinpirole, into the NAc shell is sufficient to maintain intracranial self-administration; however rats freely self-administer the combination of the D1 and D2 agonists (Ikemoto et al., 1997). The synergistic actions of D1 and D2 receptor stimulation can also be observed on a neuronal level. Walters et al (1987) observed that D1 receptors had a permissive role on postsynaptic D2 receptor activation, and White (1987) found that D1 stimulation enabled the inhibition of NAc neurons by a D2 agonist. Hopf et al. (2003) demonstrated that coactivation of D1 and D2 receptors increased spike firing in NAc neurons while stimulation of either D1 or D2 receptors alone had no effect. It has been suggested that D1 receptors enable the postsynaptic rather than the presynaptic effects of D2 agonists (Wachtel et al., 1989). The mechanism of this synergism is not yet clearly understood. Whether or not both D1 and D2 receptors are found in the same cells is controversial, and results have been reported favoring both views (reviewed by Deng et al., 2006). Nevertheless, the D1/D2 synergy is a solid experimental observation from several different laboratories over many years and was reproduced in this study.
The findings shown in Figure 5 indicate that CART peptide blocks or reduces the LMA induced by simultaneous activation of both D1 and D2 receptors. Thus, CART peptide could reduce the D2 component of the D1/D2 synergy. However, since CART peptide has no effect on D2 receptor activation alone (Figure 3), one could argue that the effect of CART peptide on the D1/D2 synergy resulted from a reduction of the effects of the enabling effects of D1 receptors on postsynaptic D2 receptor activation. But, CART peptide increased the LMA induced by D1 receptor activation. More recently So et al (2007) showed that activation of D1-D2 oligomers resulted in a unique signal involving calcium; but as noted above, it is not known if the D1 and D2 receptors occur in the same cells in the NAc. Thus the mechanism of the blunting effect of CART peptide is not clear.
Other neurotransmitters may be involved. A series of studies by David and colleagues examined the effects of blocking or activating NMDA and non-NMDA glutamatergic receptors, and group I, group II, and group III metabotropic glutamate (mGlu) receptors on LMA produced by D1, D2, or D1/D2 receptor activation in the NAc (David and Abraini, 2001; 2002; David et al., 2004; Rouillon et al. 2008a,b). Interestingly, These studies suggest that only activation of NMDA receptors (David et al., 2004) or group I mGlu receptors (Rouillon et al., 2008a,b) potentiated LMA produced by D1 receptor activation and decreased that produced by co-activation of D1 and D2 receptors, as did CART peptide. Other studies have investigated the role of various glutamatergic projections to the NAc on LMA produced by D1, D2 or D1/D2 receptor activation (Rouillon et al., (2008a,b). It was found that the basolateral amygdala and the prefrontal cortex had tonic facilitatory and tonic inhibitory control over hyperlocomotion mediated by D1 receptor activation and D1/D2 receptor activation, respectively (Rouillon et al., 2008b). If CART peptide interferes with the normal function of the glutamate system, then it is possible that some aspect of that could produce the findings in this study. Also, it has been noted that group III mGlu receptors are negatively linked to adenylate cyclase (David and Abraini, 2002), presumably through Gi, and simultaneous activation of group III mGlu receptors and CART receptors could reduce mGlu effects by competition for Gi proteins. While these ideas suggest possible mechanisms for the CART peptide’s effect on the D1/D2 receptor synergy, additional experiments will be required to explore the possibilities.
In the data reported here, a D1 and D2 synergism was required to see the CART peptide-induced reduction of the dopamine agonist effects on LMA. This has validity in that dopamine released from nerve terminals is likely to stimulate more than one type of dopamine receptor at the synapse. The CART peptide inhibition of the D1/D2 synergism is an important factor in attempting to unravel modulatory mechanisms in the NAc and should be examined further in future studies.
Acknowledgments
The authors acknowledge the support of NIH grants RR00165, DA00418, DA15162 and support of the Georgia Research Alliance.
References
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Braun AR, Chase TN. Obligatory D-1/D-2 receptor interaction in the generation of dopamine agonist related behaviors. Eur J Pharmacol. 1986;131:301–306. doi: 10.1016/0014-2999(86)90588-1. [DOI] [PubMed] [Google Scholar]
- Choi KH, Zarandi B, Todd KG, Biondo AM, Greenshaw AJ. Effects of AMPA/kainate receptor blockade on responses to dopamine receptor agonists in the core and shell of the rat nucleus accumbens. Psychopharmacology (Berl) 2000;150:102–111. doi: 10.1007/s002130000391. [DOI] [PubMed] [Google Scholar]
- David HN, Abraini JH. Differential modulation of the D1-like- and D2-like dopamine receptor-induced locomotor responses by group II metabotropic glutamate receptors in the rat nucleus accumbens. Neuropharmacology. 2001a;41:454–463. doi: 10.1016/s0028-3908(01)00082-x. [DOI] [PubMed] [Google Scholar]
- David HN, Abraini JH. Group III metabotropic glutamate receptors and D1-like and D2-like dopamine receptors interact in the rat nucleus accumbens to influence locomotor activity. Eur J Neurosci. 2002;15(5):869–75. doi: 10.1046/j.1460-9568.2002.01919.x. [DOI] [PubMed] [Google Scholar]
- David HN, Sissaoui K, Abraini JH. Modulation of the locomotor responses induced by D1-like and D2-like dopamine receptor agonists and D-amphetamine by NMDA and non-NMDA glutamate receptor agonists and antagonists in the core of the rat nucleus accumbens. Neuropharmacology. 2004;46:179–191. doi: 10.1016/j.neuropharm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat. 2006;32:101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides. 2008;42:339–343. doi: 10.1016/j.npep.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Jones D, Vicentic A, Hue G, Rye D, Kuhar MJ. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50(7):858–64. doi: 10.1016/j.neuropharm.2005.12.007. Epub 2006 Feb 2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland MD, Lowe AS, Reavill C, James MF, Leslie RA, Williams SC. Mapping the effects of the selective dopamine D2/D3 receptor agonist quinelorane using pharmacological magnetic resonance imaging. Neuroscience. 2005;133:315–326. doi: 10.1016/j.neuroscience.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Jones DC. The role of CART in the reward/reinforcing properties of psychostimulants. Peptides. 2006;27:1993–2004. doi: 10.1016/j.peptides.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55–102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides. 2007;41:65–72. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine self-administration in rats. Behav Brain Res. 2008;191(2):266–71. doi: 10.1016/j.bbr.2008.03.039. Epub PMID: 18485497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–373. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kim S, Yoon HS, Kim JH. CART peptide 55–102 microinjected into the nucleus accumbens inhibits the expression of behavioral sensitization by amphetamine. Regul Pept. 2007;144:6–9. doi: 10.1016/j.regpep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55–102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed] [Google Scholar]
- Kimmel HL, Thim L, Kuhar MJ. Activity of various CART peptides in changing locomotor activity in the rat. Neuropeptides. 2002;36:9–12. doi: 10.1054/npep.2002.0884. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391(1):115–32. [PubMed] [Google Scholar]
- Marie-Claire C, Laurendeau I, Canestrelli C, Courtin C, Vidaud M, Roques B, Noble F. Fos but not Cart (cocaine and amphetamine regulated transcript) is overexpressed by several drugs of abuse: a comparative study using real-time quantitative polymerase chain reaction in rat brain. Neurosci Lett. 2003;345:77–80. doi: 10.1016/s0304-3940(03)00307-0. [DOI] [PubMed] [Google Scholar]
- Meyer ME. Effects of intraaccumbens dopamine agonist SK&F38393 and antagonist SCH23390 on locomotor activities in rats. Pharmacol Biochem Behav. 1993;45:843–847. doi: 10.1016/0091-3057(93)90130-l. [DOI] [PubMed] [Google Scholar]
- Meyer ME. Mesolimbic 7-OH-DPAT affects locomotor activities in rats. Pharmacol Biochem Behav. 1996;55:209–214. doi: 10.1016/s0091-3057(96)00066-4. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Van Hartesveldt C, Potter TJ. Locomotor activity following intra-accumbens microinjections of dopamine D1 agonist SK&F 38393 in rats. Synapse. 1993;13:310–314. doi: 10.1002/syn.890130403. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson . The rat brain in stereotaxic coordinates. Academic Press Inc; New York: 1986. [Google Scholar]
- Robertson GS, Robertson HA. Synergistic effects of D1 and D2 dopamine agonists on turning behaviour in rats. Brain Res. 1986;384:387–390. doi: 10.1016/0006-8993(86)91178-9. [DOI] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9(10):747–58. doi: 10.1038/nrn2493. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillon C, Abraini JH, David HN. Prefrontal cortex and basolateral amygdala modulation of dopamine-mediated locomotion in the nucleus accumbens core. Exp Neurol. 2008a Jul;212(1):213–7. doi: 10.1016/j.expneurol.2008.04.002. Epub 2008 Apr 15. [DOI] [PubMed] [Google Scholar]
- Rouillon C, Degoulet M, Chevallier K, Abraini JH, David HN. Modulation by group I mGLU receptor activation and group III mGLU receptor blockade of locomotor responses induced by D1-like and D2-like receptor agonists in the nucleus accumbens. Brain Res. 2008b Mar 10;1198:44–54. doi: 10.1016/j.brainres.2008.01.025. Epub. [DOI] [PubMed] [Google Scholar]
- So CH, Verma V, O’Dowd BF, George SR. Desensitization of the dopamine D1 and D2 receptor hetero-oligomer mediated calcium signal by agonist occupancy of either receptor. Mol Pharmacol. 2007;72(2):450–62. doi: 10.1124/mol.107.034884. Epub 2007 May 22. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P. Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport. 2002;13:1215–1218. doi: 10.1097/00001756-200207020-00029. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Hu XT, Galloway MP, White FJ. D1 dopamine receptor stimulation enables the postsynaptic, but not autoreceptor, effects of D2 dopamine agonists in nigrostriatal and mesoaccumbens dopamine systems. Synapse. 1989;4:327–346. doi: 10.1002/syn.890040409. [DOI] [PubMed] [Google Scholar]
- Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science. 1987;236:719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- White FJ. D-1 dopamine receptor stimulation enables the inhibition of nucleus accumbens neurons by a D-2 receptor agonist. Eur J Pharmacol. 1987;135:101–105. doi: 10.1016/0014-2999(87)90764-3. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Kim S, Park HK, Kim JH. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 2007;53:344–351. doi: 10.1016/j.neuropharm.2007.05.014. [DOI] [PubMed] [Google Scholar]