Abstract
Polychlorinated biphenyls (PCBs), a major class of persistent organic pollutants, are metabolized to hydroxylated PCBs. Several hydroxylated PCBs are substrates of cytosolic phase II enzymes, such as phenol and hydroxysteroid (alcohol) sulfotransferases; however, the corresponding sulfation products have not been isolated and characterized. Here we describe a straightforward synthesis of a series of ten PCB sulfate monoesters from the corresponding hydroxylated PCBs. The hydroxylated PCBs were synthesized by coupling chlorinated benzene boronic acids with appropriate brominated (chloro-)anisoles, followed by demethylation with boron tribromide. The hydroxylated PCBs were sulfated with 2,2,2-trichloroethyl chlorosulfate using DMAP as base. Deprotection with zinc powder/ammonium formate yielded the ammonium salts of the desired PCB sulfate monoesters in good yields when the sulfated phenyl ring contained no or one chlorine substituent. However, no PCB sulfate monoesters were isolated when two chlorines were present ortho to the sulfated hydroxyl group. To aid with future quantitative structure activity relationship studies, the structures of two 2,2,2-trichloroethyl-protected PCB sulfates were verified by X-ray diffraction.
Keywords: Biaryls; Polychlorinated biphenyls (PCBs); Sulfates; 2,2,2-Trichloroethyl (TCE) group; Metabolites
1. Introduction
Polychlorinated biphenyls (PCBs) were manufactured commercially in large quantities and used in numerous technical applications, for example as lubricants, cooling fluids, flame retardants, adhesives, and plasticizers (Hansen 1999; Robertson and Hansen 2001). PCBs are still in use as dielectric fluids in capacitors and transformers. Their wide-spread industrial use and physicochemical properties, such as lipophilicity, semi-volatility and stability towards biological, chemical and thermal degradation, have resulted in widespread environmental contamination. PCBs have also been associated with a broad range of adverse human health effects, such as (neuro-)developmental toxicity (Kodavanti 2004) and carcinogenicity (Silberhorn et al. 1990). The production of PCBs was banned in the United Stated in the late 1970s because of these environmental and public health concerns.
Especially lower chlorinated PCBs undergo oxidative metabolism to hydroxylated PCBs catalyzed by cytochrome P-450 enzymes (Letcher et al. 2000). Some hydroxylated PCB metabolites persist in blood, liver and other tissues of humans, where they can reach levels that are comparable to PCB blood levels (Bergman et al. 1994; Hovander et al. 2006; Park et al. 2007). They potently inhibit the activity of phenol sulfotransferases (SULT) and, thus, may interfere with the sulfation of endogenous and exogenous compounds (Kester et al. 2000; Schuur et al. 1998a; Schuur et al. 1998b; Schuur et al. 1998c; van den Hurk et al. 2002; Wang et al. 2005; Wang et al. 2006). Similarly, hydroxylated PCBs are inhibitors of hydroxysteroid (alcohol) sulfotransferases, such as human SULT2A1 (Liu et al. 2006). There is also evidence that some hydroxylated PCBs are substrates for SULTs. Sacco and James demonstrated that several hydroxylated PCBs are sulfated by polar bear liver cytosol (Sacco and James 2005). Liu et al. recently reported that two hydroxylated PCBs, 4-hydroxy-2′,3,5-trichlorobiphenyl and 4′-hydroxy-2,3′,4,5′-tetrachloro-biphenyl, are substrates for SULT2A1 (Liu et al. 2006).
Very little is currently known about the biological properties and metabolic disposition of PCB sulfates. While many phenyl sulfates are water soluble and readily excreted, calculated octanol/water partition coefficients indicate that PCB sulfates may retain significant lipophilic properties (James 2001). The first chemical synthesis of a series of lower chlorinated PCB sulfate monoesters described herein provides a source of sufficient quantities of these metabolites for detailed study of their chemical and biochemical properties, and how these properties may relate to the metabolic disposition and detoxication of PCBs and hydroxylated PCBs.
2. Materials and methods
2.1. Chemicals and Instruments
All of the chlorinated benzene boronic acids, the brominated phenols and tetrakis(triphenylphosphine)palladium(0) were obtained from Fisher Scientific (Fairlawn, New Jersey, USA). 4-Hydroxy biphenyl (7a) was purchased from Sigma-Aldrich chemical company (St. Louis, MO, USA). All hydroxylated PCBs 7 were synthesized as described previously (Lehmler and Robertson 2001; McLean et al. 1996). Chlorosulfuric acid 2,2,2-trichloroethyl ester was synthesized according to the method by Hedayatullah and co-workers (Hedayatu et al. 1971). The 1H and 13C NMR spectra were recorded on a multinuclear Bruker DRX 400 Digital NMR Bruker spectrometer at ambient temperature. All 1H and 13C chemical shifts are reported in parts per million (ppm) relative to internal tetramethylsilane (Me4Si). Melting points were determined using a MelTemp apparatus and are uncorrected. The gas chromatography-mass spectra (GC-MS) were recorded using a Thermo Voyager EI instrument. High-resolution mass spectra (HR-MS) were measured using an Autospec ESI-MS instrument at the University of Iowa Mass Spectrometry Facility. Infrared spectra (IR) were recorded on a NEXUS 670 FT-TR instrument. UV/Vis spectra were measured using a Perkin Elmer Lambda 650 UV/Vis spectrometer at 23 °C (UV/Vis spectral data of the corresponding hydroxylated PCBs 7 are shown in parentheses for comparison). The characterization of selected compounds is provided below. The characterization of all other compounds is provided in the supplementary material.
2.2. General procedure for the synthesis of sulfuric acid 2,2,2-trichlororo-ethyl (TCE) esters of hydroxylated phenols and PCBs
A solution of 2,2,2-trichloroethyl chlorosulfate (3.2 mmol) in anhydrous DCM (5 mL) was added slowly at 0°C to a solution of phenols 1a,b or hydroxylated PCB 7a-l (Lehmler and Robertson 2001; McLean et al. 1996) (3 mmol) and 1.5 equivalents of 4-N,N′-dimethylaminopyridine (DMAP, 4.5 mmol) in anhydrous DCM (15 mL) (Liu et al. 2004). The reaction mixture was stirred for 30 minutes at 0°C, allowed to warm to ambient temperature and stirred for an additional 10 hours. The solvent was removed under reduced pressure and the residue was dissolved in ethyl acetate (20 mL). The ethyl acetate solution was washed with distilled water (20 mL), 1M HCl solution (2 × 20 mL) and distilled water (20 mL). The organic phase was dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the residue was purified by column chromatography on silica gel using a mixture of n-hexanes and chloroform (8 : 1 to 5 : 1, v/v) as eluent. The TCE esters 2a-b and 8a-l were obtained in good yields ranging from 75% to 94%.
2.2.1. Sulfuric acid 4′-chloro-biphenyl-4-yl 2,2,2-trichloroethyl ester (8b)
White solid; mp: 108–109 °C; 1H NMR (400 MHz, CDCl3): δ/ppm 4.86 (s, 2H, CH2), 7.42 (AA′XX′ system, 2H, J ~ 8.6 Hz), 7.43 (AA′XX′ system, 2H, J ~ 8.6 Hz), 7.47 (AA′XX′ system, 2H, J ~ 8.6 Hz), 7.59 (AA′XX′ system, 2H, J ~ 8.6 Hz). 13C NMR (100 MHz, CDCl3): δ/ppm 80.4 (CH2), 92.3 (CCl3), 121.5 (2×CH), 128.4 (2×CH), 128.6 (2×CH), 129.1 (2×CH), 134.1, 137.9, 139.9, 149.6 (CAr-OSO3). IR (film): 3027, 2975, 1480, 1409, 1389, 1215, 1153, 1095, 989, 821 cm−1. EI-MS m/z (relative intensity, %): 414 (35, C14H10Cl4O4S•+), 284 (25), 217 (10), 203 (100), 175 (46), 149 (15), 139 (35).
2.3. General procedure for the synthesis of ammonium salts of chlorinated biphenyl sulfates
Ammonium formate (0.77 g, 12 mmol) was added to a solution of the (chlorinated) biphenyl TCE sulfate 2 or 8 (2 mmol) in methanol (5 mL) (Liu et al. 2004). Zinc dust (0.26 g, 4 mmol) was added after the ammonium formate had dissolved completely, and the reaction mixture was stirred until the TCE ester 2 or 8 was consumed completely as determined by TLC (usually within 30 minutes). The solution was filtered through Celite and concentrated under reduced pressure at temperatures below 35 °C. The product was purified by column chromatography on silica gel using a mixture of chloroform, methanol and ammonium hydroxide (8 : 1 : 0.2, v/v) as eluent. The solvent was removed under reduced pressure at temperature below 35 °C to yield the final products as a white solid with yields ranging from 83% to 97%. The Rf values of all PCB sulfates were approximately Rf = 0.3 (CHCl3 : CH3OH : NH4OH = 10 : 2 : 0.5, v/v).
2.3.1. Sulfuric acid mono-(4′-chloro-biphenyl-4-yl) ester, ammonium salt (9b)
White solid; mp: 250 °C (dec.); 1H NMR (400 MHz, CD3OD): δ/ppm 7.38 (AA′XX′ system, 2H, J ~ 9.0 Hz), 7.40 (AA′XX′ system, 2H, J ~ 8.9 Hz), 7.56 (2 overlapping AA′XX′ systems, 4H, J ~ 8.8 Hz). 13C NMR (100 MHz, CD3OD): δ/ppm 122.9 (2×CH), 128.7 (2×CH), 129.4 (2×CH), 129.9 (2×CH), 134.3, 137.7, 140.6, 153.9 (CAr-OSO3). IR (KBr): 3235, 3079, 1246, 1061 cm−1. UV/Vis: λ9b,max(MeOH) = 258 nm, ε9b = 2.42×104 L·mol−1·cm−1 (λ7b,max(MeOH) = 267 nm, ε7a = 2.29×104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 282.9844, calculated for C12H8(35)ClO4S 282.9832.
2.4. Single crystal structure determination of 8e and 8k
Crystals of the TCE-protected PCB sulfates 8e and 8k suitable for crystal structure analysis were obtained by slow crystallization from methanol. X-ray diffraction data were collected at 90.0(2) K on a Nonius KappaCCD diffractometer. Raw data were integrated, scaled, merged and corrected for Lorentz-polarization effects using the Denzo-SMN package (Otwinowski and Minor 1997). The structures were solved by direct methods (Sheldrick 2008) and missing atoms were located in difference Fourier maps (Sheldrick 2008). Refinement was carried out against F2 by weighted full-matrix least-squares. Hydrogen atoms were found in difference maps but subsequently placed at calculated positions and refined using appropriate riding models. Non-hydrogen atoms were refined with anisotropic displacement parameters. Atomic scattering factors were those of SHELXL (Sheldrick 2008), as taken from the International Tables for Crystallography vol. C (Wilson 1992). The crystal data and the related parameters are summarized in Table 2. Additional crystallographic data have been deposited with the Cambridge Crystallographic Data Center as Supplementary Publications CCDC 687167 (8e) and CCDC 719235 (8k). Copies of the data can be obtained free of charge on application to the CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K. (fax, (+44)1223-336- 033; e-mail, deposit@ccdc.cam.ac.uk).
Table 2.
X-ray crystallographic data for PCB TCE sulfate diesters 8e and 8k.
| Property | 8e | 8k |
|---|---|---|
| Formula | C14H9Cl5O4S | C14H7Cl7O4S |
| M | 450.52 | 519.42 |
| T/K | 90.0(2) | 90.0(2) |
| wavelength | 0.71073 Å | 0.71073 Å |
| Space group | Monoclinic, P21/c | Monoclinic, P21/n |
| a (Å) | 9.0188(2) | 8.8951(2) |
| b (Å) | 10.5961(2) | 17.8153(4) |
| c (Å) | 18.2405(3) | 12.3093(3) |
| α(°) | 90 | 90 |
| β (°) | 90.9292(8) | 104.5587(11) |
| γ (°) | 90 | 90 |
| V (Å3) | 1742.91(6) | 1888.01(8) |
| Z | 4 | 4 |
| Calculated density (mg·m−3) | 1.717 | 1.827 |
| Absorption coefficient (mm−1) | 0.968 | 1.181 |
| F(000) | 904 | 1032 |
| Crystal size (mm) | 0.37×0.33×0.26 | 0.25×0.24×0.20 |
| θ range (°) | 2.22 to 27.48 −11 ≤ h ≤ 11 |
2.06 to 27.48 −11 ≤ h ≤ 11 |
| Limiting Indices | −13 ≤ k ≤ 12 −23 ≤ l ≤ 23 |
−23 ≤ k ≤ 17 −15 ≤ l ≤ 15 |
| Reflections collected/unique | 23838/3984 | 22255/4327 |
| R(int) | 0.0353 | 0.0337 |
| Completeness to θ = 25.00 | 99.9% | 99.9% |
| Max. and minx. transmission | 0.787 and 0.654 | 0.798 and 0.757 |
| Data/restraints/parameters | 3984/0/217 | 4327/0/235 |
| Goodness-of-fit on F2 | 1.070 | 1.098 |
| Final R indices I>2σ(I) | R1 = 0.0306; wR2 = 0.0694 | R1 = 0.0481; wR2 = 0.1153 |
| R indices (all data) | R1 = 0.0403; wR2 = 0.0741 | R1 = 0.0853; wR2 = 0.1330 |
| Largest diff. peak and hole (eÅ−3) | 0.322 and −0.484 | 0.888 and −0.668 |
3. Results and discussion
3.1. Synthesis
A variety of sulfation reagents and conditions have been reported in the literature. For examples, sulfur trioxide complex with pyridine or tertiary amines are commonly used for the sulfation of different alcohols, including saccharide derivatives (Nishino and Nagumo 1992; Petitou and van Boeckel 2004; Pires et al. 2001) and organic compounds with phenolic moieties, such as phenols (Hanson et al. 2006; Hearse et al. 1969; Ragan 1978), flavanoids (Gunnarsson and Desai 2002), flavonoids (Gunnarsson and Desai 2003) and steroids (Santos et al. 2003). However, these sulfation methods have drawbacks, such as tedious purification procedures and low yields. In recent years, novel sulfation methods have been developed to overcome these problems. In particular substituted alkyl chlorosulfates, including isobutyl, neopentyl and 2,2,2-trichloroethyl chlorosulfate (TCE-Cl), have been used to obtain sulfate diesters which form the desired sulfate monoesters upon deprotection in good-to-excellent yields (Liu et al. 2004; Simpson and Widlanski 2006).
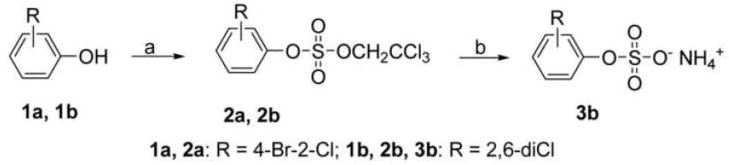
In order to obtain PCB sulfates to investigate their biological role in the metabolism and toxicity of PCBs, we employed the 2,2,2-trichloroethyl (TCE)-protection method to synthesize a series of sulfate metabolites of several lower chlorinated PCBs. As shown in Scheme 1, we initially synthesized the brominated TCE sulfate diester 2b and investigated its coupling with chlorinated benzene boronic acids 4 to obtain the desired PCB TCE sulfate diesters. However, the Suzuki coupling of 2b with chlorinated benzene boronic acids 4 failed because the TCE group is unstable under the reaction conditions employed.
Scheme 1.
Sulfation of substituted phenols ((a) 2,2,2-trichloroethyl chlorosulfate, DMAP, dry CH2Cl2, 10 h; (b) Zn powder, HCO2NH4, MeOH). Sulfate monoester 3b was identified in situ using TLC, but could not be isolated.
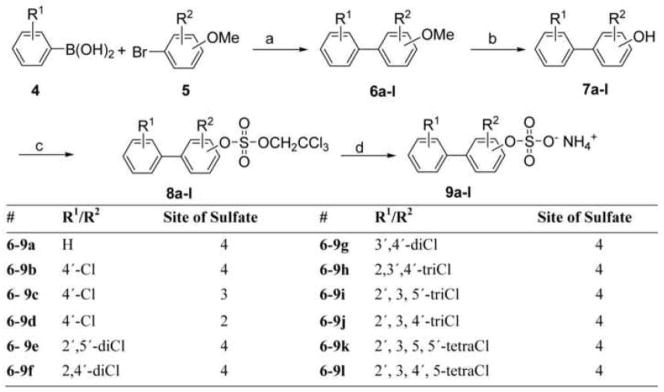
In an alternate approach, we first prepared the hydroxylated biphenyl derivatives 7 and introduced the sulfate group in the final steps of the synthesis. As shown in Scheme 2, a series of hydroxylated PCB derivatives (7a-l) were synthesized using the Suzuki-coupling of chlorinated benzene boronic acids 4 and appropriate brominated (chloro)-anisoles 5, followed by demethylation with boron tribromide (Lehmler and Robertson 2001; McLean et al. 1996). Subsequently, TCE-protected PCB sulfates diesters 8a-j were synthesized from the hydroxylated PCBs 7a-l by sulfation with 2,2,2-trichloroethyl chlorosulfate as sulfation reagent and DMAP as base. The sulfation reactions proceeded in good-to-excellent yields ranging from 75 to 94% (Table 1).
Scheme 2.
Synthesis of hydroxylated PCB sulfate ammonium salts ((a) 2 mol% Pd(PPh3)4, K2CO3, Toluene, 80 °C, 24h; (b) 1M BBr3 in CH2Cl2, 10h; (c) 2,2,2-trichloroethyl chlorosulfate, DMAP, dry CH2Cl2, 10 h; (d) Zn powder, HCO2NH4, MeOH).
Table 1.
Synthesis of hydroxylated PCB sulfate ammonium salt.
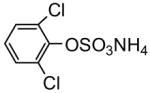
| # | TCE sulfate diester (2 and 8) | Yield/% | # | Sulfate monoester (3 and 9) | Yield/% |
|---|---|---|---|---|---|
| 2a |
|
83 | |||
| 2b |
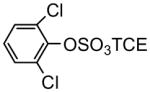
|
77 | 3b |
|
–a |
| 8a |
|
84 | 9a |
|
97 |
| 8b |
|
85 | 9b |
|
90 |
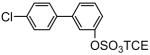
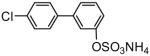
| 8c |
|
81 | 9c |
|
93 |
| 8d |
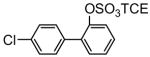
|
75 | 9d |
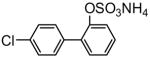
|
83 |
| 8e |
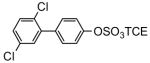
|
81 | 9e |
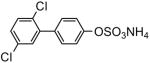
|
92 |
| 8f |
|
77 | 9f |
|
97 |
| 8g |
|
79 | 9g |
|
94 |
| 8h |
|
83 | 9h |
|
96 |
| 8i |
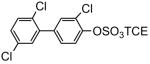
|
85 | 9i |
|
97 |
| 8j |
|
85 | 9j |
|
85 |
| 8k |
|
83 | 9k |
|
–a |
| 8l |
|
94 | 9l |
|
–a |
The respective PCB sulfate monoesters are unstable and easily degrade to the corresponding hydroxylated PCBs.
Zinc powder-ammonium formate is an efficient and mild deblocking system and can be employed with halogenated aromatic compounds without dehalogenation (Liu et al. 2004). Therefore, the TCE group was removed in the last step of the synthesis by reductive elimination using this system, with yields ranging from 83% to 97% for PCB TCE esters 8a-j (Table 1). Although the deprotection of the PCB TCE sulfate diesters 9k and 9l yield PCB sulfate monoesters according to TLC analysis, we were unable to isolate the desired product after column chromatography on silica gel with chloroform-methanol-ammonium hydroxide as eluent. Instead, the product rapidly degraded in solution to the hydroxylated starting materials 7k and 7l. Similarly, the chlorinated phenol TCE diester 2b did not yield the desired sulfate monoester, which suggests that the sulfate monoesters of phenolic compounds with two chlorine atoms in ortho position to the sulfated phenol are unstable under the conditions required for the isolation of solid products.
One possible explanation for this observation is the increasing degree of chlorination in the hydroxylated phenyl ring, which increases the acidity (pKa value: di-ortho < mono-ortho < non-ortho chloro (Tampal et al. 2002)). As a result, the hydroxylated PCBs 7k and 7l are excellent leaving groups, thus resulting in the instability of the corresponding PCB sulfate monoesters. Similarly, the stability of phenolic sulfate monoesters has been shown to correlate with the pKa of the phenol and the length of the CAr-O and O-S bond length of the sulfate monoester (Brandao et al. 2005). This interpretation is also supported by the C-O and S-O bond length observed in the molecular structures of TEC sulfate diesters 8e and 8k (see below).
The structures of the sulfate esters were confirmed by NMR, IR, and UV/Vis. In the IR spectra, we observed typical absorption bands at 1200–1220 cm−1 and 1420–1460 cm−1 (S=O of TCE-protected sulfate diesters 8), 1000–1010 cm−1 (O-S-O of TCE-protected sulfate diesters 8), 1230–1250 cm−1 and 1440–1490 cm−1 (S=O of sulfate monoester ammonium salts 9), and 1060–1070 cm−1 (O-S-O of sulfate monoester ammonium salts 9) (Ragan 1978). In the UV/Vis spectra, the λmax values of the hydroxylated PCBs 7 were always greater than the λmax values of the corresponding sulfates 9, with the difference ranging from 2 to 10 nm. These differences in λmax may be useful for the detection of PCB sulfate monoesters 9 with UV/Vis detectors.
3.2. Solid state molecular structure of PCB TCE sulfate diesters
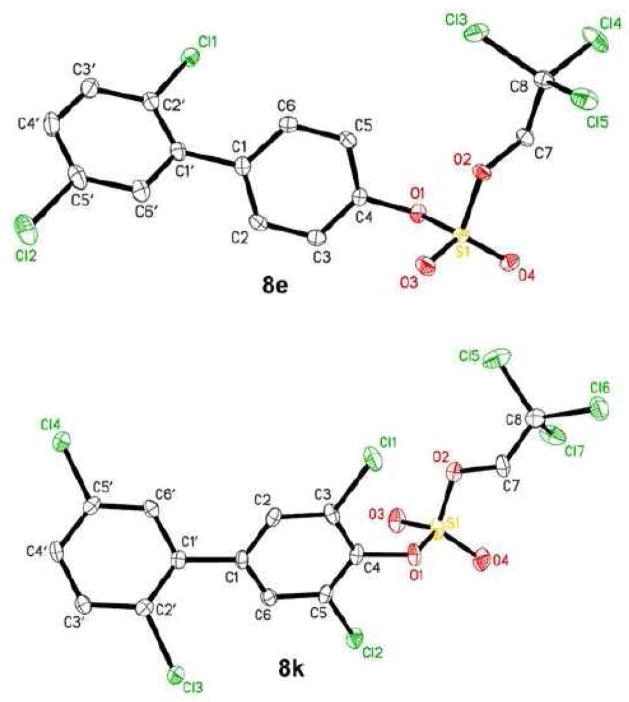
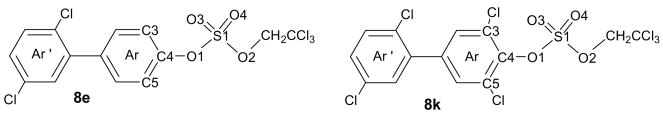
The availability of structural information of PCB sulfate monoesters would be valuable for quantitative structure activity relationship (QSAR) studies of their interaction with SULTs. Unfortunately, we were unable to obtain crystals of the PCB sulfate monoesters suitable for X-ray crystal structure determination due to their instability under the conditions employed for crystallization. Instead, we obtained single crystals of two PCB TCE sulfate diesters. The molecular structure and the labeling scheme of selected PCB sulfate diesters (8e and 8k) is shown in Figure 1. Relevant X-ray crystallographic data and selected bond lengths, angles and dihedral angles are reported in Tables 2 and 3, respectively. To the best of our knowledge, no crystal structures of similar mixed alkyl aryl esters of sulfuric acid have been reported previously.
Figure 1.
Molecular structure of sulfuric acid 2′,5′-dichloro-biphenyl-4-yl 2,2,2-trichloroethyl ester (8e) and 2′,3,5′,5-tetrachloro-biphenyl-4-yl 2,2,2-trichloroethyl ester (8k) showing the atom labeling scheme. Displacement ellipsoids of 8e and 8k are drawn at the 50% probability level.
Table 3.
Selected bond length, bond angles and dihedral angles of PCB TCE sulfate monoesters 8e and 8k.
 | |||
|---|---|---|---|
| Property | 8e | 8k | |
| Bond length (Å) | C4-O1 | 1.426(2) | 1.405(4) |
| S1-O1 | 1.5853(13) | 1.600(2) | |
| S1-O2 | 1.5684(12) | 1.564(3) | |
| S1-O4 | 1.4162(13) | 1.415(3) | |
| Bond angles (°) | O1-S1-O2 | 102.82(7) | 101.65(13) |
| O1-S1-O3 | 110.14(7) | 110.29(14) | |
| O1-S1-O4 | 104.55(7) | 104.36(14) | |
| O2-S1-O3 | 105.25(7) | 105.42(15) | |
| O2-S1-O4 | 110.17(7) | 110.25(15) | |
| O3-S1-O4 | 122.32(8) | 122.93(16) | |
| Dihedral angles (°) | Ar-Ar′ | 52.13(6) | 50.03(10) |
| Deviation of O1 from Ar plane (Å) | 0.092(5) | 0.128(5) | |
Note: Ar and Ar′ represent the aromatic rings of the biphenyl moiety.
The length of the C4-O1 and S1-O1 bonds of diesters 8e and 8k differed significantly (Table 3). While the C4-O1 bond of 8k was significantly shorter compared to 8e (1.405 Å versus 1.426 Å), the S1-O1 bond of 8k was longer compared to 8e (1.600 Å versus 1.585 Å). These differences in the bond lengths of 8e and 8k are due to the two electronegative chlorine subtituents ortho to the sulfate group of 8k. This results in a more positve partial charge on the C-4 carbon atom and, ultimately, a shorter C4-O1 bond length. At the same time, the reduced electron density on the O1 atom contributes to a longer and weaker S1-O1 bond. The increasing weakness of the S1-O1 bond is due to an increasing number of electronegative substituents (i.e., chlorines) in the phenyl ring system, and explains, at least in part, why we were unable to isolate the PCB sulfate monoesters corresponding to the di-ortho substituted TCE sulfate diesters 8k and 8l. Similarly, the C-O bond length of aromatic sulfuric acid monoesters (and ultimately their stability) correlated with the C-O bond length and, ultimatley, the pKa value of the corresponding phenol (Brandao et al. 2005).
The O1 atom of the two PCB TCE sulfate diesters was in the plane of the phenyl ring, with deviations from the ring plane of 0.092 Å and 0.128 Å for 8e and 8k, respecively. The bond lengths of the S1-O1 and S1-O2 ester bonds were similar for both compounds and ranged from 1.564 Å to 1.600 Å. In contrast, the S-O bond length in aromatic sulfate monoesters ranged from 1.611 to 1.653 Å (Brandao et al. 2005), which is slightly longer than the bond length observed for 8e and 8k. The S1-O3 and S1-O4 bond lengths were shorter in the diesters (1.409 Å to 1.416 Å) compared to monoesters (1.427 to 1.445 Å) (Brandao et al. 2005) due to the double bond character of these bonds.
The dihedral angle between the phenyl rings of a PCB congener determine its three dimensional structure and, thus, its affinity to cellular targets, such as nuclear transcription factors (Lehmler et al. 2002; Vyas et al. 2006a). The solid state dihedral angles between the two phenyl rings of PCB TCE sulfate diesters were smaller compared to the structurally-related PCB metabolites. For example, the solid state dihedral angle between the two phenyl rings of 8e (52.13°) was smaller compared to the corresponding methoxylated PCB (59.92°) (Vyas et al. 2006b). These deviations from the energetically most favorable conformations of 8e and 8k are likely due to crystal packing effects, which allow the molecule to adopt an energetically unfavorable conformation (i.e., dihedral angle) to maximize intermolecular interactions and, thus, the lattice energy in the crystal.
4. Conclusions
Hydroxylated PCBs are emerging as an important, but frequently overlooked, aspect of PCB toxicity. Little is known about the disposition of this group of PCB metabolites and their toxicity, both in rodent animal models and humans. In recent years, some hydroxylated PCBs 7 have been documented to inhibit cytosolic SULTs, whereas other hydroxylated PCBs 7 appear to be substrates for SULTs. Here, we report the first chemical synthesis of a series of PCB sulfate monoesters 8 in a four step synthesis from chlorinated benzene boronic acids 4 and brominated (chloro-)anisoles 5. Suzuki coupling of boronic acids 4 with brominated anisoles 5, followed by demethylation with BBr3 yielded the desired hydroxylated PCBs 7. Subsequently, sulfation with 2,2,2-trichloroethyl chlorosulfate and deprotection gave the desired PCB sulfate monoesters 9a–j in good-to-excellent yields. The ammonium sulfate monoesters 9 are unstable over extended periods of time and degrade to the corresponding hydroxylated PCBs. This is in particular true for PCB sulfate monoesters with two chlorine substituents ortho to the sulfate group. Most likely this is due to the increased acidity of the phenolic ring system. In summary, this series of PCB sulfate monoesters is available to study their physicochemical properties, their disposition in vivo and their interaction with SULTs and other enzymes.
Supplementary Material
Acknowledgments
This research was supported by grants ES05605, ES012475 and ES013661 from the National Institute of Environmental Health Sciences, NIH. Contents of this manuscript are solely the reponsibility of the authors and do not necessarily represent the official views of the NIEHS/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergman A, Klasson-Wehler E, Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994;102:464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao TAS, Priebe JP, Damasceno AS, Bortoluzzia AJ, Kirby AJ, Nome F. Bond length-reactivity correlations for sulfate monoesters. The crystal structure of potassium 4-nitrophenyl sulfate, C6H4KNO6S. J Mol Struct. 2005;734:205–209. [Google Scholar]
- Gunnarsson GT, Desai UR. Interaction of designed sulfated flavanoids with antithrombin: Lessons on the design of organic activators. J Med Chem. 2002;45:4460–4470. doi: 10.1021/jm020132y. [DOI] [PubMed] [Google Scholar]
- Gunnarsson GT, Desai UR. Exploring new non-sugar sulfated molecules as activators of antithrombin. Bioorg Med Chem Lett. 2003;13:679–683. doi: 10.1016/s0960-894x(02)01055-7. [DOI] [PubMed] [Google Scholar]
- Hansen LG. The ortho side of PCBs: Occurrence and disposition. Boston: Kluwer Academic Publishers; 1999. [Google Scholar]
- Hanson SR, Whalen LJ, Wong CH. Synthesis and evaluation of general mechanism-based inhibitors of sulfatases based on (difluoro)methyl phenyl sulfate and cyclic phenyl sulfamate motifs. Bioorg Med Chem. 2006;14:8386–8395. doi: 10.1016/j.bmc.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse DJ, Olavesen AH, Powell GM. Preparation and characterization of a series of 35S-labelled aryl sulphate esters for metabolic studies. Biochem Pharmacol. 1969;18:173–180. doi: 10.1016/0006-2952(69)90022-7. [DOI] [PubMed] [Google Scholar]
- Hedayatu M, Leveque JC, Denivell L. Action of sulfuryl chloride on some phenols and alcohols - alkylated and arylated phenol chlorosulfates. CR Acad Sci, Paris Ser C. 1971;273:1444–1447. [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Bignert A, Faengstroem B, et al. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern Slovakia. Environ Sci Technol. 2006;40:3696–3703. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- James MO. Polychlorinated biphenyls: Metabolism and metabolites. In: Robertson LW, Hansen LG, editors. PCBs: Recent advances in environmental toxicology and health effects. Lexington, KY: University of Kentucky Press; 2001. pp. 35–46. [Google Scholar]
- Kester MHA, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, et al. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: A novel pathway explaining the estrogenic activity of PCBs. Endocrinol. 2000;141:1897–1900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS. Intracellular signaling and developmental neurotoxicity. In: Zawia NH, editor. Molecular Neurotoxicology: environmental agents and transcription-transduction coupling. Boca Roton, FL: CRC press; 2004. pp. 151–182. [Google Scholar]
- Lehmler H-J, Parkin S, Robertson LW. The three-dimensional structure of 3,3′,5′-trichloro-4-methoxybiphenyl, a “coplanar” polychlorinated biphenyl (PCB) derivative. Chemosphere. 2002;46:485–488. doi: 10.1016/s0045-6535(01)00177-1. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Robertson LW. Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere. 2001;45:1119–1127. doi: 10.1016/s0045-6535(01)00052-2. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Klasson-Wehler E, Bergman A. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. In: Paasivirta J, editor. The handbook of environmental chemistry Vol. 3 Part K: New types of persistent halogenated compounds. Berlin Heidelberg: Springer Verlag; 2000. pp. 315–359. [Google Scholar]
- Liu Y, Apak TI, Lehmler H-J, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lien IFF, Ruttgaizer S, Dove P, Taylor SD. Synthesis and protection of aryl sulfates using the 2,2,2-trichloroethyl moiety. Org Lett. 2004;6:209–212. doi: 10.1021/ol036157o. [DOI] [PubMed] [Google Scholar]
- McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Nishino T, Nagumo T. Anticoagulant and antithrombin activities of oversulfated fucans. Carbohydr Res. 1992;229:355–362. doi: 10.1016/s0008-6215(00)90581-0. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. In: Carter CW Jr, Sweet RM, editors. Methods in Enzymology. Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Park J-S, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, et al. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in pregnant women from Eastern Slovakia. Environ Health Perspect. 2007;115:20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitou M, van Boeckel CAA. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew Chem Int Ed. 2004;43:3118–3133. doi: 10.1002/anie.200300640. [DOI] [PubMed] [Google Scholar]
- Pires L, Gorin PAJ, Reicher F, Sierakowski MR. An active heparinoid obtained by sulphation of a galactomannan extracted from the endosperm of Senna macranthera seeds. Carbohydr Polymers. 2001;46:165–169. [Google Scholar]
- Ragan MA. Phenol sulfate esters-ultraviolet, infrared, H-1 and C-13 nuclear magnetic-resonance spectroscopic investigation. Can J Chem. 1978;56:2681–2685. [Google Scholar]
- Robertson LW, Hansen LG. Recent advances in the environmental toxicology and health effects of PCBs. Lexington: University Press of Kentucky; 2001. [Google Scholar]
- Sacco JC, James MO. Sulfonation of environmental chemicals and their metabolites in the polar bear (Ursus maritimus) Drug Metab Dispos. 2005;33:1341–1348. doi: 10.1124/dmd.105.004648. [DOI] [PubMed] [Google Scholar]
- Santos GAG, Murray AP, Pujol CA, Damonte EB, Maier MS. Synthesis and antiviral activity of sulfated and acetylated derivatives of 2 beta, 3 alpha-dihydroxy-5 alpha-cholestane. Steroids. 2003;68:125–132. doi: 10.1016/S0039-128X(02)00166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur AG, Brouwer A, Bergman; Coughtrie MWH, Visser TJ. Inhibition of thyroid hormone sulfation by hydroxylated metabolites of polychlorinated biphenyls. Chem-Biol Interact. 1998a;109:293–297. doi: 10.1016/s0009-2797(97)00140-3. [DOI] [PubMed] [Google Scholar]
- Schuur AG, Legger FF, van Meeteren ME, Moonen MJH, van Leeuwen-Bol I, Bergman A, et al. In vitro inhibition of thyroid hormone sulfation by hydroxylated metabolites of halogenated aromatic hydrocarbons. Chem Res Toxicol. 1998b;11:1075–1081. doi: 10.1021/tx9800046. [DOI] [PubMed] [Google Scholar]
- Schuur AG, van Leeuwen-Bol I, Jong WMC, Bergman A, Coughtrie MWH, Brouwer A, et al. In vitro inhibition of thyroid hormone sulfation by polychlorobiphenylols: Isozyme specificity and inhibition kinetics. Tox Sci. 1998c;45:188–194. doi: 10.1006/toxs.1998.2504. [DOI] [PubMed] [Google Scholar]
- Sheldrick GM. A short history of SHELX. Acta Cryst. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20:439–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Simpson LS, Widlanski TS. A comprehensive approach to the synthesis of sulfate esters. J Am Chem Soc. 2006;128:1605–1610. doi: 10.1021/ja056086j. [DOI] [PubMed] [Google Scholar]
- Tampal N, Lehmler H-J, Espandiari P, Malmberg T, Robertson LW. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) Chem Res Toxicol. 2002;15:1259–1266. doi: 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- van den Hurk P, Kubiczak GA, Lehmler HJ, James MO. Hydroxylated polychlorinated biphenyls as inhibitors of the sulfation and glucuronidation of 3-hydroxy-benzo[a]pyrene. Environ Health Perspect. 2002;110:343–348. doi: 10.1289/ehp.02110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas SM, Parkin S, Lehmler H-J. 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl (PCB 180) Acta Cryst. 2006a;E62:o2905–o2906. [Google Scholar]
- Vyas SM, Parkin S, Lehmler H-J. 2,5-Dichloro-4′-methoxybiphenyl. Acta Cryst. 2006b;E62:o4162–o4163. [Google Scholar]
- Wang L-Q, Lehmler H-J, Robertson LW, Falany CN, James MO. In vitro inhibition of human hepatic and cDNA-expressed sulfotransferase activity with 3-hydroxybenzo[a]pyrene by polychlorobiphenylols. Environ Health Perspect. 2005;113:680–687. doi: 10.1289/ehp.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-Q, Lehmler H-J, Robertson LW, James MO. Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate. Chem Biol Interact. 2006;159:235–246. doi: 10.1016/j.cbi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Wilson AJC. Vol C: Mathematical, physical and chemical tables. Kluwer Academic Publishers; Holland: 1992. International tables for crystallography. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.