Abstract
Concerns about infections caused by orthopoxviruses, such as variola and monkeypox viruses, drive ongoing efforts to develop novel smallpox vaccines that are both effective and safe to use in diverse populations. A subunit smallpox vaccine comprising vaccinia virus membrane proteins A33, B5, L1, A27 and aluminum hydroxide (alum) ± CpG was administered to non-human primates, which were subsequently challenged with a lethal intravenous dose of monkeypox virus. Alum adjuvanted vaccines provided only partial protection but the addition of CpG provided full protection that was associated with a more homogeneous antibody response and stronger IgG1 responses. These results indicate that it is feasible to develop a highly effective subunit vaccine against orthopoxvirus infections as a safer alternative to live vaccinia virus vaccination.
Keywords: Smallpox, Monkeypox, Vaccinia virus, Subunit vaccine, CpG, Aluminum hydroxide, Baculovirus expressed proteins
1. Introduction
Smallpox was eradicated worldwide by a 1970s campaign led by the World Health Organization [1]. However, the possibility of accidental or purposeful re-introduction of variola virus has led governments to stockpile live vaccinia virus (VACV) vaccines like ACAM2000™ derived from the Dryvax® vaccine [2], [3]. Serious side effects can accompany live vaccinia-based vaccines, especially in immunocompromised people and those with common skin diseases. This has encouraged efforts to develop safer smallpox vaccines [4], [5], [6], [7]. Modified vaccinia Ankara (MVA), a highly attenuated smallpox vaccine under development [8], may be safer but requires high doses. Zoonotic human infections with monkeypox virus (MPXV) in the USA [9] further illustrate the need for safe and effective vaccines against other poxviruses. Our approach has been to develop and test the efficacy of a subunit protein-based vaccine.
Identification of target antigens for a subunit vaccine is challenging because poxviruses encode hundreds of proteins and their complex life cycle produce two infectious forms: mature virus (MV) and extracellular virus (EV) [10], [11]. MV is an enveloped virus with many surface proteins required for infectivity [11]. EV has an additional membrane surrounding the MV particle with another set of unique membrane proteins [10], [11]. Both forms are important in viral acquisition and spread. Subunit vaccines under development usually contain antigens from both envelopes [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. Here we report that vaccination of non-human primates (NHP) with purified protein ectodomains of A33 and B5 derived from EV plus L1 and A27 derived from MV, combined with the adjuvants Alhydrogel and CpG, provided full protection of NHPs from lethal intravenous challenge with MPXV. Our results clearly show the feasibility of developing safe and effective subunit vaccines for human use against smallpox and monkeypox.
2. Materials and methods
2.1. Non-human primates
Two separate NHP vaccination and challenge studies were performed in cynomolgus macaques (Macaca fascicularis) with three- or two-dose regimens. The 3-dose study involved 30 macaques (16 females, 14 males) obtained from Three Spring Scientific (Perkasie, PA) then vaccinated and challenged at Southern Research Institute (SRI, Frederick, MD). The 2-dose study involved 12 macaques (7 females, 5 males) obtained from Charles River Laboratories (Reno, NV), quarantined (3 months) and vaccinated at University of Maryland School of Medicine (Baltimore, MD) then challenged at SRI. All animal facilities are approved by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International, procedures were in accordance with relevant federal policies and guidelines, and protocols were approved by Institutional Animal Care and Use Committees.
2.2. VACV proteins
For this study, recombinant proteins were produced by infecting whole insect larvae with recombinant baculoviruses (details in a manuscript in preparation). Briefly, recombinant A33, B5, L1, and A27 [24], [25], [26] were produced by infecting insect larvae (cabbage looper moth, Trichoplusia ni) [27], [28] with recombinant baculoviruses (Autographa californica multiple nuclear polyhedrosis virus) expressing individual histidine-tagged VACV proteins. After metal affinity chromatography, additional ion exchange polishing and formulation chromatographic steps were added to ensure very high purity proteins (>98% by digitized Coomassie image analysis) that were largely free of contaminating host proteins/proteases. The protein preparations were verified to have endotoxin levels below FDA guidance levels.
2.3. Vaccinations
Vaccines comprised three or four antigens (20 or 100 μg each) adsorbed to aluminum hydroxide at 8.25 or 16.5 μg aluminum ion/μg protein (Alhydrogel, Accurate Chemical & Scientific Corp., Westbury, NY). Some formulations included B-Class CpG oligodeoxynucleotide TLR9 agonists (500 μg/vaccine dose), namely CPG7909 (sequence 5′-TCG TCG TTT TGT CGT TTT GTC GTT-3′) and CpG10104 (sequence 5′-TCG TCG TTT CGT CGT TTT GTC GTT-3′) (Coley Pharmaceutical Group, now Pfizer Inc.), in the 3-dose and 2-dose studies respectively. These CpG's differ by a single nucleotide and have similar activity in mice and NHP (H. Davis, unpublished). The change to CpG 10104 in the second study occurred because CPG 7909 had been dedicated for immune therapy in oncology indications by Pfizer. For the 3-dose study, monkeys (5/group; Table 1 ) were intramuscularly vaccinated at 0, 4, and 12 weeks with 1 mL of vaccine containing 3 proteins (ABL, 100 μg each) plus CpG/alum or 4 proteins (ABLA, 20 or 100 μg each) plus alum or CpG/alum. In the 2-dose study, monkeys (3/group; Table 2 ) were vaccinated at 0 and 4 weeks with 4 proteins (ABLA, 100 μg each) plus CpG/alum (2 different alum ratios). Both studies included a non-vaccinated control group (CpG/alum without proteins) and a positive control group receiving Dryvax® (Lot # 4020075; CDC) administered at a single time (day 0) by scarification (∼2.5 × 105 pfu live VACV) with 15 pricks between the shoulder blades using bifurcated needle. (At the time of initiation of these studies, Dryvax was the only FDA approved vaccine for smallpox. ACAM2000 has since replaced Dryvax and is considered to have similar immunogenicity.) All monkeys were bled prior to vaccination, 2 weeks after each vaccination, and just prior to challenge for immunogenicity measures.
Table 1.
Three-dose vaccination study (5 monkeys/group).
| Vaccine formulation |
Monkey number | PRNT50 d | Mean/median number of lesions |
|||||
|---|---|---|---|---|---|---|---|---|
| Groupa | Proteinb | Protein amount (μg/protein) | Adjuvantc | Number of lesions in individual monkeys |
||||
| Day 6 | Day 9 | Day 12 | ||||||
| 1. Control | – | – | CpG/alum | 4342 | <10 | TNTCe | TNTCe | TNTCeB |
| 4348 | <10 | 116 | TNTCeA | |||||
| 4354 | <10 | TNTCe | TNTCeA | |||||
| 4357 | <10 | TNTCe | TNTCe | TNTCeC | ||||
| 4364 |
<10 |
TNTCe |
TNTCeA |
|||||
| 2. Dryvax | Dryvax | – | – | 7/2 | 0/0 | 0/0 | ||
| 4347 | 208 | 2 | 0 | 0 | ||||
| 4353 | 140 | 0 | 0 | 0 | ||||
| 4362 | 351 | 0 | 0 | 0 | ||||
| 4369 | <10 | 31 | 0h | 0 | ||||
| 4371 |
313 |
2 |
2 |
0 |
||||
| 3. ABLA(100) | ABLA | 100 | CpG/alum | 11/3 | 32/17 | 3/0 | ||
| 4345 | 1,895 | 3 | 26 | 0h | ||||
| 4351 | >10,240 | 15 | 7 | 4h | ||||
| 4360 | 297 | 1 | 17 | 0h | ||||
| 4363 | 1,847 | 0 | 2 | 0h | ||||
| 4367 |
132 |
34 |
108 |
10h |
||||
| 4. ABLA(20) | ABLA | 20 | CpG/alum | 11/3 | 25/21 | 12/0 | ||
| 4346 | 6,613 | 3 | 49 | 0h | ||||
| 4352 | 1,836 | 0 | 0 | 0 | ||||
| 4361 | 3,744 | 3 | 12 | 4h | ||||
| 4368 | 281 | 6 | 21 | 0h | ||||
| 4370 |
326 |
45 |
99 |
54h |
||||
| 5. ABLA (alum only) | ABLA | 100 | Alum only | 35/15 | 95/73 | 100/14f | ||
| 4344 | 544 | 4 | 7 | 13h | ||||
| 4350 | 1,728 | 0 | 2 | 16h | ||||
| 4356 | 455 | 128 | 312 | TNTCg | ||||
| 4359 | 279 | 15 | 73 | 14h | ||||
| 4366 |
123 |
29 |
80 |
8h |
||||
| 6. ABL(100) | ABL | 100 | CpG/alum | 34/19 | 167/179 | 180/224 | ||
| 4343 | 287 | 19 | 180 | 224h | ||||
| 4349 | 1,054 | 46 | 323 | 12h | ||||
| 4355 | 247 | 10 | 179 | 404h | ||||
| 4358 | 30 | 2 | 2 | 0 | ||||
| 4365 | 193 | 95 | 152 | 258h | ||||
Group number and abbreviations of vaccine type used in text.
Protein where A,B,L,A stands for A33, B5, L1, A27, respectively.
Adjuvant where CpG/alum stands for CPG 7909 and aluminum hydroxide (Alhydrogel).
PRNT50, pre-challenge (day 121) 50% plaque reduction neutralizing titer against MPXV.
TNTC, too numerous to count. (A) Three control animals needed to be euthanized on day 11; (B) 1 on day 13; and (C) 1 on day 15.
For calculation of mean and median value, the lesion count was set at 500 for the monkey with TNTC lesions on day 12.
One in ABLA/alum only needed to be euthanized on day 13.
Lesions present are mostly or only scabs.
Table 2.
Two-dose vaccination study (3 monkeys/group).
| Vaccine formulation |
Monkey number | PRNT50 d | Number of lesions in individual monkeys |
|||||
|---|---|---|---|---|---|---|---|---|
| Groupa | Proteinb | Protein amount (μg/protein) | Adjuvantc | Day 6 | Day 10 | Day 14 | ||
| A. Control | – | – | CpG/alum | 4372 | <10 | TNTCe | ||
| 4376 | <10 | TNTCe | ||||||
| 4380 | <10 | TNTCe | ||||||
| B. Dryvax | Dryvax | – | – | 4381 | 383 | 13 | 0f | 0f |
| 4382 | 102 | 21 | 0 | 0 | ||||
| 4383 | 497 | 18 | 0f | 0 | ||||
| C. ABLA(8.25) | ABLA | 100 | CpG/alum(8.25) | 4377 | 9,387 | 0 | 5 | 0f |
| 4378 | 151 | 5 | 11 | 0f | ||||
| 4379 | 1,440 | 0 | 13 | 0 | ||||
| D. ABLA(16.5) | ABLA | 100 | CpG/alum(16.5) | 4373 | 1,800 | 36 | 0f | 0f |
| 4374 | 7,235 | 24 | 21f | 0f | ||||
| 4375 | 1,984 | 0 | 0 | 0 | ||||
Group letter and abbreviations of vaccine type used in text.
Protein where ABLA stands for A33, B5, L1, A27.
Adjuvant where CpG/alum stands for CpG 10104 and aluminum hydroxide (Alhydrogel), used at either 8.25 μg of aluminum ion/μg of protein as in the study in Table 1 or at 16.5 μg of aluminum ion/μg of protein to increase the amount of aluminum hydroxide.
PRNT50, pre-challenge 50% plaque reduction neutralizing titer against MPXV.
TNTC, too numerous to count. The three control animals needed to be euthanized on day 8.
Lesions present are mostly or only scabs.
2.4. MPXV challenge
Anesthetized monkeys were challenged 5 weeks (3-dose study) or 4 weeks (2-dose study) after the last subunit vaccine dose by intravenous infusion into the saphenous vein of 1.0 mL media containing 2 × 107 pfu MPXV (NR523, BEI Research Resources Repository, Manassas, VA). The challenge inoculum, which was back titered on Vero E6 cells using a plaque assay to confirm dose, was selected to be lethal and indeed all control animals and one monkey receiving ABLA/alum required euthanasia upon meeting a predetermined set of criteria of disease progression. Post-challenge, blood was drawn every third day and DNA extracted from 200 μL of blood for viral load (VL) determination by real-time PCR [16]. The detection limit of the viral load assay was 5000 genome copies/mL blood. Animals were monitored for activity, temperature, weight, appetite, and development of pock lesions.
2.5. Antibody ELISA
Standard ELISA determinations used as capture antigens either purified VACV (0.6 μg/mL in PBS) or recombinant proteins (A33, B5, or L1 at 2.5 μg/mL; or A27 at 0.625 μg/mL) in PBS coated overnight at 4 °C. After blocking, 2-fold dilutions of sera were incubated for 1 h at 37 °C. After extensive washes, the secondary antibody, HRP-conjugated goat anti-monkey IgG (KPL) at 1:2000 was incubated for 1 h at 37 °C followed by color development with ABTS substrate (Sigma) for ∼20 min at RT. IgG isotypes were determined by coating plates with non-his tagged versions of B5 or L1 at 1 μg/mL and after incubation with sera, HRP-conjugated anti-human total IgG, IgG1, IgG2, IgG3, or IgG4 (Binding Site, Birmingham, UK) diluted at 1:1000 was added and incubated for 1 hr at 37 °C.
2.6. Depletion of sera of anti-L1 antibodies
Recombinant L1 protein (1 mg) was coupled to 0.5 g of CNBr-activated Sepharose 4B beads (Amersham Biosciences) following the manufacturer's instructions. Mock-coupled beads were processed similarly except without protein addition. Beads were washed and resuspended in 500 μL PBS. Equal volumes of sera from the 5 monkeys in each of the ABLA/CpG/alum or ABL/CpG/alum groups in the 3-dose study were combined, heat inactivated, and 20 μL of pooled serum was incubated with 180 μL of L1-coupled or mock-coupled sepharose beads overnight at 4 °C. Beads were pelleted, the supernatant collected, and L1 depletion confirmed by L1 or A27 ELISA as above, except plates were coated with each protein at 1 μg/mL. Supernatants, stored at 4 °C, were used for neutralization experiments.
2.7. Virus neutralization
For MXPV 50% plaque neutralization reduction titers (PNRT50), 4-fold dilutions of sera (in 225 μL of media) were mixed with 225 μL purified MPXV MV (450 pfu/well). After overnight incubation at 37 °C, 100 μL was removed and plated in triplicate on Vero cells for titering. VACV (strain WR) MV was used in neutralization studies with the L1-depleted and control depleted sera. MV neutralization assays were carried out in triplicate in a final volume of 100 μL containing ∼200 pfu of MV, dilutions of sera from individual monkeys at 1:20 to 1:2000, and incubated for 2 h at 37 °C. Samples of 90 μL were titered on confluent wells of a 6-well tissue culture plate. After 36–48 h, wells were stained with crystal violet, plaques counted, and normalized to control wells that contained MV incubated in media alone. EV neutralization was carried out with VACV EV. EV was isolated from VACV (strain IHDJ) infected RK13 cells in MEM (without FBS) as previously described [29]. The media from infected cells was clarified by low speed centrifugation and EV was titered in the presence of the MV neutralizing monoclonal antibody (mAb) 2D5. EV neutralization assays were carried out in triplicate in a final volume of 100 μL containing ∼200 pfu EV, the indicated dilution of sera from individual monkeys, the anti-L1 mAb 2D5 (to neutralize contaminating MV), and with or without 10 μL of guinea pig complement (Complement Technology, Inc., Tyler, TX) for 30 min at 37 °C. 90 μL of each sample were then titered.
2.8. Enzyme-linked immunospot (ELISPOT) assay
Using a commercial human ELISPOT (Cell Sciences, Norwood, MA) with demonstrated cross-reactivity with non-human primates, IFN-γ-secreting peripheral blood mononuclear cells (PBMC) obtained from vaccinated macaques from the 2-dose study were measured. Isolated PBMC were added to blocked wells at a density of 1 × 105 cells/well in 100 μL complete RPMI growth medium containing 5 × 105 pfu VACV plus 5% FBS. Triplicate wells of unstimulated PBMC or PBMC stimulated with Con A or staphylococcus endotoxin B were included as negative and positive controls. After overnight incubation at 37 °C in 5% CO2, plates were washed and IFN-γ-secreting spot forming cells (SFC) were counted using the AID ELISPOT Reader System (Cell Technology, Inc., Columbia, MD) and expressed as number of IFN-γ-secreting cells/106 PBMC.
2.9. Statistical analysis
For the 3-dose study (5/group), statistical analyses were performed with SAS™ Version 9.1 using an alpha = 0.05, except where indicated. The dilution ratios for ELISA proteins at an optical density (OD) = 0.2 were calculated by point-point regression between ELISA OD readings immediately <0.2 and >0.2 OD. The dilution values calculated at OD = 0.2 were used for statistical analyses. A dilution of 0 was given where ELISA optical density readings were <0.2. Group median dilutions, PRNT50 levels, and viral loads were compared by Kruskal-Wallis ANOVA. Pair-wise comparisons for significant differences in viral loads across groups were performed for Groups 1–6 (Dunn's test). Significant changes in PRNT50 levels from a baseline level at day 98 were tested by Wilcoxon Rank Signed Test. Kaplan-Meier survival plots were calculated for vaccination groups following MPXV challenge (days 0–27) and survival curves for each vaccination group were compared by Cox-Mantel test; Spearman's rank-order correlation test was used on the log[PRNT50] versus lesion numbers and viral loads at days 6 and 9 post-challenge (GraphPad Prism™, Version 5). The effect of vaccination group on weight and temperature was tested with a repeated measures analysis of variance (PROC MIXED, SAS™).
3. Results
3.1. Vaccine safety
The adjuvanted subunit vaccines were well tolerated by all animals with no adverse reactions.
3.2. Challenge with MPXV
3.2.1. Negative and positive vaccine controls
We analyzed clinical symptoms and survival post-challenge (Fig. 1A), lesion counts (Fig. 1B), and viral loads (Fig. 2 ) in both vaccinated and non-vaccinated animals. The non-vaccinated control animals (Group 1) exhibited typical signs of MPXV disease and experienced depression, lethargy, and pock lesions after challenge that became too numerous to count (TNTC) by day 6 (Table 1 and Fig. 1B). Peak viral loads (VL) were between 107 and 109 genome copies/mL by day 9 or 12 (Fig. 2). These animals met endpoint criteria for euthanasia between days 11 and 15 (Fig. 1A). Animals vaccinated with the positive control gold standard Dryvax vaccine (Group 2), all survived and exhibited little or no clinical signs of infection; the few pock lesions that developed healed quickly (Fig. 1B), and had no measurable VL at any time except for animal #4353 with a VL of ∼2 × 104 genome copies/mL on day 3 post-challenge (p.c.) (Fig. 2). When we compared Group 2 with Group 1, there were statistically lower viral loads in Group 2 at all time points (Dunn's multiple comparison test; P < 0.05). Similar results were seen for negative and positive control groups in the smaller 2-dose study where all non-vaccinated control monkeys (Group A) met endpoints that required euthanasia on day 8 p.c. and the Dryvax vaccine protected all the monkeys (Group B) from MPXV disease (Table 2).
Fig. 1.
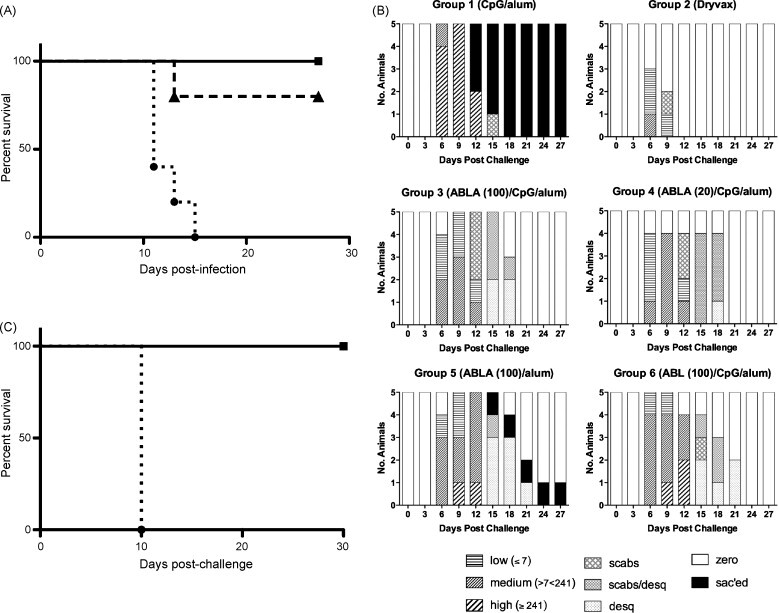
Kaplan-Meier survival analyses and pock lesion summary. (A) Kaplan-Meier survival plots following MPXV challenge for the 3-dose study with 5 monkeys/group. Solid line with squares: Dryvax (Group 2), ABLA/CpG/alum (Groups 3 and 4), and ABL/CpG/alum (Group 6); dashed line with triangles: ABLA/alum (Group 5); dotted line with circles: CpG/alum control (Group 1). (B) Frequency of each lesion category for vaccination groups at days 0–27 post-challenge. The upper and lower quartiles of lesion frequencies for numeric lesion data were calculated and plotted. The 25th (1 to ≤7 lesions) and 75th percentiles (≥241 lesions) were used to recode the numeric lesion data into low (≤25th), medium (25–75th) and high (≥75th) lesion categories. For this analysis, lesion counts that were recorded as “TNTC” (too numerous to count) were set at ≥500 lesions. High lesions (≥241) were observed for all animals in Group 1 by day 9 post-challenge. High lesions were observed in 1 of 5 animals in Group 5 by days 9 and 2 of 5 animals in Group 6 by day 12. All animals in Group 3 experienced low (≤7) to medium (<241) lesions by day 9. All lesions of surviving animals in Groups 2–6 were healed by day 24, post-challenge. C. Kaplan-Meier survival plots following MPXV challenge for the 2-dose study with 3 monkeys/group. Solid line with squares: Dryvax (Group B) ABLA/CpG/alum (Groups C and D); dotted line with circles: CpG/alum control (Group A).
Fig. 2.
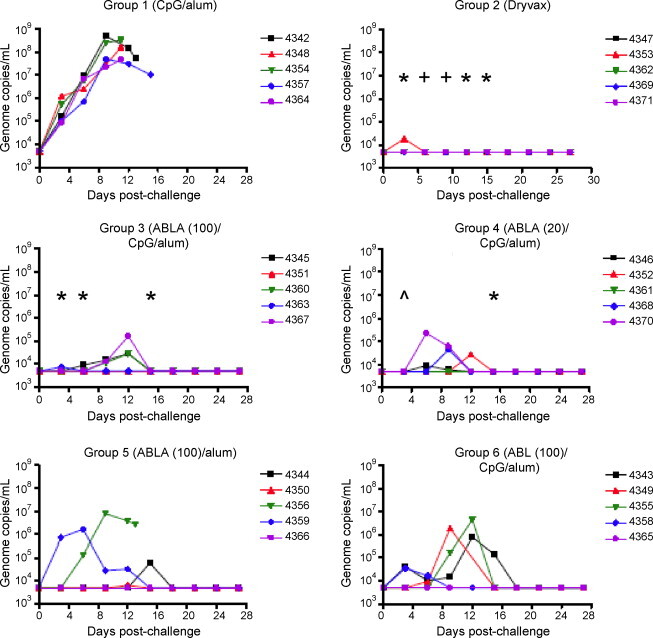
Viral load on days 0–27 post-challenge. Graphed are the post-challenge viral load by group and monkey. The detection limit of the viral load assay was 5000 genome copies/mL blood. Median VL and inter-quartile ranges were determined and Dunn's multiple comparison test was performed on vaccination pairs where a significant main effect of vaccination groups was found. A higher viral load was found for Group 1 compared to (i) Group 2 (Dryvax) at days 3–15 (P < 0.05); (ii) Group 3 (ABLA(100)/CpG/alum) at days 3, 6, and 15 (P < 0.05); and (iii) Group 4 (ABLA(20)/CpG/alum) at days 3 and 15 (P < 0.05). *P < 0.05, ^P < 0.01, +P < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.2.2. Subunit vaccine provides protection
All animals vaccinated with three doses of subunit vaccines containing CpG/alum (Groups 2, 4, and 6) survived (; P = 0.0015 relative to control Group 1), and all but one in Group 5 (ABLA/alum) survived (; P = 0.0088) (Fig. 1A). Groups vaccinated with ABLA/CpG/alum also had statistically lower VL than controls at days 3, 5, and 12 p.c. (Dunn's multiple comparison test; P < 0.05; Fig. 2, Groups 3 and 4). In all groups, monkeys with the highest VL exhibited the most lesions.
3.2.3. Benefit of CpG
In the 3-dose study, addition of CpG to the tetravalent vaccine resulted in more consistent control of infection than with alum as the sole adjuvant as indicated by fewer lesions (Table 1 and Fig. 1B) and lower VL (Fig. 2; Groups 3 and 4 versus 5). Indeed, with just alum, the VL at all time points p.c. were not significantly different than non-vaccinated controls (Dunn's multiple comparison test; P > 0.05). Also, a dose of 20 μg/protein with CpG and alum gave equivalent results for survival, lesions, and VL as a dose of 100 μg/protein (Groups 3 versus 4) indicating that lower antigen doses can be used when CpG is included. In the 2-dose study we compared CpG containing vaccines with two doses of alum, either 8.25 aluminum ion/μg of protein (as used in the 3-dose study) or 16.5 (Table 2, Groups C and D). We found no difference in protection (Fig. 1C and Table 2), although only 3 monkeys per group may be inadequate to detect differences.
3.2.4. Trivalent versus tetravalent protein vaccines and three versus two-dose regimen
The CpG/alum adjuvanted trivalent vaccine protected animals from death, as did the tetravalent vaccines (Fig. 1B, Groups 3, 4, and 6), but there were fewer lesions and lower VL with ABLA (20 or 100 μg) than with ABL (100 μg), suggesting a role for A27 in protection. In the 2-dose study, the tetravalent vaccine (100 μg/protein) with CpG/alum provided excellent protection. One monkey showed no lesions at any time tested (Fig. 1C and Table 2). While this study was run separately from the 3-dose study, the results were similar for both.
3.3. Vaccine immunogenicity and correlation with protection
Since protection from secondary poxvirus infections is primarily antibody-mediated [16], [30], [31], we focused on evaluating antibody responses (Supplementary Figure). In the 3-dose study, animals vaccinated with a single Dryvax scarification developed VACV-specific antibodies early (day 14) but as expected, titers did not increase at later times (Supplementary Figure). Monkeys vaccinated with subunit vaccines had no detectable VACV-specific antibody responses post-prime but exhibited strong responses by day 42 (2 weeks post 1st boost). These titers increased further by day 98 (2 weeks post 2nd boost). While there were no significant differences between groups in antibody titers to individual proteins, due to high variability and small number of animals, we did note trends indicating that 100 μg/protein of the tetravalent vaccine was better than 20 μg/protein, and CpG/alum was better than alum alone, with the highest titers being found with ABLA(100)/CpG/alum (Supplementary Figure).
3.3.1. Neutralization activity correlates with protection
The tetravalent vaccines adjuvanted with CpG/alum (Groups 3 and 4) were the only formulations that yielded better pre-challenge neutralization titers against MPXV MV than non-vaccinated controls (P < 0.01) (Table 1). The 100 μg antigen dose developed the best pre-challenge responses, which did not increase appreciably post-challenge. In contrast, while pre-challenge PRNT50 values for Dryvax were not “statistically significant” (but at similar levels to published studies [32], [33]), the post-challenge (days 9, 18 and 27 p.c.) PRNT50 values with Dryvax increased over pre-challenge values (P < 0.05) and were significantly higher than non-vaccinated controls (P < 0.001). They were also higher than the titers of the ABL/CpG/alum group (P < 0.05). There was an inverse relationship between pre-challenge PRNT50 and post-challenge VL or lesion numbers (Fig. 3 ).
Fig. 3.
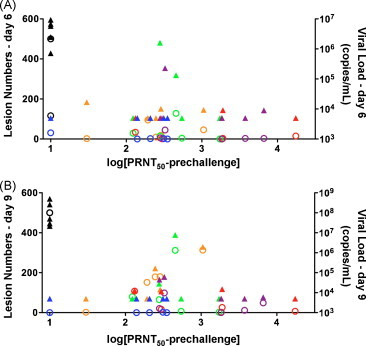
Relationship between PRNT50, lesion number, and viral load. Post-challenge lesion counts (open circles) and viral loads (solid triangles) at day 6 (A) and day 9 (B) are plotted against the log of the pre-challenge PRNT50. Various colored symbols represent each different vaccination group: Group 1 (adjuvant only) black; Group 2 (Dryvax) blue; Group 3 (ABLA(100)/CpG/alum) red; Group 4 (ABLA(20)/CpG/alum) purple; Group 5 (ABLA/alum) green; Group 6 (ABL/CpG/alum) orange. For this analysis, “TNTC” lesions were set at 500 lesions. Correlations between pre-challenge neutralization (log[PRNT50]) and day 6 lesions (r = −0.5433 P = 0.0019) and viral loads (r = −0.3997 P = 0.0287) and day 9 lesions (r = −0.3929 P = 0.0318) and viral loads (r = −0.3928 P = 0.0318) were statistically significant.
3.3.2. Antibody isotype contributes to protection
CpG, a Th1 adjuvant, had a clear effect on protection (Table 1, Groups 3 and 4 versus 5). Although antibody isotype is a less clear indicator of Th-bias in primates than mice, we examined anti-B5 and anti-L1 specific responses after the third vaccine dose (day 98). We found IgG1 and IgG2, but not IgG3 and IgG4, which possibly were not detected due to use of human reagents. The anti-B5 isotype response was more homogeneous in vaccines formulated with CpG/alum than with alum alone. Moreover, the CpG/alum adjuvanted vaccine showed a consistent IgG1:IgG2 ratio >2 (Fig. 4A), whereas the ratio of IgG1:IgG2 for alum alone was closer to one (Fig. 4B). Importantly, IgG1 responses alone were consistently high in all NHPs vaccinated with CpG/alum adjuvanted vaccine, while only 2 of 5 NHPs vaccinated with alum only showed high IgG1 titers. Antibody isotype determines function, and IgG1 antibodies are known to activate complement [34]. When we used concentrations of sera that did not efficiently neutralize EV, addition of active complement consistently enhanced EV neutralization in animals vaccinated with ABLA(100)/CpG/alum but had an enhancing effect in only 2 of 5 animals that received ABLA(100)/alum (Fig. 4C).
Fig. 4.
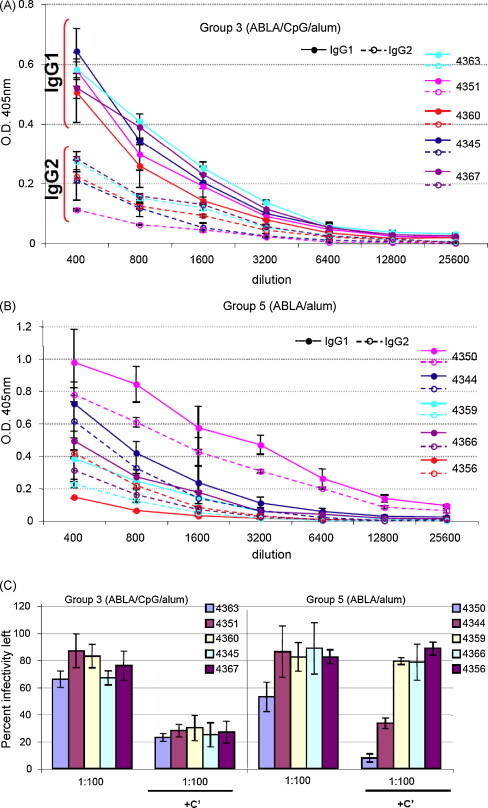
Protein vaccines adjuvanted with CpG/alum provide higher IgG1 to IgG2 ratios that result in more consistent complement-enhanced neutralization of EV. (A) IgG1 and IgG2 isotype responses to B5 in Group 3 (ABLA(100)/CpG/alum) at day 98 (2 weeks after the second boost). (B) IgG1 and IgG2 isotype responses to B5 in Groups 5 (ABLA/alum) at day 98 (2 weeks after the second boost). Solid lines with solid symbols: IgG1 responses. Dashed lines with open symbols: IgG2 responses. (C) EV neutralization by NHP sera on day 98 (2 weeks after the second boost) in the absence and presence of complement (C′). Error bars represent standard deviation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.3.3. Anti-A27 antibodies do not neutralize virus
While the trivalent and tetravalent vaccines adjuvanted with CpG/alum protected NHP from death after lethal challenge with MPXV, we were surprised that pock lesion counts with the trivalent vaccine (Group 6) were much higher than with the tetravalent vaccine (Group 3, Table 1). In mice, A27 could not substitute for L1 in a trivalent vaccine, possibly because the anti-A27 antibodies do not neutralize MV [20]. To determine whether the monkeys developed a neutralizing antibody response to A27, we depleted anti-L1 antibodies by passing pooled sera from the vaccinated groups through L1-coupled (or uncoupled) sepharose beads. ELISA showed no remaining anti-L1 titers, but titers to A27 were unchanged (data not shown). We found that if L1 antibodies were depleted, sera no longer was able to neutralize MV (data not shown), indicating that the monkeys did not develop neutralizing response to A27.
3.3.4. Role of cellular immunity for protection by the protein-based vaccine is unclear
Interferon (IFN)-γ ELISPOT after VACV re-stimulation of PBMCs was used to assess cellular immunity. PBMCs from monkeys in 2-dose study were isolated ∼3 weeks after the boost and prior to challenge. As expected for a live vaccine, Dryvax induced strong cellular responses with 80–130 IFN-γ SFC/106 PBMC for all 3 monkeys. In contrast, only one of the 6 animals that received the tetravalent subunit vaccine with CpG/alum had a measurable, albeit low response (30 IFN-γ SFC/106 PBMC).
4. Discussion
Recent life-threatening complications [35], [36] with the FDA approved live VACV vaccine highlight the need for safer vaccines against orthopoxviruses, for which the threat of infection exists through zoonosis (e.g., monkeypox) or inadvertent or purposeful release of smallpox. The highly attenuated MVA, which does not produce infectious virions in human cells, is thought to be a safer attenuated VACV vaccine [8]. However, it continues to rely on a live virus and requires 1000-times the dose of the current live VACV vaccine. Subunit vaccines comprising recombinant protein antigens may be safe for all individuals and could be a useful future alternative, particularly if live virus vaccines are no longer acceptable at those times. Such a subunit vaccine would be useful in the pre-event setting to provide baseline immunity should newly introduced orthopoxviruses cause serious human infections. Also, a protein vaccine might enhance the safety of a live vaccinia vaccine in the event that a fully replication competent vaccine be deemed necessary to control a significant smallpox outbreak. Alternatively, it could also be useful to boost responses in the older population who had received childhood smallpox vaccines, leaving potentially limited supplies of live vaccine for those at greatest risk. Herein, we have shown the ability of a trivalent (A33, B5, and L1 (ABL)) or tetravalent (ABL + A27 (ABLA)) protein-based adjuvanted vaccine to elicit humoral immune responses in monkeys that protect against lethal MPXV challenge.
While a direct correlate of immunity to poxvirus infections is still unknown, our data suggest that the best results are obtained when the protein vaccine elicits a high amount of IgG1 isotype antibodies that are able to neutralize EV in the presence of complement. These results are consistent with findings by the Crotty group showing the importance of the Fc domain in antibody protection of poxvirus infections [37], [38]. They found that anti-B5 mAbs (that poorly neutralized EV on their own) were quite potent at neutralizing EV in the presence of complement. Furthermore, treatment of mice with anti-B5 mAbs with complement fixing isotypes were better at protecting mice from VACV challenge. In future studies of smallpox vaccines in humans or NHPs, inducing a high titer of IgG1 antibodies may correlate with improved protection for the vaccine and thus IgG1 titers should be evaluated.
Our earlier work in mice indicated that ABL and ABLA (both formulated with CpG/alum) provided similar protection from intranasal challenge with VACV or ectromelia virus [20]. Thus, we were surprised that the tetravalent vaccine clearly outperformed the trivalent protein vaccine for clinical outcomes (pock lesions and VL) in NHPs. The role of A27 is not clear since we found that anti-A27 antibodies did not neutralize MV. It is noteworthy that a recent report from the Moss group found neutralizing antibodies to A27, but the protein was still less protective than the L1 protein [39]. T-helper epitopes within A27 may improve responses to other antigens that enhance protection. Another possibility is that the addition of A27 resulted in physiochemical changes in the tetravalent vaccine formulation that enhanced protection. Studies to better understand this are underway. Our results are consistent with others using the same antigens. ABL/CpG/alum provided similar protection to that reported by the Moss group with ABL/QS21 (a saponin adjuvant) [18], although it is important to note several differences in study design. These include adjuvant formulation, vaccination schedules (dose number and time between doses), the number of NHP, and relative lethality of challenge dose. Likewise, our ABLA results are similar to those obtained in a smaller study by the Franchini group using MPXV orthologs of the same four VACV proteins used here [16].
Aluminum hydroxide, a well-established adjuvant, provides reasonably good protection, but adding CpG provides additional benefit and improved protection. CPG7909 is a B-Class CpG oligodeoxynucleotide that has proven in clinical studies to significantly enhance (∼5–10-fold) humoral responses against several different antigens [40], [41]. CpG10104, a related molecule with similar pharmacological effects, is currently under development as an adjuvant for infectious disease vaccines. While not compared side-by-side in the same study, our results support their similar activities. The Franchini study involved DNA prime/protein boost and a slightly higher MPXV challenge dose, but two groups were vaccinated with protein adjuvanted with either aluminum hydroxide (n = 3) or CpG (2 mg/animal of CPG7909, also known as CpG2006; n = 4). More than 25 lesions were seen in 3 of 3 (protein/alum) and 3 of 4 (protein/CpG) challenged monkeys in the Franchini study and 3 of 5 (protein/alum) monkeys in our study, but only 4 of 16 (protein/CpG/alum) challenged monkeys in our studies. Thus, there appears to be a benefit for including both alum and CpG in subunit vaccine formulations.
Intravenous challenge has been widely used to study smallpox therapies and vaccines because it more closely mimics some aspects of smallpox disease [15], [16], [18], [23], [30], [42], [43], [44], [45], [46], [47], [48]. However there are disadvantages. The challenge dose is high and essentially starts the disease process at a stage closer to the secondary viremic phase of smallpox, therefore setting a very high bar for a preventative vaccine. Conversely, the outcome of intravenous challenge may depend more heavily on antibody responses against MV. Other NHP challenge models, like respiratory challenge, are being developed [49], [50], [51], [52] and should be useful to further test effectiveness of subunit orthopoxvirus vaccines. For FDA approval of future generation smallpox vaccines by the “animal rule”, multiple animal models with various modes of challenge will likely be needed.
In conclusion, we found that as few as two doses of an adjuvanted protein-based subunit vaccine protected NHP from a lethal MPXV challenge. Such a vaccine would be valuable in a setting where it is difficult to screen large populations to identify those with increased risk of complications from live VACV vaccination. It could also be used to safely provide baseline poxvirus immunity and for immunization of individuals refusing VACV.
Acknowledgements
We thank the veterinarians and the animal care technicians at SRI and University of Maryland for their work with the NHPs. We also thank Manuel Ponce de Leon and Chwang Hong Foo for the L1-coupled sepharose beads. This work was supported by Public Health Service grants UC1-AI067129, U54-AI057168 (Middle Atlantic Regional Center of Excellence in Biodefense and Emerging Infectious Diseases), and U01-AI077913 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2010.07.030.
Appendix A. Supplementary data
(A) Timeline of vaccination of monkeys (5/group) in the 3-dose study. (B) VACV-specific antibody responses to purified vaccinia virions over time. Sera were taken 7 days prior to vaccination (−7) or 14 days after initial vaccination and boosts. Reciprocal endpoint titers were determined as the highest dilution that was 3-time the standard deviation above the mean OD of the negative control. (C) Individual ELISAs by day and by monkey. Based on this data, median and inter-quartile ranges of antibodies to the proteins at each time point were calculated and underwent statistical analysis. Statistical comparisons of the protein vaccinated groups with the unvaccinated group revealed some subtle differences between the protein vaccination groups. At 14 days after the first vaccination, the groups with statistically significant elevated antibody levels to the each of the protein components of the vaccine were seen in the tetravalent and trivalent groups given 100 μg of each protein along with CpG/alum (Groups 3 and 6). Despite the differences in mean and median lesion counts seen after challenge in animals in these two groups (Table 1), when compared to the control group they continued to have statistically significant elevated antibody levels to each protein component after the first and second boosts. The group that received ABLA and alum only (Group 5) had statistically significant elevated antibody levels to the A33, B5, and A27 (but not L1) after the 2nd and 3rd boost vaccinations. Thus, the antibody response to L1 may have been not as robust as in groups that received the same amount of protein adjuvanted with CpG/alum. The group that received the lower amounts of proteins with CpG/alum (Group 4) had statistically significant elevated antibody levels to the B5 protein on day 14 after the initial vaccination and then to A27 (but not A33, B5, or L1) after the 2nd and 3rd boost vaccinations. Thus, the antibody response to A33, B5, and L1 was different than what was generated by the vaccine with an increased amount of protein adjuvanted with CpG/alum. Despite these differences, NHPs in Group 4 had similar lesion counts as Group 3, which received 100 μg of each protein (Table 1).
References
- 1.Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi ID . 1st ed. World Health Organization; Geneva: 1988. Smallpox and its eradication. [Google Scholar]
- 2.Greenberg R.N., Kennedy J.S. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Investig Drugs. 2008;17(4):555–564. doi: 10.1517/13543784.17.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC Notice to readers: newly licensed smallpox vaccine to replace old smallpox vaccine. MMWR Morb Mortal Wkly Rep. 2008;57(8):207–208. [Google Scholar]
- 4.Rosenthal S.R., Merchlinsky M., Kleppinger C., Goldenthal K.L. Developing new smallpox vaccines. Emerg Infect Dis. 2001;7(6):920–926. doi: 10.3201/eid0706.010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poland G.A. Smallpox vaccines: from first to second to third generation. Lancet. 2005;365(9457):362–363. doi: 10.1016/S0140-6736(05)17840-4. [DOI] [PubMed] [Google Scholar]
- 6.Wiser I., Balicer R.D., Cohen D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine. 2007;25(6):976–984. doi: 10.1016/j.vaccine.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Artenstein A.W. New generation smallpox vaccines: a review of preclinical and clinical data. Rev Med Virol. 2008;18(4):217–231. doi: 10.1002/rmv.571. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy J.S., Greenberg R.N. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines. 2009;8(1):13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 10.Smith G.L., Vanderplasschen A., Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83(12):2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 11.Condit R.C., Moussatche N., Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- 12.Hooper J.W., Custer D.M., Schmaljohn C.S., Schmaljohn A.L. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 13.Hooper J.W., Custer D.M., Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306(1):181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogg C., Lustig S., Whitbeck J.C., Eisenberg R.J., Cohen G.H., Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78(19):10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper J.W., Thompson E., Wilhelmsen C., Zimmerman M., Ichou M.A., Steffen S.E., et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heraud J.M., Edghill-Smith Y., Ayala V., Kalisz I., Parrino J., Kalyanaraman V.S., et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006;177(4):2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 17.Sakhatskyy P., Wang S., Chou T.H., Lu S. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology. 2006;355(2):164–174. doi: 10.1016/j.virol.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogg C.N., Americo J.L., Lustig S., Huggins J.W., Smith S.K., Damon I., et al. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007;25(15):2787–2799. doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornburg N.J., Ray C.A., Collier M.L., Liao H.X., Pickup D.J., Johnston R.E. Vaccination with Venezuelan equine encephalitis replicons encoding cowpox virus structural proteins protects mice from intranasal cowpox virus challenge. Virology. 2007;362(2):441–452. doi: 10.1016/j.virol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Y., Aldaz-Carroll L., Ortiz A.M., Whitbeck J.C., Alexander E., Lou H., et al. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine. 2007;25:1214–1224. doi: 10.1016/j.vaccine.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berhanu A., Wilson R.L., Kirkwood-Watts D.L., King D.S., Warren T.K., Lund S.A., et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol. 2008;82(7):3517–3529. doi: 10.1128/JVI.01854-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman D.R., Goudsmit J., Holterman L., Ewald B.A., Denholtz M., Devoy C., et al. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J Virol. 2008;82(14):6829–6837. doi: 10.1128/JVI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper J.W., Ferro A.M., Golden J.W., Silvera P., Dudek J., Alterson K., et al. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine. 2009;28:494–511. doi: 10.1016/j.vaccine.2009.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldaz-Carroll L., Whitbeck J.C., Ponce de Leon M., Lou H., Hirao L., Isaacs S.N., et al. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J Virol. 2005;79(10):6260–6271. doi: 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aldaz-Carroll L., Whitbeck J.C., Ponce de Leon M., Lou H., Pannell L.K., Lebowitz J., et al. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005;341(1):59–71. doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Aldaz-Carroll L., Xiao Y., Whitbeck J.C., Ponce de Leon M., Lou H., Kim M., et al. Major neutralizing sites on vaccinia virus glycoprotein B5 are exposed differently on variola virus ortholog B6. J Virol. 2007;23:23. doi: 10.1128/JVI.00374-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connell K.P., Kovaleva E., Campbell J.H., Anderson P.E., Brown S.G., Davis D.C., et al. Production of a recombinant antibody fragment in whole insect larvae. Mol Biotechnol. 2007;36(1):44–51. doi: 10.1007/s12033-007-0014-4. [DOI] [PubMed] [Google Scholar]
- 28.Kovaleva E.S., O’Connell K.P., Buckley P., Liu Z., Davis D.C. Recombinant protein production in insect larvae: host choice, tissue distribution, and heterologous gene instability. Biotechnol Lett. 2009;31(3):381–386. doi: 10.1007/s10529-008-9883-2. [DOI] [PubMed] [Google Scholar]
- 29.Bell E., Shamim M., Whitbeck J.C., Sfyroera G., Lambris J.D., Isaacs S.N. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325(2):425–431. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 31.Panchanathan V., Chaudhri G., Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J Virol. 2006;80(13):6333–6338. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marriott K.A., Parkinson C.V., Morefield S.I., Davenport R., Nichols R., Monath T.P. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26(4):581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey S.E., Newman F.K., Kennedy J.S., Ennis F., Abate G., Hoft D.F., et al. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax in healthy vaccinia-naive adults. Vaccine. 2009;27(10):1637–1644. doi: 10.1016/j.vaccine.2008.11.079. [DOI] [PubMed] [Google Scholar]
- 34.Ward E.S., Ghetie V. The effector functions of immunoglobulins: implications for therapy. Ther Immunol. 1995;2(2):77–94. [PubMed] [Google Scholar]
- 35.CDC Household transmission of vaccinia virus from contact with a military smallpox vaccinee--Illinois and Indiana, 2007. MMWR Morb Mortal Wkly Rep. 2007;56(19):478–481. [PubMed] [Google Scholar]
- 36.CDC Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(19):532–536. [PubMed] [Google Scholar]
- 37.Benhnia M.R., McCausland M.M., Moyron J., Laudenslager J., Granger S., Rickert S., et al. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J Virol. 2009;83(3):1201–1215. doi: 10.1128/JVI.01797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benhnia M.R., McCausland M.M., Laudenslager J., Granger S.W., Rickert S., Koriazova L., et al. Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5. J Virol. 2009;83(23):12355–12367. doi: 10.1128/JVI.01593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogg C.N., Americo J.L., Earl P.L., Resch W., Aldaz-Carroll L., Eisenberg R.J., et al. Disparity between levels of in vitro neutralization of vaccinia virus by antibody to the A27 protein and protection of mice against intranasal challenge. J Virol. 2008;82(16):8022–8029. doi: 10.1128/JVI.00568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper C.L., Davis H.L., Morris M.L., Efler S.M., Adhami M.A., Krieg A.M., et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 41.Mullen G.E., Ellis R.D., Miura K., Malkin E., Nolan C., Hay M., et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS One. 2008;3(8):e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 43.Chen N., Li G., Liszewski M.K., Atkinson J.P., Jahrling P.B., Feng Z., et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Earl P.L., Americo J.L., Wyatt L.S., Anne Eller L., Montefiori D.C., Byrum R., et al. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;10:10. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigam P., Earl P.L., Americo J.L., Sharma S., Wyatt L.S., Edghill-Spano Y., et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;14:14. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci USA. 2008;105(31):10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huggins J., Goff A., Hensley L., Mucker E., Shamblin J., Wlazlowski C., et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53(6):2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan R., Goff A., Frimm A., Corrado M.L., Hensley L.E., Byrd C.M., et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother. 2009;53(5):1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81(12):1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80(11):5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J Gen Virol. 2009;90(Pt 9):2266–2271. doi: 10.1099/vir.0.010207-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Timeline of vaccination of monkeys (5/group) in the 3-dose study. (B) VACV-specific antibody responses to purified vaccinia virions over time. Sera were taken 7 days prior to vaccination (−7) or 14 days after initial vaccination and boosts. Reciprocal endpoint titers were determined as the highest dilution that was 3-time the standard deviation above the mean OD of the negative control. (C) Individual ELISAs by day and by monkey. Based on this data, median and inter-quartile ranges of antibodies to the proteins at each time point were calculated and underwent statistical analysis. Statistical comparisons of the protein vaccinated groups with the unvaccinated group revealed some subtle differences between the protein vaccination groups. At 14 days after the first vaccination, the groups with statistically significant elevated antibody levels to the each of the protein components of the vaccine were seen in the tetravalent and trivalent groups given 100 μg of each protein along with CpG/alum (Groups 3 and 6). Despite the differences in mean and median lesion counts seen after challenge in animals in these two groups (Table 1), when compared to the control group they continued to have statistically significant elevated antibody levels to each protein component after the first and second boosts. The group that received ABLA and alum only (Group 5) had statistically significant elevated antibody levels to the A33, B5, and A27 (but not L1) after the 2nd and 3rd boost vaccinations. Thus, the antibody response to L1 may have been not as robust as in groups that received the same amount of protein adjuvanted with CpG/alum. The group that received the lower amounts of proteins with CpG/alum (Group 4) had statistically significant elevated antibody levels to the B5 protein on day 14 after the initial vaccination and then to A27 (but not A33, B5, or L1) after the 2nd and 3rd boost vaccinations. Thus, the antibody response to A33, B5, and L1 was different than what was generated by the vaccine with an increased amount of protein adjuvanted with CpG/alum. Despite these differences, NHPs in Group 4 had similar lesion counts as Group 3, which received 100 μg of each protein (Table 1).


