Abstract
Cytotoxic T lymphocytes (CTL) represent one of the front lines of defense for the immune system, killing virus-infected and tumor-transformed cells. CTL use at least two mechanisms to induce apoptosis in their targets, one mediated by perforin and granzymes, and the other triggered by the death ligand, CD95-ligand (CD95L). Here we used an in vivo cytotoxicity assay to measure specific clearance of antigen-bearing target cells in mice that had previously been immunized with non-infectious cell-associated-antigens. We found that perforin was dispensable for efficient clearance of antigen-bearing cells from immunized mice, but only if CD95/CD95L was functional; however, there was a delay in target cell clearance in the absence of perforin. Additionally, we observed approximately 35% target cell clearance in the absence of both perforin and CD95L which was only slightly abrogated in the presence of a neutralizing anti-TNF antibody. The presence of a dominant negative Fas-Associated Death Domain (FADD) did not block target cell clearance and therefore cannot be attributed to known death receptors. Taken together these data suggest that perforin- and CD95L-dependent killing are complementary at early time points, can each compensate for the absence of the other at later time points and that there is an additional component of antigen-restricted CTL killing independent of both perforin, CD95L and TNFα.
Keywords: death receptor/ligand, perforin, antigen-restricted killing, in vivo cytotoxicity
Introduction
Cytotoxic T lymphocytes (CTL) play a crucial beneficial role in the elimination/destruction of virus-infected and tumor-transformed cells, but a detrimental role in the development of many autoimmune diseases and transplant rejection. At least two known pathways have been identified for the induction of target cell apoptosis: i) the Ca2+-dependent, granule-mediated pathway that relies on the discharge of perforin and granzyme proteases to induce apoptosis via a modified intrinsic mechanism and ii) CD95-ligand (CD95L)/CD95 death ligand/receptor interactions that trigger the extrinsic pathway (for reviews see 1–3).
Engagement of the T-cell receptor (TCR) results in specialized granules in the CTL migrating toward the site of contact between the effector and target cells. These lysosome-like granules contain granzymes, perforin and CD95L. Following migration of granules to the site of contact 2, 4, exocytosis of granule contents into the intercellular space between killer and target results in rapid death, within 30 minutes in vitro 5. Perforin is necessary for granzymes to induce apoptosis via caspase-dependent and caspase-independent pathways. CD95L is translocated from cytolytic granules to the surface of CTL upon engagement of the TCR. In nonlymphoid cells CD95L induces death in CD95-bearing targets by an extrinsic death pathway without any further additional requirements and therefore represents a promiscuous form of killing with no requirement for antigen (Ag) presentation.
There have been numerous conflicting reports suggesting the relative importance of various components of the CTL’s killing machinery 6–8 (reviewed in 1–3), illustrating the need for further dissection of the activities and potential synergies between granzyme/perforin- and CD95L-dependent killing by CTL. Here, we show in a non-infectious model that CTL primed to cell-associated Ag require both perforin and CD95L for maximal target cell killing at four hours but that the two cannot account for all Ag-dependent target cell deletion in vivo. The perforin-dependent system showed significant but incomplete clearance at four hours, whereas the CD95/CD95L system was less efficient at rapid clearance but capable of target cell clearance at 20 hours. For rapid clearance, these two systems likely represent complementary, non-redundant, killing mechanisms.
Results and Discussion
CTL-mediated killing of target cells is restricted to Ag-bearing targets
CD95L, when expressed by many cell types, imparts cytotoxic activity against CD95-expressing cells 9–13. However, in CTL and NK cells CD95L is sequestered specialized granules until engagement of TCR with Ag-loaded MHC I. We first asked was if CD95L mediated killing of target cells by CTL was restricted to targets bearing appropriate Ag. Lymphocytes were purified from Ad5E1-MEC immunized wild type (WT) mice, expanded in vitro and subsequently cultured with 3[H]-labeled target cells from WT or lpr (CD95-deficient) mice that were pulsed with the immunodominant epitope E1B192–200(Ag) or control peptide. As shown in Figure 1A, neither WT or lpr targets not bearing Ag were killed by CTL whereas there was specific killing against Ag-bearing targets, confirming that CD95-dependent and -independent killing was restricted to Ag-bearing targets.
Figure 1. CTL-mediated killing is restricted to Ag-bearing targets.
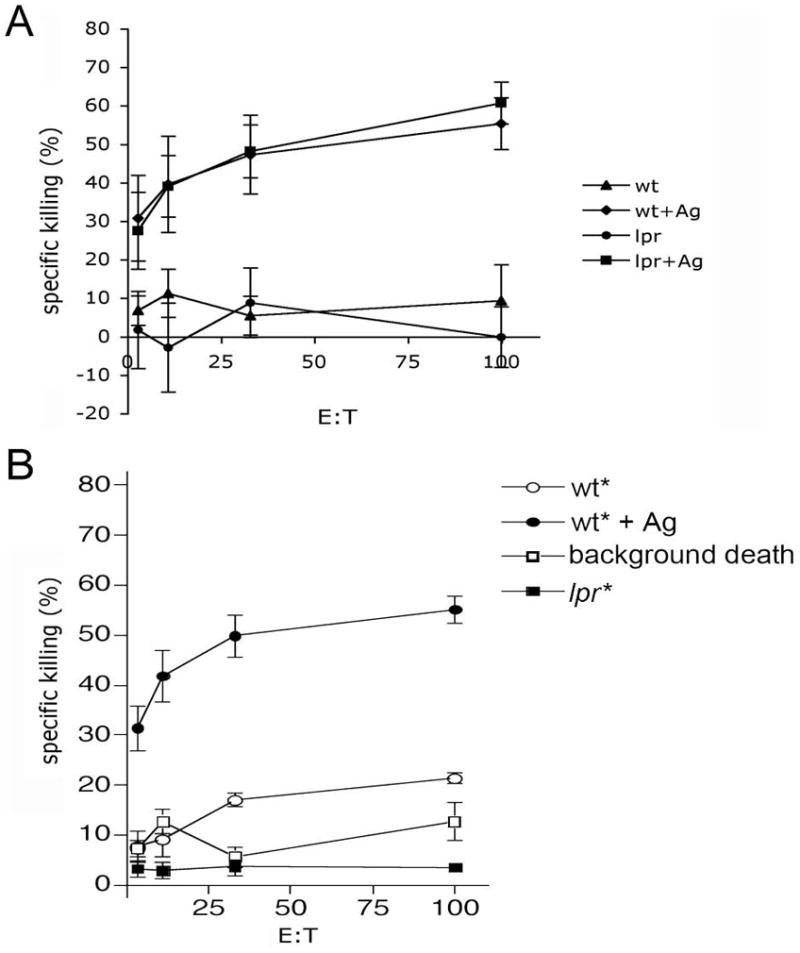
A) Splenocytes from Ad5E1-MEC-immunized mice were expanded in vitro and cultured with 3[H]labeled target cells from WT and CD95−/− mice that were pulsed with E1B192–200 (Ag) peptide. OVA257–264 pulsed non-labeled cells were used as negative control. Specific killing was assessed by JAM assay after 4 hours. B) Non-specific “bystander” killing was assessed by JAM assay as described above using 3[H]labeled cells without Ag (bystander) in the presence of non-3[H]labeled Ag-bearing target. Data are expressed as mean +/− s.e.m. (n=3 per group, representative experiment of 3 experiments).
We next asked if under conditions where the CD95L system becomes activated (i.e. in the presence of Ag-bearing targets) would CTL become promiscuous and capable of killing non Ag-bearing bystander cells. To address this question, 3[H]-labeled target cells pulsed with irrelevant peptide were used in combination with Ag-bearing targets. Specific killing of Ag-bearing targets was observed at all effector:target (E:T) ratios (Figure 1B, closed circles), but no killing of either WT or lpr bystander cells was detected (Figure 1B, open circles and closed squares). These observations are consistent with early reports regarding bystander killing 14, 15 and suggest that once a CTL becomes capable of utilizing CD95L (which is Ag-dependent), it retains the requirement for its targets to have appropriate Ag.
Perforin is dispensable for Ag-dependent target cell clearance in vivo
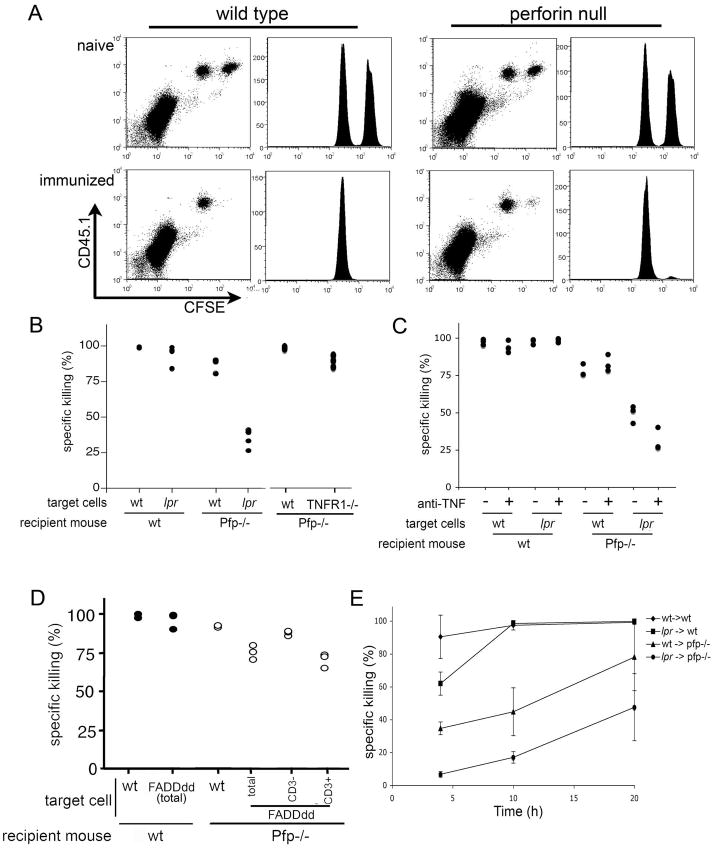
Our results prompted us to investigate Ag-restricted killing by CD95L in vivo. C57Bl/6 and perforin−/− mice were immunized by subcutaneous injection of lethally irradiated Tap-deficient Ad5E1 mouse embryonic cells (MEC). Seven days after the initial challenge, naïve and immunized mice were administered with a mixture of carboxyfluorescein diacetate succinimidyl ester (CFSE)high cells pulsed with Ag (E1B192–200) and CFSEmed cells pulsed with irrelevant peptide (OVA257–264). Twenty hours later, splenocytes were recovered from recipient mice and, as shown in Figure 2A, both Ag-bearing and irrelevant targets were recovered in similar ratios from naïve WT and perforin−/− mice. Importantly, target cells bearing Ag were efficiently deleted from both WT and perforin−/− immunized mice at 20 hours, suggesting that perforin was dispensable for Ag-dependent killing in this system. Our observations are consistent with those reported by Barchet et al.6 showing clearance of gp33-specific target cells at 20 hours by virus-specific CTL in LCMV-immunized WT and perforin−/− animals. We also found that killing was completely CTL-MHC class I dependent as depletion of CD8 but not CD4 T cells completely prevented clearance of the target cells in both strains of mice (data not shown).
Figure 2. Perforin is dispensable for Ag-dependent killing in vivo and both perforin and CD95/CD95L are required for optimal MHC I-restricted killing of Ag-bearing targets in vivo.
A) Ad5E1-MEC immunized WT and perforin−/− mice received equal numbers of CFSEhigh Ag-pulsed targets and CFSEmed control cells. Twenty hours later clearance of Ag-pulsed target cells was determined in the spleen by flow cytometry, using CFSEmed control cells as internal standard. Data are shown from one naïve and immunized recipient mouse of each strain and are representative of three separate experiments with n=3–4 per group. B) Ad5E1-MEC immunized WT and perforin−/− mice received equal numbers of CFSEhigh Ag-pulsed targets and CFSEmed control cells from indicated mouse strains. Twenty hours later clearance of Ag-pulsed target cells was determined in the spleen by flow cytometry, using CFSEmed control cells as internal standard. C) Four hours prior to transfer of labeled target and control cells, immunized animals were administered neutralizing anti-TNF antibody or isotype control. Specific clearance Ag-bearing wild type and lpr target cells in wild type and perfrin-null recipient mice in the presence or absence of neutralizing anti-TNF was assessed 20 hours after transfer. D) Clearance of FADDddlckexpressing targets in immunized WT and perforin−/− mice was determined. Killing of FADDddlck target cells was further dissected according to CD3+ targets that expressed FADDddlck and CD3- target cells that did not express the transgene. Clearance of non-lck expressing targets cells from FADDddlck mice was comparable to WT targets. E) Kinetics of perforin and CD95/CD95L mediated clearance. Immunized WT and perforin−/− mice received WT or CD95−/− targets and AG-specific killing was determined at indicated time-points. Data are expressed as mean +/− s.e.m. (n=3 per group, representative experiment of 3 experiments).
Dissection of Ag-restricted killing mechanisms in vivo
To further dissect the requirements for perforin-independent Ag-restricted killing in vivo we first analyzed CTL induction and cytolytic effector molecules expression in WT and perforin−/− mice. Both WT and perforin−/− mice showed a similar percentage of E1B192–200 specific interferon (IFN)γ-producing CD8+ T cells following immunization (Supplemental Figure 1A) with comparable expression of effector molecules TNFα and CD95L (Supplemental Figure 1B). Immunized WT mice cleared both WT and lpr Ag-pulsed targets within 20 hr of transfer. However, while immunized perforin−/− mice efficiently cleared WT targets, clearance of lpr targets was significantly reduced, indicating that together perforin- and CD95-mediated killing accounts for the majority of target cell killing in immunized mice. Interestingly, however, there remained approximately 35% specific killing in the absence of these two triggers (Figure 2B) and 6. Although perforin and granzymes are intricately associated and considered to work in concert, it is possible that one or more of the granzymes kill in the absence of perforin. In vitro evidence indicates that perforin is not an absolute requirement for the entrance granzymes into the target cell 16, 17. However, current research still indicates that perforin is essential for cytolysis 1, 18.
Tumour necrosis factor (TNF)α, a lymphocyte-derived apoptotic cytokine, was excluded as a major contributor to CTL-induced killing in vivo as no reduction in Ag-dependent killing of TNF-receptor (TNFR1)−/− target cells was seen, which is in line with previously published findings 19. In order to investigate the possibility that killing by a TNF-dependent mechanism could account for the 35% killing in the absence of perforin and CD95. We performed similar experiments as above in which immunized animals were administered neutralizing anti-TNFα antibody along with target and control cells. As shown in Figure 2C, there was a slight reduction in the clearance of Ag-bearing targets in the presence of anti-TNF indicating that TNFα contributes to Ag-restricted killing, but cannot account for all of the perforin- and CD95-inpdendent killing of Ag-bearing target cells.
To further rule out the possible involvement of other death ligands, targets were used from mice expressing a dominant negative of Fas-Associated Death Domain (FADD), FADDdd, under the T cell specific promoter lck. FADDdd binds death domains of the death receptors but fails to activate caspase-8, thereby inhibiting receptor-mediated apoptosis. Ag-bearing FADDddlck expressing target cells were efficiently deleted in immunized WT mice similar to Ag-bearing WT target cells (Figure 2D). In contrast, perforin−/− mice showed reduced killing of FADDdd expressing targets.
Results from our in vivo cytotoxicity assays suggest that there is a mechanism of Ag-specific killing employed by CTL that is not mediated by perforin, CD95L or TNFα. It is possible that the remaining cytotoxic activity (~30%) against Ag-bearing targets cannot be attributed to any known apoptotic initiator(s). TNF-related apoptosis inducing ligand (TRAIL), another member of the TNF superfamily of death ligands, has been implicated as an important player in apoptotic death in the immune system; however, as we did not detect expression of TRAIL in activated CD8+ cells in immunized animals (Supplemental Figure 1B) TRAIL is unlikely to be involved in target cell clearance in this system.
Perforin- and CD95L-dependent killing are both required for rapid clearance of Ag-bearing targets in vivo
Although perforin was dispensable for clearance of Ag-bearing targets cells by 20 hours, there remained the possibility of differential involvement of the perforin- and CD95-dependent systems at earlier time points. Strikingly, in WT mice deletion of Ag-bearing targets was virtually complete by 4 hours (Figure 2E). Mice lacking a functional CD95 system, but retaining active perforin, showed reduced killing at the earliest time point, but complete clearance of targets at 10 hours. This observation is in agreement with the findings of Barber et al. that indicate depressed clearance of WT target cells in LCMV immunized perforin−/− animals at one and four hours by virus-specific CTL20. Deletion of WT targets was reduced in perforin−/− mice, presumably due to less efficient rapid clearance of targets due to CD95L. It is interesting to note that at 4 hours the combined killing by perforin (~50%) and CD95L (~35%) approaches that when both of these modalities were present. Therefore, it is possible that they provide complementary killing mechanisms that that are nonredundant in the early induction of apoptosis during Ag-restricted killing. There is also a possibility that characteristics of the target cell may also influence the mechanism(s) and kinetics by which target cells are cleared, for example by the expression of viral inhibitors of apoptosis or oncogenes.
In summary, here we demonstrated that CTL primed to cell-associated Ag in the absence of infection require both perforin- and CD95L-dependent arms of CTL’s arsenal for maximal target cell deletion in vivo at four hours and that the contribution of each of these triggers may be additive and therefore represent complementary death inducing systems at early time points. At later times, perforin-mediated killing efficiently compensates for a lack of CD95L/CD95 killing; however, CD95L/CD95 cannot completely replace the perforin-dependent arm, even at 20 hours. We observed no Ag-independent killing in vivo, thus excluding bystander killing and the involvement of secreted cytotoxic factors. These observations provide mechanistic insights into CTL killing in vivo that may influence therapeutic approaches to regulate CTL activity in non-infectious scenarios such as transplantation, autoimmune diseases and autologous tumour cell vaccination.
Materials and Methods
Reagents, cell lines and mice
C57Bl/6 (B6); B6.MRL-Tnfrsf6lpr/J (lpr); B6.129-Tnfrsf1atm1Mak/J (TnfR1−/−); B6.129S6-Tnftm1Gkl/J (TNF−/−); C57Bl/6-Prf1tm1Sdz (perforin−/−); B6.SJL-PtprcaPep3b/BoyJ (CD45.1) mice were purchased from Jax Mice and Services (Bar Harbor, ME). FADDddlck mice21 were generously provided by Dr. Craig Walsh (University of California, Irvine). Mice were bred and maintained at the LIAI or CCHMC under specific pathogen-free conditions in accordance with guidelines of AALAC International or UK Home Office. C57Bl/6 TAP+/+ and TAP−/− mouse embryo cell (MEC) lines expressing human adenovirus type 5 early region (Ad5E1) were cultured as described22. In vivo CD4+ cell depletion was performed as described before 23,24. Unless stated otherwise, antibodies were purchased from BD Pharmingen (La Jolla, CA) and general chemicals from Sigma. CFSE was purchased from Molecular Probes (Eugene, OR). Rat anti-TNFα IgG1 antibody (MP6-XT22)25 was generously provided by Dr. F. Finkelman (CCHMC, Cinncinati).
In vivo cytotoxicity assay
Clearance of Ag-bearing targets relative to target cells with irrelevant Ag in experimental mice was performed as previously described 26. Briefly, recipient mice were immunized by subcutaneous injection of irradiated (3000 rad) Tap deficient Ad5E1 MEC cells (1 × 107). Seven days later, CD45.1+ target cells were isolated from congenic mice and labeled with high (6.25 μM) or low (0.625 μM) concentrations of CFSE. Brightly labeled cells were pulsed with E1B192–200 peptide (VNIRNCCYI), and the other population with irrelevant peptide (OVA257–264 SIINFEKL). Cells were i.v. injected in a 1:1 ratio into immunized or control mice. Spleens were harvested at indicated times and CFSE-labeled cells enumerated by flow cytometry after gating on CD45.1+ cells. Ag-specific clearance was compared directly with clearance of targets with irrelevant peptide in each recipient animal. In experiments involving neutralizing anti-TNF, antibody or isotype control (2 mg/mouse) was injected i.v. four hours before target cells were transferred25.
Assessment of CTL induction and activation
The frequency of E1B192–200-specific T cells was determined by intracellular cytokine staining upon stmulation with E1B192–200 peptide (5μg/ml) for five hours in the presence of brefeldin A as described before. Surface staining for CD8 and CD95L and intracellular staining for IFNγ, TNFα, CD95L and TRAIL were performed as described 26.
Ex vivo killing and bystander killing assays
In vitro cytolytic activity of E1B-specific CTL was evaluated by JAM assay as described27. Briefly, splenocytes from immunized mice were isolated and stimulated for six days with irradiated syngeneic TAP-sufficient 5E1 MEC. Target cells were prepared by culturing splenocytes from indicated mouse strains with conA for 48 hours; 3H-thymidine was added for the final 12 hours. Restimulated splenocytes were cultured in various ratios with target cells. To assess bystander killing, 3H-labeled target cells loaded with irrelevant peptide were combined with Ag-bearing non-3H-labeled targets and re-stimulated splenocytes. Specific killing was calculated as ((spontaneous cpm – experimental cpm) × 100)/spontaneous cpm 23, 26.
Supplementary Material
Frequency (A) and effector molecules expression (B) of E1B192–200 specific CD8+ T cells in Ad5E1-MEC immunized WT and perforin−/− mice as determined by intracellular IFNg staining in combination with TNFa, TRAIL and CD95L. Data are shown from one naïve and immunized recipient mouse of each strain and are representative of three separate experiments with n=3–4 per group.
Acknowledgments
This work was supported with funding from the Medical Research Council (UK) to MJP, NIH/NIAID (R21 AI079545) to EMJ, NIH (RO1 AI44828) to DRG and NIH (R01 CA081261, R01 AI076972) to SPS. The authors thank Dr. L. Bouchier-Hayes for critical reading of the manuscript.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References cited
- 1.Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251–62. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 3.Trambas CM, Griffiths GM. Delivering the kiss of death. Nat Immunol. 2003;4:399–403. doi: 10.1038/ni0503-399. [DOI] [PubMed] [Google Scholar]
- 4.Stinchcombe JC, Griffiths GM. The role of the secretory immunological synapse in killing by CD8+ CTL. Semin Immunol. 2003;15:301–5. doi: 10.1016/j.smim.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–9. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 6.Barchet W, Oehen S, Klenerman P, et al. Direct quantitation of rapid elimination of viral antigen-positive lymphocytes by antiviral CD8(+) T cells in vivo. Eur J Immunol. 2000;30:1356–63. doi: 10.1002/(SICI)1521-4141(200005)30:5<1356::AID-IMMU1356>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Regner M, Pavlinovic L, Koskinen A, Young N, Trapani JA, Mullbacher A. Cutting edge: Rapid and efficient in vivo cytotoxicity by cytotoxic T cells is independent of granzymes A and B. J Immunol. 2009;183:3740. doi: 10.4049/jimmunol.0900466. [DOI] [PubMed] [Google Scholar]
- 8.Sutton VR, Estella E, Li C, et al. A critical role for granzyme B, in addition to perforin and TNFalpha, in alloreactive CTL-induced mouse pancreatic beta cell death. Transplantation. 2006;81:146–54. doi: 10.1097/01.tp.0000191939.68451.d9. [DOI] [PubMed] [Google Scholar]
- 9.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 10.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 11.Igney FH, Behrens CK, Krammer PH. The influence of CD95L expression on tumor rejection in mice. Eur J Immunol. 2003;33:2811–21. doi: 10.1002/eji.200324176. [DOI] [PubMed] [Google Scholar]
- 12.Pinkoski MJ, Droin NM, Lin T, Genestier L, Ferguson TA, Green DR. Nonlymphoid Fas ligand in peptide-induced peripheral lymphocyte deletion. Proc Natl Acad Sci U S A. 2002;99:16174–9. doi: 10.1073/pnas.262660999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stassi G, Di Liberto D, Todaro M, et al. Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol. 2000;1:483–8. doi: 10.1038/82725. [DOI] [PubMed] [Google Scholar]
- 14.Maimone MM, Morrison LA, Braciale VL, Braciale TJ. Features of target cell lysis by class I and class II MHC-restricted cytolytic T lymphocytes. J Immunol. 1986;137:3639–43. [PubMed] [Google Scholar]
- 15.Wei WZ, Lindquist RR. Alloimmune cytolytic T lymphocyte activity: triggering and expression of killing mechanisms in cytolytic T lymphocytes. J Immunol. 1981;126:513–6. [PubMed] [Google Scholar]
- 16.Motyka B, Korbutt G, Pinkoski MJ, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103:491–500. doi: 10.1016/s0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 17.Pinkoski MJ, Hobman M, Heibein JA, et al. Entry and trafficking of granzyme B in target cells during granzyme B-perforin-mediated apoptosis. Blood. 1998;92:1044–54. [PubMed] [Google Scholar]
- 18.Waterhouse NJ, Sutton VR, Sedelies KA, et al. Cytotoxic T lymphocyte-induced killing in the absence of granzymes A and B is unique and distinct from both apoptosis and perforin-dependent lysis. The Journal of cell biology. 2006;173:133–44. doi: 10.1083/jcb.200510072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Bar-Haim E, Machlenkin A, et al. In vivo rejection of tumor cells dependent on CD8 cells that kill independently of perforin and FasL. Cancer Gene Ther. 2004;11:237–48. doi: 10.1038/sj.cgt.7700678. [DOI] [PubMed] [Google Scholar]
- 20.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 21.Walsh CM, Wen BG, Chinnaiyan AM, O’Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439–49. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 22.Schoenberger SP, van der Voort EI, Krietemeijer GM, Offringa R, Melief CJ, Toes RE. Cross-priming of CTL responses in vivo does not require antigenic peptides in the endoplasmic reticulum of immunizing cells. J Immunol. 1998;161:3808–12. [PubMed] [Google Scholar]
- 23.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 24.Dialynas DP, Wilde DB, Marrack P, et al. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 25.Via CS, Shustov A, Rus V, Lang T, Nguyen P, Finkelman FD. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J Immunol. 2001;167:6821–6. doi: 10.4049/jimmunol.167.12.6821. [DOI] [PubMed] [Google Scholar]
- 26.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 27.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. Journal of immunological methods. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frequency (A) and effector molecules expression (B) of E1B192–200 specific CD8+ T cells in Ad5E1-MEC immunized WT and perforin−/− mice as determined by intracellular IFNg staining in combination with TNFa, TRAIL and CD95L. Data are shown from one naïve and immunized recipient mouse of each strain and are representative of three separate experiments with n=3–4 per group.