Abstract
Thy-1 is a cell surface protein important in immunologic and neurologic processes, including T cell activation and proliferation, and neuronal outgrowth. In murine thymocytes, Thy-1 is downregulated in response to norepinephrine (NE) through posttranscriptional destabilization of its mRNA mediated by βAR/AC/cAMP/PKA signaling. In this study we investigated factors involved in NE/cAMP mediated Thy-1 mRNA destabilization in S49 thymoma cells, and identified a region containing two copies of the AUUUA regulatory element (ARE), a motif commonly associated with mRNA decay, in the Thy-1 mRNA 3′ UTR. Insertion of the Thy-1 ARE region into a reporter gene, resulted in cAMP induced destabilization of the reporter gene mRNA. RNA-protein binding studies revealed multiple Thy-1 ARE binding proteins, including AUF1, HuR, and TIAR. RNA silencing of HuR enhanced cAMP mediated downregulation of Thy-1 mRNA, in contrast, silencing AUF1 had no effect. Immunoblotting revealed multiple proteins phosphorylated by PKA as a result of NE or cAMP signaling. These results reveal that the machinery of NE/cAMP modulation of Thy-1 mRNA decay involves a cAMP responsive ARE in its 3′ UTR and multiple site specific ARE binding proteins. These findings add to our knowledge of Thy-1 mRNA regulation and provide insight into the regulation of ARE containing mRNAs, which impacts stress-related immunosuppression.
Keywords: T cells, Cell Surface Molecules, Thy-1, Neuroimmunology, Signal Transduction, mRNA regulation, RNA binding proteins, HuR
Introduction
The effect of psychogenic stress on the regulation of the immune system has become an area of increasing research (Chambers et al., 1993; Nance and Sanders, 2007; Kin and Sanders, 2006; Kohm and Sanders, 2001; Ader et al, 1995; Chambers and Schauenstein, 2000). The perception of stress can lead to the release of catecholamines (CA) and other neuromodulators by the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, which can act directly on immune cells to affect their development and function. The CA, norepinephrine (NE), binds to βARs on immune cells, and results in altered regulation of cytokines, e.g. TNF-α, IL-2, IFN-γ, and IL-4, as well as cytokine receptors, e.g. TNFR and IL-2R, and other cell surface proteins, such as Thy-1 (Ignatowski et al., 1996; Meltzer et al., 2004; Sekut et al., 1995; Feldman et al., 1987, Guiro et al., 1997; Frohman et al., 1988; Wajeman-Chao et al., 1998). We have shown that NE binding to βARs in T lymphocytes inhibits lymphocyte activation and downregulates expression of Thy-1 protein (Wajeman-Chao et al., 1998, Cook-Mills et al., 1992, 1995a, 1995b).
Thy-1 protein (CD-90), initially recognized as a biomarker for T lymphocytes and abundantly present in brain tissue in mice, is a glycophosphatidylinositol (GPI)-anchored cell surface protein, and its catalog of expression now includes thymocytes, neurons, fibroblasts, keratinocytes, endothelial cells, hematopoietic, mesenchymal stem cells, and some cancer cells (Xue et al., 1990; Sanders et al., 2008; Haeryfar and Hoskin, 2004; Rege and Hagood, 2006a; Barker and Hagood, 2008; Chambers, 1985). Although a specific Thy-1 ligand has not been characterized, the use of anti-Thy-1 antibodies, both stimulatory and inhibitory, and Thy-1 null mice, have confirmed its importance in a wide range of cellular events, including apoptosis, adhesion, differentiation, and wound healing (Rege and Hagood, 2006a; Koumas et al., 2003; Leposavic et al., 2008; Marmor et al., 1999; Lung et al., 2005; Haeryfar et al., 2005; Heuber et al., 1997).
In the nervous system, Thy-1 is found in certain areas of the brain, on retinal ganglion cells, and on neurons. The use of knockout mice, has revealed the importance of Thy-1 in neuronal regeneration, neurite outgrowth, and in brain functions, such as memory and learning (Rege and Hagood, 2006a; Rege and Hagood 2006b). Thy-1 expression on hematopoietic progenitor cells, thymocytes, and T cells has been shown to be important in T lymphocyte development and proliferation, and its recruitment to immunological synapses has suggested an importance for Thy-1 in T lymphocyte signal transduction and activation (Rege and Hagood, 2006a; Rege and Hagood 2006b). The plethora of Thy-1 associated T cell activities implies that disruption of Thy-1 expression, which is observed in response to the stress mediator NE, may affect T cell dysfunction.
Thy-1 gene expression can be regulated transcriptionally by promoter hypermethylation, posttranscriptionally by an as yet unidentified trans-acting suppressor, or posttranslationally through shedding of the molecule (Sanders et al., 2008; Rege and Hagood, 2006; Xue et al., 1990; Deglon et al., 1995; Hagood et al., 2005). In mouse thymocytes and S49 thymoma cells (derived from a Balb/c mouse thymoma), NE signaling through the βAR/adenylyl cyclase (AC)/cAMP/protein kinase A (PKA) pathway downregulates Thy-1 expression posttranscriptionally by altering Thy-1 mRNA stability (Wajeman-Chao et al., 1998).
The complexities of posttranscriptional gene regulation involve interactions between RNA binding proteins and specific sequences in their target mRNAs. The regulatory sequences in an mRNA are most commonly located in the 5′ and 3′ untranslated regions (UTRs) and can alter gene expression by regulating translation and mRNA stability. A highly characterized mRNA regulatory sequence in the 3′ UTR, the AU-rich element (ARE) is an important factor in mRNA decay, and is a region of particular interest in this study (Wilusz et al., 2001; Zhang et al., 2002; Bevilacqua et al., 2003). ARE sequences vary, but in general consist of one or more copies of the AUUUA pentamer arranged singly within a U rich region (Class I; common for transcription factor and cell cycle regulatory factor genes), or as multiple overlapping pentamers (Class II; common for cytokine genes) (Wilusz et al., 2001; Zhang et al., 2002; Barreau et al., 2006). U rich, non-AUUUA regions have also been classified as AREs (Class III) (Wilusz et al., 2001; Zhang et al., 2002). Regulation of mRNA stability by ARE regions involves binding of various ARE binding proteins (AREbps) to the ARE sequence. Many AREbps have been identified based on their ability to either promote mRNA stability, e.g. HuR, promote mRNA decay, e.g. AUF1, TTP, KSRP, or to inhibit translation, e.g. TIA-1 and TIAR (Wilusz et al., 2001; Zhang et al., 2002; Bevilacqua et al., 2003; Barreau et al., 2006). In response to stimuli, alterations in the function of these proteins through changes in expression, subcellular localization, or posttranslational modification, can impact their effectiveness in mRNA turnover regulation (Bevilacqua et al., 2003; Wang et al., 2002; Doller et al., 2008b; Abdelmohsen et al., 2007; McMullen et al., 2003; Wilson et al., 2003; Johnson et al., 2002; Esclatine et al., 2004).
In these studies we sought to elucidate the mechanisms involved in NE or cAMP mediated Thy-1 mRNA destabilization in S49 cells. We hypothesized that the decay process involves cAMP responsive elements in the Thy-1 mRNA 3′ UTR to which specific RNA binding proteins could bind, and because Thy-1 mRNA decay is not conserved in a S49 cell mutant in functional PKA, the decay process requires PKA-dependent protein phosphorylation. We have identified a 116 nucleotide ARE containing region in the 3′ UTR of Thy-1 mRNA. Insertion of this ARE sequence into the 3′ UTR of GFP mRNA resulted in the destabilization of the reporter gene when stimulated by cAMP. This same Thy-1 ARE sequence specifically bound multiple cytoplasmic proteins, including AUF1, HuR, and TIAR. By employing the technique of RNA silencing, we found that HuR is involved in Thy-1 mRNA regulation by cAMP. These results add to our knowledge of CA/cAMP mediated mRNA decay by revealing a cAMP responsive ARE within Thy-1 mRNA and identifying associated RNA binding proteins. Many immune cell effector molecules, including cytokines and cell surface proteins contain AREs in their 3′ UTRs and are modulated following NE release (Sekut et al., 1995; Guirao et al., 1997; Wajeman-Chao et al., 1998, Bevilacqua et al., 2003). Thus, the molecules associated with the events responsible for NE/cAMP mediated Thy-1 mRNA decay are likely to be important in stress mediated regulation of immune cells.
Methods
Cell Culture
Mouse thymocytes were isolated from pathogen free male BALB/c mice aged 4-6 weeks. Mice were killed by cervical dislocation, thymuses were surgically removed and minced, and thymocytes were isolated by passing the minced thymuses through nylon mesh. Thymocytes were cultured in RPMI 1640 medium (Hyclone) supplemented with 0.3% gentamycin (Gibco), 0.4% fungizone (Gibco), 0.1% penicillin/streptomycin (Gibco), and 0.1% Tylosine (Sigma) in a humidified atmosphere containing 5% CO2 at 37°C. These procedures comply with federal and institutional guidelines.
BALB/c mouse derived S49 thymoma wild type cells, and PKA-deficient mutant S49 cell clones (kin-), described previously (Wajeman-Chao et al., 1998), were maintained at a density of 1 × 105 - 2 × 106 cell/ml in suspension in RPMI 1640 medium (Hyclone) supplemented with 22 % DMEM (CellGro), 10 % heat inactivated FBS (CellGro), 0.3 % gentamycin (Gibco), 0.4 % fungizone (Gibco), 0.1 % penicillin/streptomycin (Gibco), and 0.1 % Tylosine (Sigma) in a humidified atmosphere containing 5 % CO2 at 37 °C. Cells were exposed to norepinephrine (150 μM, freshly prepared in 10 mM HCl), 8-bromoadenosine 3′,5′-cyclic monophosphate (8-bromo cAMP) (600 μM, freshly prepared in RPMI 1640 medium), and nadolol (20 μM in RPMI, Sigma) for the indicated times at a starting cell density of 1 × 106 cell/mL. Cell viabilities, determined by trypan blue exclusion, were at least 95 % at the start of experiments. S49 wt cells transfected with plasmids expressing short hairpin RNA or GFP were grown in the same medium supplemented with 1 μg/mL puromycin (Sigma) or 0.4 μg/μL G418 (Calbiochem) respectively.
Cell Transfections
Plasmids
For the reporter gene assay, the GFPARE and GFPLongARE constructs were derived by inserting Thy-1 nucleotide bases 821-937 and bases 821-1550 respectively at the BCl1 site in the MCS region of the Amaxa plasmid, PmaxFC-Green-C vector (Genscript Corporation, Piscatway, NJ, USA; Web:www.genscript.com)). The resulting plasmids contained either the Thy-1 ARE (bases 821-937) or the Thy-1 LongARE (bases 821-1550) in the 3′ UTR of GFP. The control GFP construct contained no additional inserts. For the silencing experiments, plasmids containing puromycin resistance, and a short hairpin sequence against HuR (SHDNAC-TRCN0000112088), AUF1 (SHDNAC-TRCN0000103739), or against a nonspecific sequence, control (SHC002V) were purchased from Sigma.
Transfection
Transfections were performed using program C-13/C-013 of the Amaxa Nucleofector device according to manufacturer's instructions. S49 wt cells (2×106) were resuspended in 100 μL Cell Line Nucleofector Solution (Amaxa) with plasmid DNA (0.5 μg GFPARE, 0.5 μg GFPLongARE, 1 μg GFPControl, 2 μg control, 2 μg siAUF1, 4 μg siHuR). Following transfection, cells were cultured as described above.
Preparation of protein extracts
Cytoplasmic protein
Cytoplasmic extract was prepared using HEPES based lysis buffer (10 mM HEPES, 3 mM MgCl2, 40 mM KCl, 5 % glycerol, 0.2% NP-40) supplemented with 0.5 μl Protease Inhibitor Cocktail (Sigma), 1 mM PMSF (Sigma), and 10 μl Phosphatase Inhibitor Cocktails 1 and 2 (Sigma) per mL of buffer. Following exposure, cells were collected by centrifugation at 1000 RPM for 7 min, washed in ice cold PBS, and lysed at a ratio of 10 μl buffer to 1×106 cells for 20 minutes on ice. The lysate was centrifuged at 4° C for 5 min at 12000 RPM, and the supernatant was aliquoted and stored at -80 °C.
Total cellular proteins
Total cellular protein was prepared using Cell Extraction Buffer (Biosource #FNN0011) supplemented with 1 mM PMSF (Sigma), and 0.5 μl Protease Inhibitor Cocktail (Sigma), and 10 μl Phosphatase Inhibitor Cocktails 1 and 2 (Sigma) per mL of buffer. Cells were isolated, washed in ice cold PBS, and lysed on ice for 30 min at a ratio of 10 μl buffer to 1×106 cells. Insoluble fractions (DNA) were separated by centrifugation at 4 °C for 10 min at 14000 RPM and discarded. The supernatant was aliquoted and stored at -80 °C.
RNA probe synthesis
The template for ARE probe synthesis was obtained by SmaI digestion of pG7p(A) plasmids containing the 116 nucleotide Thy-1 ARE insert (ACC ACT AGC TGT CAT TTT GTA CTC TGT ATT TAT TCC AGG GCT GCT TCT GAT TAT TTA GTT TGT TCT TTC CCT GGA GAC CTG TTA GAA ACA TAA GGG CGT ATGG) or the TNF-α ARE insert (CCC TCT ATT TAT ATT TGC ACT TAT TAT TTA TTA TTT ATT TAT TAT TTA TTT ATT TGC TTA TGA ATG TAT TTA TTT GGA AGG CCG GGG TGT CCT GGA GGA CCC AGT GTG GGA AGC TGT CTT CAG ACA GAC ATG TTT TCT GTG AAA ACG GAG CTG AGC TGT CC). The ARE region was PCR amplified using forward T7 promoter primer (Sigma) and reverse primer Thy-1 3′ TACCGAATTCCATACGCCCTTTAT (Sigma Genosys) to generate a template for transcription for Thy-1 riboprobe, or TNF-α 3′ GTACCGAATTCGGACAGCTC (Sigma Genosys) for TNF-α riboprobe. The template for control GFP probe was purchased from IDT (CTA CAG CTA ATA CGA CTC ACT ATA GGG GCC TGA CCT TCA GCC CCT ACC TGC TGA GCC ACG TGA TGG GCT ACG GCT TCT ACC ACT TCG GCA CCT ACC CCA GCG GCT ACG AGA ACC CCT TCC TGC ACG CCA TCA ACA ACG GCG GCT ACA CCA ACACCC GCA TCG AGA AGT AC). Transcription reactions contained 1 μg of PCR template, 50U T7 polymerase (Invitrogen), 0.2 mM each ATP, CTP, GTP and 0.125 mM of UTP (Roche), and 50 μCi of 32P UTP (PerkinElmer Life Sciences, for labeled riboprobes) or 200 μM UTP (non radioactive probes) in a total volume of 40 μL. Reactions were carried out for 30 min. at 37 °C. Unincorporated nucleotides were removed using Sephadex G-25 mini Quick Spin TNA columns (GE Healthcare, radiolabeled) or with TE-100 columns (Clonetech, non radiolabeled).
Electromobility shift assay (EMSA)
Increasing amounts of cytoplasmic protein extract (5, 10, and 20 μg) and 50,000 cpm 32P labeled Thy-1 RNA probe were incubated in Binding Buffer (10 mM HEPES, 3 mM MgCl2, 40 mM KCl, 5 % glycerol, 0.2 % NP-40, 2 μL/mL 1 M DTT, 10 μL/mL Heparin (1 mg/mL)) for 1 hour on ice in a total volume of 25 μL. The reaction was stopped by the addition of 10X Stop Solution (50 % glycerol, 0.2 % bromophenol blue) and samples were separated by nondenaturing PAGE (4 %) in 0.25X TBE at 150 V for 3 hours and visualized by autoradiography.
In Vitro UV crosslink assay
20 μg cytoplasmic protein extract and 50,000 cpm 32P labeled RNA probe were incubated in Binding Buffer for 20 min at RT in a final volume of 25 μL. RNA-protein complexes were covalently bound by irradiation with UV light (250 nm, 150 V, placed 3-5 cm above the sample) for 15 min on ice. Unbound mRNA was digested by incubating with 4 μL RNase A/T1 cocktail (Ambion) for 30 min at 37 °C. RNA-protein complexes were separated by 10 % SDS-PAGE, unless otherwise noted, overnight at 70 mV, transferred to nitrocellulose membrane via semi-dry transfer for two hours at 150 mA, and visualized by autoradiography.
Competition assay
UV-crosslinking assay was performed as described above. 32P labeled probe was mixed with 10, 50 or 100 pmol of cold competing probe. Reactions were analyzed as described before and visualized by autoradiography.
UV crosslink IP assay
UV crosslinking was performed with 40 μg protein and 100,000 cpm 32P labeled RNA probe. Following RNase digestion, 500 μL IP buffer (10 mM Tris-HCl pH 8, 250 mM NaCl, 0.5 % NP-40, 1 mM EDTA) containing 5 μg of antibodies against AUF1 (Upstate), HuR (Molecular Probes), TIAR (Santa Cruz Biotechnology), or multiple isoforms of actin (Santa Cruz Biotechnology) was added to 30 μL protein A/G agarose beads (Santa Cruz Biotechnology) and incubated overnight at 4 °C. Protein/RNA complexes were recovered by centrifugation, washed 4 times in ice cold PBS, and resuspended in loading buffer. Protein-RNA complexes were resolved by 10 % SDS-PAGE and detected by autoradiography.
Thy-1 biotin pull down assay
Thy-1 riboprobe (400 pmoles) was annealed to a biotinylated oligo (biotin-ATT GGG CCC GAC GTC GCA TGC TCC TCT AGA, Sigma Genosys) and incubated with cytoplasmic extract (500 μg). The mixture was run through a magnetized streptavidin column (Miltenyl MACS streptavidin kit) and the bound proteins were eluted with a high salt buffer (1M NaCl). Eluted fractions, resolved by 10 % SDS-PAGE, were analyzed by western blotting.
Western blotting
Protein samples (20 μg) were resolved by electrophoresis on 10 % SDS polyacrylamide gels, and transferred to nitrocellulose membrane. Membranes were blocked in TBST (10 mM Tris/base pH 7.6, 0.1 M NaCl, 0.1 % Tween-20) with 5 % membrane blocking agent (GE Healthcare) for 1 h at room temperature and treated with primary antibodies, rabbit anti-AUF1 1/1000 (Upstate), goat anti-actin 1/1000 (Santa Cruz Biotechnology), goat anti-TIAR 1/1000 (Santa Cruz Biotechnology), mouse anti-HuR 1/500 (Molecular Probes), or rabbit anti-phospho-PKA substrate (pRRXS/T) 1/1000 (Cell Signaling) overnight at 4°C. After washing with TBST, membranes were incubated with the corresponding horseradish peroxidase labeled secondary antibody for 1 h at room temperature, and detected with the ECL Plus Western Blotting detection reagents (GE Healthcare). Luminescence was visualized on Kodak Biomax MS film.
RNA extraction and Quantitative Real-time PCR (qPCR)
Following exposures, cells were collected by centrifugation (1000 RPM for 7 min) and lysed in TRIzol LS Reagent (Invitrogen), 1 ml reagent/1×107 cells. Total cellular RNA was extracted using the RNEasy Mini Prep Kit (Qiagen) according to manufacturer's instructions. cDNA was prepared from polyadenlylated mRNA by reverse transcription using Superscript III (Invitrogen) and Oligo(DT)12-18 primers (Invitrogen) according to manufacturer's instructions. The resulting cDNA was used in qPCR reactions containing gene specific primers for HuR, AUF1, Thy-1, GFP, L32, or HPRT1 and SYBR Green master mix (Applied Biosciences). Samples were run in triplicate in the 7900HT Applied Biosciences real-time PCR thermocyler using default settings. The cDNA expression levels were analyzed using the qGene software (www.biotechniques.com/softlib/qgene.html).
Primer sequences, selected from a primerbank (http://pga.mgh.harvard.edu/primerbank/) are as follows: HuR forward, 5′-GGA TGA CAT TGG GAG AAC GAAT -3′, and HuR reverse, 5′-TGT CCT GCT ACT TTA TCC CGAA-3′; AUF1 forward, 5′-GTG AAG TTG TAG ACT GCA CTC TG-3′, and AUF1 reverse, 5′- TGG CCC TTT TAG GAT CAA TGAC-3′; Thy-1 forward, 5′- CTT CTC CCT CCA TGC ATA CC-3′, and Thy-1 reverse, 5′- CTA CCC ACC ATA CGC CCT TA-3′; HPRT1 forward, 5′-CTG GTG AAA AGG ACC TCT CG-3′, and HPRT1 reverse, 5′-TGA AGT ACT CAT TAT AGT CAA GGG CA-3′; L32 forward, 5′-ACA TTT GCC CTG AAT GTG GT-3′, and L32 reverse, 5′-ATC CTC TTG CCC TGA TCC TT-3′; GFP forward, 5′-CTG ACC TTC AGC CCC TAC CT-3′, and GFP reverse, 5′-ACA CCC GCA TCG AGA AGT AC-3′. All primers were synthesized and purchased from Sigma Genosys.
Reporter gene assay
S49 wild type cells containing GFPARE, GFPLongARE, or GFPControl constructs were treated with or without 8-bromo-cAMP (600 μM) as indicated. Total cellular mRNA was extracted, and GFP mRNA levels were determined by qPCR. For RNA decay studies, cells were pretreated with α-amanitin (10 μg/mL, 4 h) (Sigma) followed by 8-bromo-cAMP (600 μM) treatment for 4 h or untreated. Cells were collected at 0, 2, and 4 h post transcriptional repression and mRNA was extracted, and analyzed by qPCR. Following normalization of GFP mRNA levels to HPRT1, decay rates were calculated by linear regression of the fraction of GFP mRNA remaining as a function of time, yielding first-order decay constants.
Data Analysis
Data are represented as the mean +/- standard deviation (SD). Each individual experiment was normalized to the untreated condition, and the normalized data for all trials were averaged. Statistical differences between the mean values of control and experimental groups were determined by two-tailed, unpaired Student's t-tests.
Results
The 3′ UTR of Thy-1 mRNA contains an ARE which affects Thy-1 mRNA stability
Previous studies in this laboratory have revealed that NE stimulation of the classical βAR/cAMP/PKA pathway in S49 thymoma cells results in a decrease in Thy-1 mRNA expression through a posttranscriptional event involving mRNA destabilization (Wajeman-Chao et al., 1998). Since we have shown that NE mediated Thy-1 mRNA decay is dependent on the production of the second messenger cAMP in response to NE, in our experimental design we elected to study the NE effect directly by using the cAMP analogue, 8-bromo cAMP.
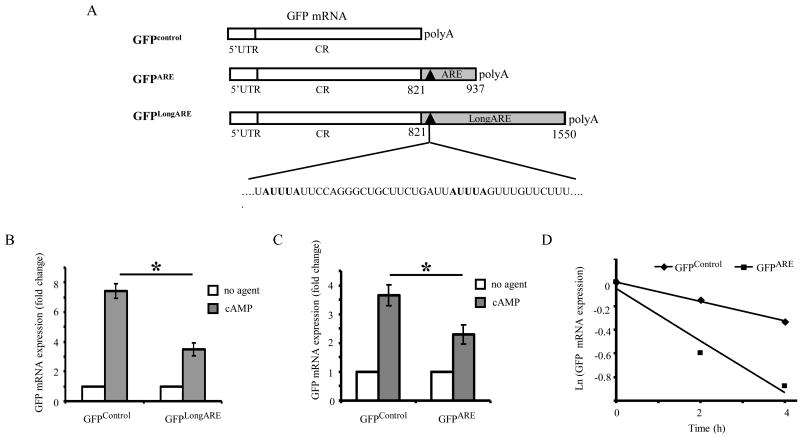
Murine Thy-1 mRNA (Genbank accession number NM_009382) is 1753 nucleotides long, and has a long 3′ UTR consisting of 1146 nucleotides. Analysis of the 3′ UTR sequence identified a putative ARE region containing two copies of the AUUUA pentamer sequence clustered within a U rich region (nucleotides 821-937), typical of a Class I ARE. To determine if the identified Thy-1 ARE region contains an mRNA regulatory element, the ARE was inserted into the 3′ UTR of a GFP reporter gene and the effect of the ARE on GFP mRNA regulation was analyzed.
Three reporter gene constructs were made (Fig. 1A), a control vector encoding for GFP protein (GFPControl), the GFP encoding vector with an insert of the Thy-1 ARE in its native context (a 729 nucleotide sequence, GFPLongARE), and the GFP encoding vector with an insert of the ARE region (a 116 nucleotide sequence, GFPARE). These vectors were stably transfected into S49 wild type cells and GFP mRNA levels were analyzed by qPCR in the presence and absence of 8-bromo cAMP treatment. In the control cells, treatment with 8-bromo cAMP led to an increase in steady state levels of GFPcontrol mRNA (Fig. 1B and 1C), however, in the presence of both the Thy-1 ARE inserts GFP mRNA levels decreased, 50 % for the LongARE (Fig. 1B) and 30 % for the ARE (Fig. 1C). These results suggest that the Thy-1 3′ UTR contains a cAMP responsive element involved in the regulation of Thy-1 mRNA.
Figure 1. Thy-1 ARE mediates destabilization of GFP mRNA in the presence of 8-bromo cAMP.
A. Schematic representation of the constructs used for the reporter gene assay. S49 cells were stably transfected with plasmids expressing GFP mRNA (GFPcontrol), or GFP mRNA with the Thy-1 LongARE sequence (GFPLongARE), or the Thy-1 ARE sequence (GFPARE) inserted in its 3′ UTR. The putative Thy-1 ARE region contains two copies of the AUUUA pentamer (bolded) clustered within a U rich sequence. The transfection efficiency for GFPARE and GFPControl were 50 %, and 10% for GFPLongARE. B. Cells containing GFPcontrol or GFPLongARE were treated in the presence or absence of 8-bromo cAMP (600 μM) for 4 h. Steady state GFP mRNA levels were determined by qPCR and normalized to HPRT1. Data was normalized to the untreated condition and the mean fold change in GFP mRNA levels ± SD for three independent experiments is represented. * p < 0.05. C. Cells containing GFPcontrol or GFPARE were treated in the presence or absence of 8-bromo cAMP (600 μM) for 6 h. Steady state GFP mRNA levels were determined by qPCR and normalized to HPRT1. Data was normalized to the untreated condition and the mean fold change in GFP mRNA levels ± SD for three independent experiments is represented. * p < 0.05. D. Cells containing GFPcontrol or GFPARE were exposed to α-amanitin for four hours followed by treatment with 8-bromo cAMP (600 μM) for 0, 2, and 4 h. GFP mRNA levels were determined by qPCR, normalized to HPRT1, and depicted as the natural log (Ln) of mRNA remaining vs time (h). Data shown is representative of 3 independent experiments.
To analyze the ability of the Thy-1 ARE to regulate GFP mRNA stability we blocked transcriptional activity with α-amanitin and compared the decay rates of GFPcontrol and GFPARE mRNAs in the presence and absence of 8-bromo cAMP (Table 1). In the absence of treatment, GFPcontrol and GFPARE mRNAs were stable with similar decay rates (Table 1). However, following 8-bromo cAMP, treatment, the GFPARE mRNA decayed at a rate faster than GFPcontrol mRNA (Fig. 1D). While the presence of the ARE alone was insufficient to cause a significant change in the GFP decay rate, upon treatment with cAMP, the Thy-1 ARE led to destabilization of this otherwise stable mRNA. Thus, the Thy-1 3′ UTR contains an ARE that acts as a cAMP responsive control element to regulate mRNA stability.
Table 1.
The effect of Thy-1 ARE on GFP mRNA decay in the presence and absence of cAMP
| GFP mRNA Decay Rate (h-1) (mean ± SD)a | ||
|---|---|---|
| No agent | cAMP | |
| GFPControl | 0.01 ± 0.12 | 0.09 ± 0.01 |
| GFPARE | 0.03 ± 0.12 | 0.22 ± 0.02b |
n=3 independent experiments
p<0.005, student's t test comparing GFPARE mRNA decay rates of cells untreated and treated with cAMP
S49 cytoplasmic proteins form RNA-protein complexes with the Thy-1 ARE
The regulation of mRNA stability is dependent not only on the presence of regulatory elements in the mRNA, but also on the proteins that bind to specific RNA regulatory elements. EMSAs of radiolabeled Thy-1 ARE probe in the presence of S49 cytoplasmic protein revealed RNA-protein complex formation (Fig. 2A). With increasing amounts of protein, an upward shift in the mobility of the labeled probe was observed, signifying that the cytoplasmic proteins form complexes with the Thy-1 ARE sequence. Two conformations of the free probe were observed and both conformations migrated more slowly in the presence of protein (Fig. 2A).
Figure 2. Detection of Thy-1 ARE-protein complexes.
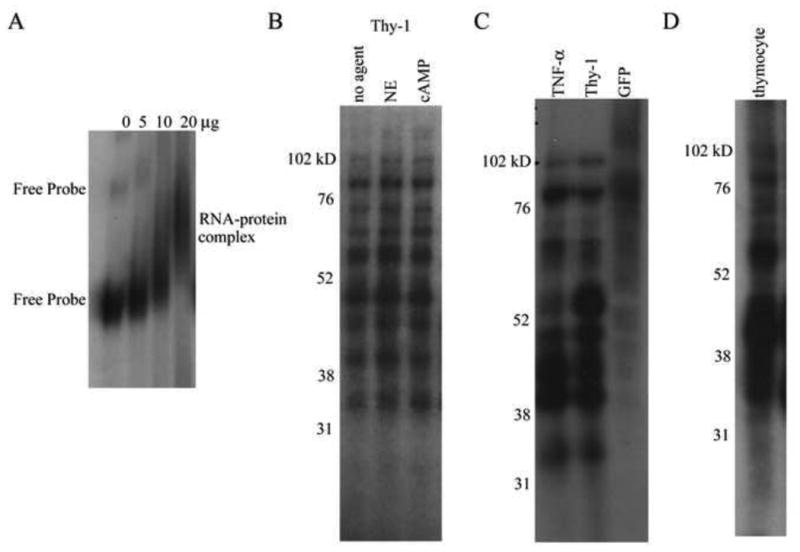
A. Radiolabeled Thy-1 ARE probe was incubated with cytoplasmic protein, and the resulting complexes were separated on nondenaturing gels and visualized by autoradiography. Addition of increasing amounts of cytoplasmic protein (0, 5, 10, and 20 μg) resulted in an upward shift in mobility of the probe indicating RNA-protein complex formation. The results shown represent one of three independent experiments. B-D. Radiolabeled RNA probes for Thy-1 ARE, TNF-α ARE, or control mRNA (obtained from GFP coding region) were incubated with 20 μg of cytoplasmic protein. RNA-protein complexes were fixed with UV irradiation, unbound RNA was digested with RNAse T1/A, and the complexes were subjected to electrophoresis under denaturing conditions and visualized by autoradiography. B. Thy-1 ARE probe crosslinked with protein from untreated, NE (150 μM), or 8-bromo cAMP (600 μM) treated S49 cells for 4 h, resolved on a 12% gel. The results shown represent one independent trial of n > 5. C. Thy-1 ARE, TNF-α ARE, and GFP probes crosslinked to protein from untreated S49 cells, resolved on a 10% gel. The results shown represent one of 3 independent experiments. D. Thy-1 ARE probe crosslinked to protein from untreated thymocytes isolated from BALB/c mice, resolved on a 12% gel. The results shown represent one of 3 independent experiments.
To characterize the proteins present in the Thy-1 ARE-protein complex, radiolabeled Thy-1 ARE probe was UV-crosslinked to S49 cytoplasmic proteins. Multiple proteins of molecular weights 115, 105, 80, 75, 60, 50-35, and 32 kD, bound to the Thy-1 ARE (Fig. 2B). The specificity of these proteins for the Thy1 ARE was shown by UV crosslink experiments using a GFP probe that does not contain the ARE sequence (Fig. 2C). Using cytoplasmic proteins isolated from murine thymocytes, a similar Thy-1 ARE binding protein profile was observed, suggesting that the two cell types contain similar proteins involved in the regulation of Thy-1 mRNA (Fig. 2D).
To determine if NE/cAMP treatment alters the profile of binding for Thy-1 AREbps, crosslinking experiments were performed with cytoplasmic extract from S49 cells treated for 30 min with 8-bromo cAMP (600 μM) or NE (150 μM). Comparison revealed identical Thy-1 AREbps bound to the RNA probe in the presence or absence of NE/cAMP stimulation (Fig. 2B). Varying the time of treatment, 2 h or 4 h, did not change the Thy-1 AREbp profile (data not shown). Thus, multiple cytoplasmic proteins can bind to the Thy-1 ARE independently of NE/cAMP treatment.
The Thy-1 ARE binding protein profile matches that of TNF-α
Having established a profile of binding proteins to the Thy-1 ARE, we compared that profile to the binding profile of the well characterized TNF-α ARE. The TNF-α ARE is known to be a major regulatory element and many of its binding proteins including AUF1, HuR, TTP, KSRP, and TIAR have been identified and characterized (Wilusz et al., 2001; Bevilacqua et al., 2003; Dean et al., 2001; Hel et al., 1998; Piecyk et al., 2000; Gueydan et al., 1999; Brewer et al., 2004; Wilson et al., 2001a, 2001b). Figure 2C compares the binding profiles of the Thy-1 ARE to the TNF-α ARE and shows that the two AREs bind S49 cytoplasmic protein of similar molecular weights, with the exception of a 60 kD protein that preferentially binds the Thy-1 ARE.
We utilized competition assays to determine if the Thy-1 AREbps and TNF-α AREbps are similar. Figure 3 shows that Thy-1 ARE competes with TNF-α ARE for the same cytoplasmic proteins. The addition of increasing amounts of cold Thy-1 probe effectively competes for the TNF-α AREbps, as is observed by a decrease in the intensity of the radiolabeled TNF-α ARE signal. Thus, the Thy-1 and TNF-α AREs have a number of common AREbps.
Figure 3. Thy-1 and TNF-α AREs compete for the same binding proteins.
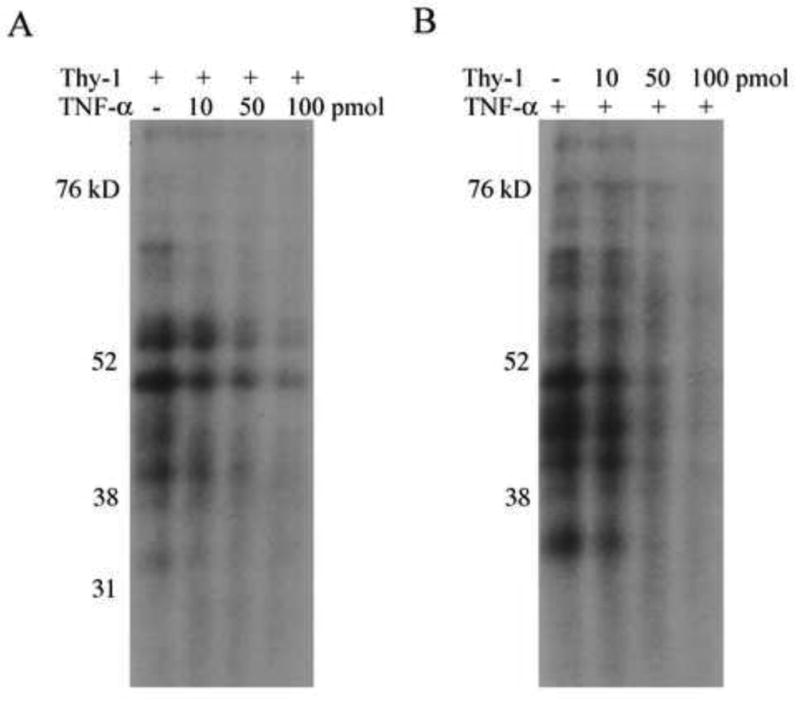
A. Radiolabeled Thy-1 ARE probe and increasing amounts of unlabeled TNF-α probe (0, 10, 50, 100 pmol) were incubated with 20 μg cytoplasmic protein. The RNA-protein complexes were fixed by UV irradiation, and the free RNA was digested with RNAse T1/A. Image shows autoradiograph of samples run on denaturing polyacrylamide gels. B. Same conditions as described in A with radiolabeled TNF-α ARE probe competed with unlabeled Thy-1 ARE probe. The results shown are from one representative experiment of three independent trials.
AUF1, HuR, and TIAR are Thy-1 ARE binding proteins
Since Thy-1 and TNF-α AREs can bind similar proteins, the S49 cytoplasmic protein extract was analyzed for the presence of specific TNF-α AREbps. Western blots identified AUF1, HuR, and TIAR in S49 cytoplasmic extract (Fig. 4A). AUF1 consists of four isoforms, p37, p40, p42, and p45, which are represented as three major bands in the 38-50 kD region of the gel since p40 and p42 resolve as a single band (Lu and Schneider, 2004). TIAR consists of two isoforms, p40 and p42, which co-migrate with AUF1, and HuR runs at 36 kD. All three proteins correlate in size with RNA-protein complexes identified in the crosslink.
Figure 4. Identification of Thy-1 ARE RNA-binding proteins by immunoprecipitation of RNA-protein complexes.
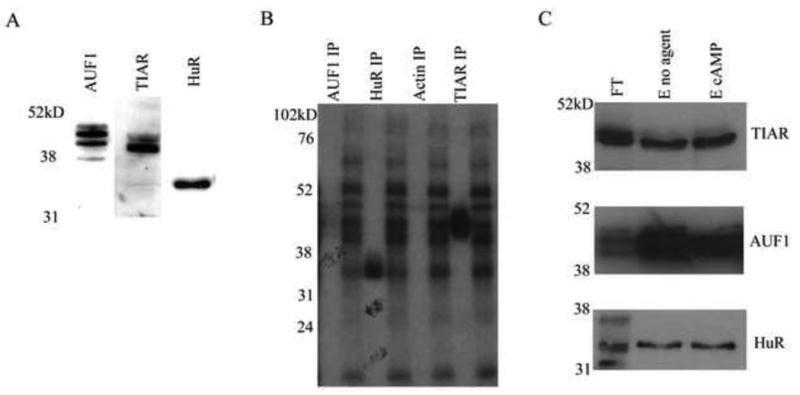
A. S49 cellular extract (20μg) was run on 10% SDS-PAGE and AUF1, HuR, and TIAR were detected by western blot. Images are representative of n > 5 independent experiments. B. Radiolabeled Thy-1 ARE probe was incubated with cytoplasmic protein and the RNA-protein complexes formed were fixed by UV irradiation. Unbound RNA was digested with RNAse T1/A. Antibodies specific for AUF1, HuR, or TIAR were used for immunoprecipitation. The immunoprecipitate was separated by SDS-PAGE and AUF1, HuR, and TIAR bound to labeled probe, were visualized by autoradiography. The results shown represent one experiment of five independent trials. C. RNA binding proteins interacting with a biotinylated Thy-1 ARE probe were isolated by affinity chromatography as described in materials and methods. S49 cytoplasmic protein extract from untreated or 8-bromo cAMP (600 μM, 30 min) treated cells were fractionated by RNA affinity chromatography. The flow through, and eluted fractions were resolved by 10% SDS-PAGE and AUF1, HuR, and TIAR were identified by western blotting. Results shown were from one representative experiment of three independent trials. The gel lanes contain eluted fractions (E) from untreated or treated extract, or flow through (FT) from untreated extract.
To determine if these AREbps bind to the Thy-1 ARE in vitro, Thy-1 ARE-protein complexes were UV-crosslinked, immunoprecipitated with specific antibodies for AUF1, HuR, and TIAR, and observed by autoradiography. Antibodies for AUF1, TIAR, and HuR each pulled down Thy-1 ARE riboprobe suggesting their ability to bind Thy-1 ARE (Fig. 4B). The specificity of this procedure was validated by the inability of an antibody for actin, a non AREbp, to pull down probe (Fig. 4B). A nonspecific band was observed in actin that migrated at 50 kD, which was also visible in control IPs using mouse, rabbit, or goat IgGs and could be the result of non-specific binding of probe to the highly abundant heavy chain (data not shown).
The interaction between AUF1, HuR, and TIAR with the Thy-1 ARE was also examined by RNA affinity chromatography. A biotinylated Thy-1 ARE probe was incubated with S49 cytoplasmic protein and the bound proteins were isolated by running the mRNA-protein complexes through a column containing immobilized streptavidin. The ARE bound proteins were eluted from the column with a high ionic strength buffer, and AUF1, HuR and TIAR, were identified in the eluate by western blotting (Fig. 4C). The non AREbp actin, was not present in the eluted fraction (data not shown). As with the UV crosslink data (Fig. 2B), no difference was observed in the ability of Thy-1 ARE to bind AUF1, HuR, or TIAR in cells treated with 8-bromo cAMP.
RNA silencing reveals the importance of HuR, but not AUF1, in the in vivo regulation of Thy-1 mRNA
Specific silencing of RNA has proven to be a valuable tool for elucidating the biological function of specific molecules. Accordingly, we used RNA silencing to study the relationship of the binding proteins AUF1 and HuR to CA/cAMP mediated Thy-1 mRNA downregulation. S49 cells were stably transfected with plasmids encoding short hairpin RNAs (shRNA) targeting HuR (siHuR) or AUF1 (siAUF1), or a control shRNA with a random nontargeting sequence (siControl). The silencing efficiency was determined by analyzing the mRNA and protein levels of HuR or AUF1 by qPCR and immunoblotting respectively. HuR mRNA levels were reduced by 50 %, and AUF1 mRNA levels were reduced by 90 % (Fig. 5A and 5C). These reductions in the mRNA levels correlated with decreases in protein expression (Fig. 5B and 5D).
Figure 5. Effect of HuR or AUF1 on the regulation of Thy-1 mRNA in S49 cells.
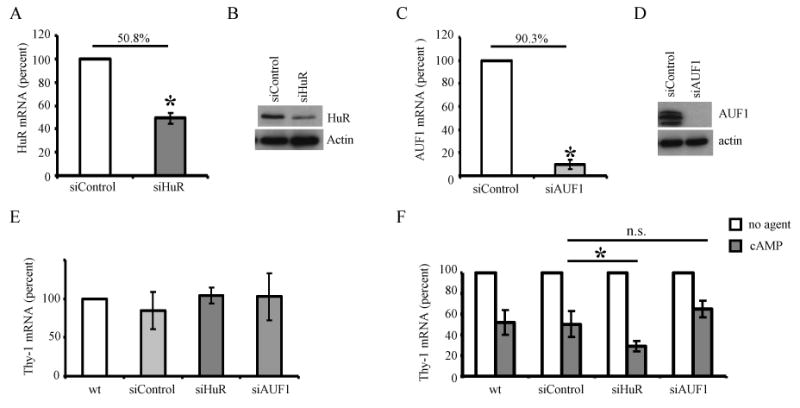
S49 cells express a nontargeting shRNA control (siControl) or a targeting shRNA against HuR (siHuR) or AUF1(siAUF1). A. HuR mRNA levels in the control and siHuR cells were analyzed by qPCR (normalized to L32). The data was normalized to the wt condition and the mean percent change ± SD for six independent experiments is represented. The percent decrease is 50.8 +/- 4.8 %. B. HuR and actin immunoblots using whole cell protein from siControl, and siHuR cells (n=6). C. AUF1 mRNA levels in the control and siAUF1 cells as detected by qPCR (normalized to L32). The data was normalized to the wt condition and the mean percent change ± SD for three independent experiments is represented. The percent decrease is 90.3 +/- 3.9 %. D. AUF1 and actin protein levels as detected by immunoblot of whole cell protein (n=3). E. Steady state levels of Thy-1 mRNA in S49 wt, siControl, siAUF1, and siHuR cells as detected by qPCR (normalized to HPRT1). F. S49 wt, siControl, siHuR, or siAUF1 cells were treated with or without 8-bromo cAMP (600 μM) for 4 h. The levels of Thy-1 mRNA were determined by qPCR (normalized to HPRT1). Data from each individual trial was normalized to the untreated condition and the mean percent change in Thy-1 mRNA levels ± SD for five independent experiments is represented. * p < 0.05.
Steady state levels of Thy-1 mRNA in the siControl cells mirrored that of the wt cells both in the absence (Fig. 5E) and in the presence of 8-bromo cAMP (Fig. 5F), demonstrating that the transfection of wt cells with a shRNA encoding plasmid does not disrupt Thy-1 mRNA regulation. In the absence of stimuli, the knockdown of HuR or AUF1 protein did not affect the steady state levels of Thy-1 mRNA (Fig. 5E), however, treatment of siHuR cells with 8-bromo cAMP (4 h, 600 μM) resulted in a significantly enhanced downregulation of Thy-1 mRNA levels (70 ± 5 %), while treatment of siAUF1 did not result in a statistically significant decrease in Thy-1 mRNA levels (35 ± 8 %), (Fig. 5F). These data suggest that although HuR and AUF1 do not seem to regulate basal Thy-1 mRNA levels, upon stimulation with cAMP, HuR is involved in the regulation of Thy-1 mRNA, possibly by protecting it from decay.
NE/cAMP treatment of S49 cells leads to PKA dependent phosphorylation of a number of proteins
We have shown that PKA, the cAMP dependent protein kinase, is essential for NE/cAMP-mediated Thy-1 mRNA decay, since S49 clonal mutant cells deficient in PKA activity (kin-) do not exhibit such mRNA decay (Wajeman-Chao et al., 1998). This observation led us to hypothesize that PKA phosphorylates an effector protein essential to NE/cAMP-mediated mRNA decay. In an effort to identify potential effector proteins, phosphorylated PKA substrates were detected by immunoblotting using an antibody that recognizes proteins containing a phosphorylated serine or threonine residue with arginine at the -3 and -2 positions (RRXpS/pT). Immunoblots for phosphorylated PKA substrates of cytoplasmic extracts from untreated S49 wt cells, or S49 wt cells treated with NE, or 8-bromo cAMP for 30 min revealed an increase in signal intensity for proteins at 60 and 102kD, and for a number of proteins in the 35-52 kD range (Fig. 6A). Since other kinases, e.g. PKC and AKT, have similar consensus sequences to PKA, we utilized S49 kin- cells to verify that this protein phosphorylation was dependent on PKA. Figure 6B shows that in protein extract from kin- cells treated with NE or 8-bromo cAMP for 30 min no increase in phosphorylated PKA substrates is observed. These results are consistent with our hypothesis that NE/cAMP signaling results in the PKA dependent phosphorylation of proteins which may be effector molecules involved in the regulation of Thy-1 mRNA decay.
Figure 6. Identification of NE/cAMP mediated phosphorylation of PKA substrates.
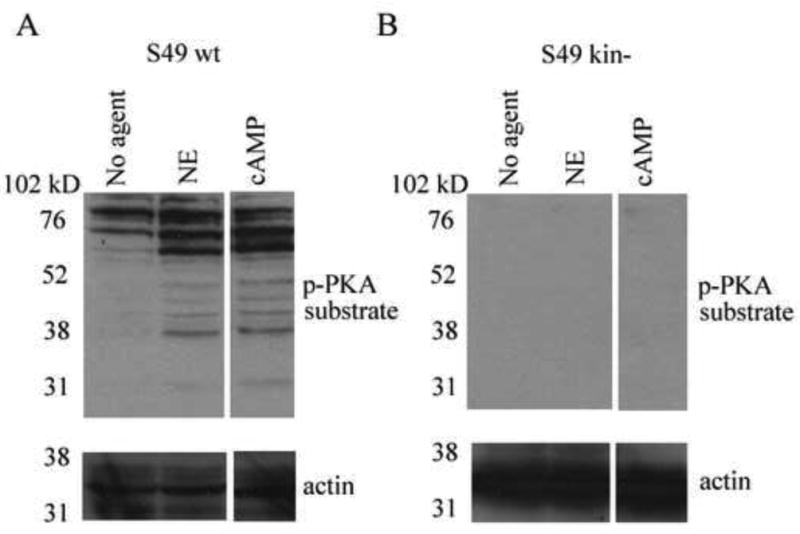
A. Immunoblot detection of phosphorylated PKA substrates and actin from S49 wild type cells (A) or kin- cells (B) stimulated with NE or 8-bromo cAMP for 30 min. Images are from a representative experiment (impertinent lanes have been removed) of 3 independent trials.
Discussion
We have shown that the stressor, NE, a neurotransmitter and hormone, inhibits T lymphocyte activation and decreases expression of Thy-1 protein (Cook-Mills et al., 1995a, 1995b; MS Thesis, Lancaster, UIC, 1993). The NE mediated downregulation of Thy-1 protein involves βAR/AC/cAMP/PKA signaling and posttranscriptional regulation of Thy-1 mRNA stability (Wajeman-Chao et al., 1998). In this report, we identify factors associated with NE mediated Thy-1 mRNA destabilization; a cAMP responsive ARE in its 3′ UTR and AREbps that bind to it, and also candidate PKA-phosphorylated proteins likely to be involved in Thy-1 mRNA regulation.
AREs are expressed in the 3′ UTR of many mRNAs and are important in the posttranscriptional regulation of various inflammatory modulators, including cytokines, chemokines, and proinflammatory proteins (Khabar, 2007). In T cells, at least 50% of the changes in gene expression, upon activation, occur through posttranscriptional regulation of mRNA stability (Cheadle et al., 2005a, 2005b). Due to the prevalence of ARE mediated mRNA regulation in T cells, we hypothesized that the NE/cAMP posttranscriptional regulation of Thy-1 mRNA involves ARE dependent decay. In this study we have identified an ARE region located in the 3′ UTR of Thy-1 mRNA, and shown its ability to destabilize a reporter gene mRNA in response to cAMP (Fig. 1D). Interestingly, the reporter gene also appeared to respond to cAMP through an activation of transcription (Fig 1B, 1C). Analysis of the reporter gene construct promoter sequence revealed the presence of CREB specific sites, and since cAMP signaling leads to the activation of CREB, it is likely that the cAMP mediated increase observed results from construct specific transcriptional activity. Transcriptional inhibition eliminated this transcriptional component, and highlighted the role of the Thy-1 ARE in posttranscriptional destabilization of the reporter gene (Table 1). These findings reveal for the first time, the presence of a destabilizing, cAMP responsive, ARE in murine Thy-1 mRNA. The human Thy-1 3′ UTR also contains a single ARE located at a similar nucleotide distance downstream of the stop codon as in the murine Thy-1 3′ UTR, suggesting that the ARE mediated regulation of Thy-1 mRNA described herein may also be observed in humans as well.
In general, mRNAs containing AREs are labile and the ARE region confers destability to the mRNA in its native context. Recent studies, however, have shown that many mRNAs containing few AREs (1 or 2) are stable under basal conditions (Hao and Baltimore, 2009), and those that contain Class I AREs may require stimulation for posttranscriptional regulation (Zhang et al., 2002). The Thy-1 mRNA is a stable message with a 12 h half-life, and its Class I type ARE, requires cAMP mediated signaling events for mRNA destabilization (Table 1).
Posttranscriptional regulation of many inflammatory genes can often be influenced by both ARE and non-ARE regulatory elements within the same message. For example, many cytokine messages including TNF-α, IL-4, and granulocyte stimulating factor, have regulatory elements located in their 5′ and 3′ UTRs, as well as in their coding regions (Stoecklin et al., 2003; Anderson, 2008). That Thy-1 mRNA contains other regulatory elements in addition to the ARE is possible and could explain differential regulation by various stimuli. One such regulatory element, responsive to fatty acids, is located in the coding region of Thy-1 mRNA (Deglon et al., 1995). In this study, we observed that the LongARE (729 nt) in response to cAMP, is more effective in downregulating GFP mRNA than the ARE alone (116 nt) (Fig. 1B and 1C), suggesting the possibility of additional cAMP responsive elements in the Thy-1 mRNA 3′ UTR. Nevertheless, the 116 nucleotide sequence (we term the ARE), by itself, is sufficient to promote cAMP mediated Thy-1 mRNA decay.
Using the Thy-1 ARE probe, multiple proteins were found to complex with the ARE, (Fig. 2), of which the AREbps, AUF1, TIAR, and HuR were identified (Fig. 4). NE or cAMP treatment did not seem to alter the ability of these proteins to bind Thy-1 ARE (Fig. 2B), however it remains possible, that NE/cAMP signaling could alter the functional activity of the AREbp in regulating Thy-1 mRNA stability. Hence, we analyzed the physiological role of the Thy-1 AREbps in vivo by RNA silencing. Since TIAR is a regulator of translational events, and not mRNA stability, in these studies we chose to study AUF1 and HuR, which are both factors directly involved in mRNA turnover.
Although AUF1 was identified as a Thy-1 AREbp (Fig. 4), siAUF1 cells did not show a significant change in Thy-1 mRNA basal levels or in 8-bromo cAMP mediated Thy-1 mRNA downregulation as compared to the siControl cells (Fig. 5). While this finding was unexpected, relative, rather than absolute levels of AUF1 isoforms could be adequate to induce changes in mRNA stability (Raineri, et al. 2004). In our studies, the short hairpin RNA targets all 4 AUF1 isoforms, leading to an absolute downregulation of AUF1, which may negate any possible regulatory effects of AUF1 on Thy-1 mRNA dynamics. Two other explanations also support these findings. First, redundancy in the function of many AREbps has been suggested (Gherzi et al., 2004), and it is possible that in the AUF1 silenced cells, a different destabilizing binding protein, like TTP, may compensate for the loss of AUF1 in these cells. Secondly, AUF1 may not be a target of the NE/cAMP mediated decay pathway; however, that AUF1 binds to the Thy-1 ARE (Fig. 4) is suggestive that it participates in the regulation of Thy-1 mRNA, but perhaps through a different signaling pathway.
In contrast to siAUF1, the knockdown of HuR led to an enhancement in 8-bromo cAMP mediated Thy-1 mRNA downregulation (Fig. 5F). HuR is a stabilizing AREbp, and is important in posttranscriptional gene regulation by regulating export of mRNAs to the cytoplasm, enhancing mRNA stability through binding to AREs in the 3′ UTR, and enhancing or suppressing translation by binding to regions in both the 3′ and 5′ UTRs (Hinman and Lou, 2008). Direct transcriptional effects by HuR have not been reported to date. Since in our studies, HuR binds the Thy-1 ARE (Fig. 4), and a decrease in its expression enhances cAMP mediated Thy-1 mRNA decay (Fig. 5F), we believe that HuR is acting as a Thy-1 mRNA stabilizing protein.
That the knockdown of HuR did not effect basal levels of Thy-1 mRNA (Fig. 5E), but did alter cAMP mediated Thy-1 regulation (Fig. 5F) suggests that signaling events initiated by cAMP lead to a decrease in the effectiveness of HuR as a stabilizing protein. A decrease in HuR function could result from impaired nuclear-cytoplasmic shuttling or decreased protein expression, which may be consequences of post translational modification, such as phosphorylation (Wang et al., 2002; McMullen et al., 2003; Doller et al., 2008a, Abdelmohsen et al., 2007, 2008). Preliminary findings in this laboratory suggest that NE/cAMP signaling decreases HuR protein expression in the cytoplasm through a mechanism involving PKA (unpublished data). Thus, a decrease in cytoplasmic HuR protein might expose Thy-1 mRNA, allowing a destabilizing protein to act. HuR can compete with the destabilizing proteins TTP and KSRP (Linker et al., 2005; Ming et al., 2001), of which, TTP mRNA is upregulated in S49 cells upon treatment with a cAMP analogue (Zambon et al., 2005). Thus, cAMP regulation of Thy-1 mRNA decay could be mediated by competition between the stabilizing protein, HuR and the destabilizing protein, TTP.
PKA is essential for CA/cAMP-dependent Thy-1 mRNA decay, as is seen in the absence of mRNA decay in S49 kin- cells (Wajeman-Chao et al., 1998). Consequently, it is likely that PKA phosphorylates an effector protein essential to CA/cAMP-mediated mRNA decay. Consistent with this hypothesis, in S49 wt cells we find a number of proteins phosphorylated by PKA in response to NE treatment which are absent in kin-cells (Fig. 6). Of interest, some of the phosphorylated proteins have molecular weights similar to the Thy-1 AREbps, including the 60 kD protein that specifically binds to the Thy-1 ARE, but not to the TNF-α ARE (Fig. 2C), and the identified AREbps, AUF1 and TIAR, that run in the 38-50 kD range (Fig. 4). AUF1 isoforms p40 and p45 have previously been identified as PKA substrates (Tolnay et al., 2000), however, a lack of Thy-1 mRNA regulation in the siAUF1 cells suggests that a different PKA modified protein is involved in the Thy-1 decay mechanism (Fig. 5E-F). Numerous PKA phosphorylated substrates, including kinases (e.g. GSK3α/β), transcription factors (e.g. CREB, NF-kB), and proteins involved in mitogen-activated protein (MAP) signaling (e.g. HePTP, and PTP-SL) have molecular weights matching those of the phosphorylated proteins identified herein (Shabb 2001). Recently, we have shown that p38 MAP kinase, a kinase involved in mRNA stability regulation, is activated by NE in S49 cells through a PKA dependent mechanism, suggesting an involvement of MAPKs in Thy-1 decay (Manuscript in preparation, M.D. LaJevic, and D.A. Chambers). Since there are many proteins phosphorylated by PKA that display the initial characteristics of the effector protein, further investigation of this group could enhance understanding of the mechanism of NE/cAMP-mediated mRNA regulation.
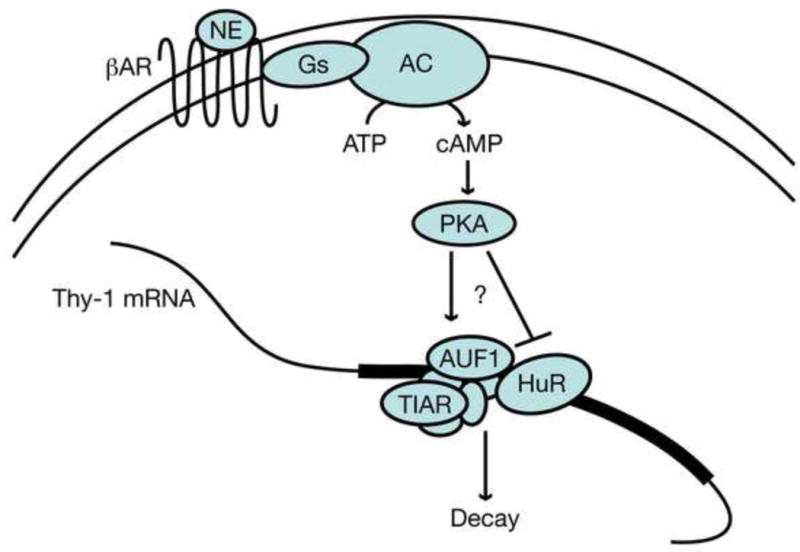
These results add to our previous mechanistic knowledge of NE mediated Thy-1 mRNA decay as shown in Figure 7. We have identified an ARE in the Thy-1 mRNA and shown that it acts as a destabilizing element in the presence of cAMP. The AREbps AUF1, HuR, and TIAR bind to the Thy-1 ARE in vitro. cAMP signaling, through PKA activation, leads to the phosphorylation of multiple proteins, which, in part, may be related to a decrease in the ability of HuR to stabilize Thy-1 mRNA. These investigations are important in understanding the dynamics of Thy-1 mRNA regulation, and may extend to understanding the NE mediated regulation of other ARE containing mRNAs, such as cytokines, that are ubiquitous and have been associated with stress mediated immune dysfunction.
Figure 7. Proposed mechanism of NE mediated Thy-1 mRNA decay.
Acknowledgments
Dr. Lloyd Graf (currently a Research Assistant Professor, Cincinnati Children's Hospital Medical Center, Pulmonary Division) designed and constructed the TNF-α and Thy-1 ARE pG7p(A) plasmids while a member of this laboratory.
We would like to thank our colleague Daniel Davis for helpful suggestions and technical advice.
Abbreviations used in this paper
- CA
catecholamine
- NE
norepinephrine
- βAR
beta-adrenergic receptor
- AC
adenylyl cyclase
- ARE
AU rich element
- AREbp
ARE binding protein
- 3′ UTR
3′ untranslated region
Footnotes
This work was supported by National Institutes of Health grant RO1 DE/AI 13684
Disclosures: The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelmohsen K, Pullmann R, JR, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmohsen K, Srikantan S, Kuwano Y, Gorospe M. miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc Natl Acad Sci U S A. 2008;105:20297–20302. doi: 10.1073/pnas.0809376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ader R, Cohen N, Felton D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet. 1995;345:99–103. doi: 10.1016/s0140-6736(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- Barker TH, Hagood JS. Getting a grip on Thy-1 signaling. Biochim Biophys Acta. 2009;1793:921–923. doi: 10.1016/j.bbamcr.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau C, Paillard L, Osborne HB. AU rich elements and associated factors; are there unifying principles? Nucleic Acids Res. 2006;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua A, Ceriani MC, Capaccioli S, Nicolin A. Post-Transcriptional regulation of gene expression by degradation of messenger RNAs. J Cell Physiol. 2003;195:356–372. doi: 10.1002/jcp.10272. [DOI] [PubMed] [Google Scholar]
- Brewer BY, Malicka J, Blackshear PJ, Wilson GM. RNA sequence elements required for high affinity binding by the zinc finger domain of tristetraprolin: conformational changes coupled to the bipartite nature of AU-rich mRNA-destabilizing motifs. J Biol Chem. 2004;279:27870–27877. doi: 10.1074/jbc.M402551200. [DOI] [PubMed] [Google Scholar]
- Chambers DA. Thy-1 epidermal cell: perspective and prospective. J Dermatol. 1985;133:24–33. doi: 10.1111/j.1365-2133.1985.tb15623.x. [DOI] [PubMed] [Google Scholar]
- Chambers DA, Cohen RL, Perlman RL. Neuroimmune modulation: signal transduction and catecholamines. Neurochem Int. 1993;22:95–110. doi: 10.1016/0197-0186(93)90002-m. [DOI] [PubMed] [Google Scholar]
- Chambers DA, Schauenstein K. Mindful immunology: neuroimmunomodulation. Immunol Today. 2000;21:168–170. doi: 10.1016/s0167-5699(99)01577-7. [DOI] [PubMed] [Google Scholar]
- Cheadle C, Fan J, Cho-Chung YS, Werner T, Ray J, Do L, Gorospe M, Becker KG. Control of gene expression during T cell activation: alternate regulation of mRNA transcription and mRNA stability. BMC Genomics. 2005a;6:75. doi: 10.1186/1471-2164-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle C, Fan J, Cho-Chung YS, Werner T, Ray J, Do L, Gorospe M, Becker KG. Stability regulation of mRNA and the control of gene expression. Ann N Y Acad Sci. 2005b;1058:196–204. doi: 10.1196/annals.1359.026. [DOI] [PubMed] [Google Scholar]
- Cook-Mills JM, Cohen RL, Perlman RL, Chambers DA. Inhibition of lymphocyte activation by catecholamines: evidence for a non-classical mechanism of catecholamine action. Immunology. 1995a;85:544–549. [PMC free article] [PubMed] [Google Scholar]
- Cook-Mills JM, Mokyr MB, Cohen RL, Perlman RL, Chambers DA. Neurotransmitter suppression of the in vitro generation of a cytotoxic T-lymphocyte response against the synergeic MOPC-315 plasmacytoma. Cancer Immunol Immunother. 1995b;40:79–87. doi: 10.1007/BF01520288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook-Mills J, Munshi HG, Perlman RL, Chambers DA. Mouse hepatitis virus infection suppresses modulation of mouse spleen T-cell activation. Immunology. 1992;75:542–545. [PMC free article] [PubMed] [Google Scholar]
- Dean JL, Wait R, Mahtani KR, Sully G, Clark AR, Saklatvala J. The 3′ untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol Cell Biol. 2001;21:721–730. doi: 10.1128/MCB.21.3.721-730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglon N, Wilson A, Desponds C, Laurent P, Bron C, Fasel N. Fatty acids regulate Thy-1 antigen mRNA stability in T lymphocyte precursors. Eur J Biochem. 1995;231:687–696. doi: 10.1111/j.1432-1033.1995.0687d.x. [DOI] [PubMed] [Google Scholar]
- Doller A, Akool el-S, Huwiler A, Muller R, Radeke HH, Pfeilschifter V, Eberhardt W. Posttranslational modification of the AU-rich element binding protein HuR by protein Kinase Cδ elicits angiotensin II-induced stabilization and nuclear export of cyclooxygenase 2 mRNA. Mol Cell Biol. 2008a;28:2608–2625. doi: 10.1128/MCB.01530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008b;20:2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Esclatine A, Taddeo B, Roizman B. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J Virol. 2004;78:8582–8592. doi: 10.1128/JVI.78.16.8582-8592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RD, Hunninghake GW, McArdle WL. Beta-adrenergic-receptor-mediated suppression of interleukin 2 receptors in human lymphocytes. J Immunol. 1987;139:3355–3359. [PubMed] [Google Scholar]
- Frohman EM, Vayuvegula B, Gupta S, van den Noort S. Norepinephrine inhibits gamma interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta2-adrenergic signal transduction mechanisms. Proc Natl Acad Sci U S A. 1988;85:1292–1296. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherzi R, Lee KY, Briata P, Wegmuller D, Moroni C, Karin M, Chen CY. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol Cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Gueydan C, Droogmans L, Chalon P, Huez G, Caput D, Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J Biol Chem. 1999;274:2322–2326. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- Guirao X, Kumar A, Katz J, Smith M, Lin E, Keogh C, Calvano SE, Lowry SR. Catecholamines increase monocyte TNF receptors and inhibit TNF through beta2-adrenoreceptor activation. Am J Physiol. 1997;273:1203–1208. doi: 10.1152/ajpendo.1997.273.6.E1203. [DOI] [PubMed] [Google Scholar]
- Haeryfar SM, Conrad DM, Musgrave B, Hoskin DW. Antibody blockade of Thy-1 (CD90) impairs mouse cytotoxic T lymphocyte induction by anti-CD3 monoclonal antibody. Immunol Cell Biol. 2005;83:352–363. doi: 10.1111/j.1440-1711.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- Haeryfar SM, Hoskin DW. Thy-1: More than a mouse pan-T cell marker. J Immunol. 2004;173:3581–3588. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–379. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Baltimore D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol. 2009;10:281–288. doi: 10.1038/ni.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hel Z, Di Marco S, Radzioch D. Characterization of the RNA binding proteins forming complexes with a novel putative regulatory region in the 3′UTR of TNF-alpha mRNA. Nucleic Acids Res. 1998;26:2803–2812. doi: 10.1093/nar/26.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuber AO, Bernard AM, Battari CL, Marguet D, Massol P, Foa C, Brun N, Garcia S, Stewart C, Pierres M, He HT. Thymocytes in Thy-1-/- mice show augmented TCR signaling and impaired differentiation. Curr Biol. 1997;7:705–708. doi: 10.1016/s0960-9822(06)00300-9. [DOI] [PubMed] [Google Scholar]
- Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65:3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowski TA, Gallant S, Spengler RN. Temporal regulation by adrenergic receptor stimulation of macrophage (M phi)-derived tumor necrosis factor (TNF) production post-LPS challenge. J Neuroimmunol. 1996;65:107–117. doi: 10.1016/0165-5728(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Stehn JR, Yaffe MB, Blackwell TK. Cytoplasmic localization of tristetraproline involves 14-3-3-dependent and –independent mechanisms. J Biol Chem. 2002;277:18029–18036. doi: 10.1074/jbc.M110465200. [DOI] [PubMed] [Google Scholar]
- Khabar KS. Rapid transit in the immune cells: the role of mRNA turnover regulation. J Leukoc Biol. 2007;81:1335–1344. doi: 10.1189/jlb.0207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine and beta-2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leposavic G, Pilipovic I, Radojevic K, Pesic V, Perisic M, Kosec D. Catecholamimes as immunomodulators: A role for adrenoceptor-mediated mechanisms in fine tuning of T-cell development. Auton Neurosci. 2008;144:1–12. doi: 10.1016/j.autneu.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005;33:4813–4827. doi: 10.1093/nar/gki797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JY, Schneider RJ. Tissue distribution of AU-rich mRNA-binding proteins involved in regulation of mRNA decay. J Biol Chem. 2004;279:12974–12979. doi: 10.1074/jbc.M310433200. [DOI] [PubMed] [Google Scholar]
- Lung HL, Bangarusamy DK, Xie D, Cheung AK, Cheng Y, Kumaran MK, Miller L, Liu ET, Guan XY, Sham JS, Fang Y, Li L, Wang N, Protopopov AI, Zabarovsky ER, Tsao SW, Stanbridge EJ, Lung ML. THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene. 2005;24:6525–6532. doi: 10.1038/sj.onc.1208812. [DOI] [PubMed] [Google Scholar]
- Marmor MD, Bachmann MF, Ohashi PS, Malek TR, Julius M. Immobilization of glycosylphosphatidylinositol-anchored proteins inhibits T cell growth but not function. Int Immunol. 1999;11:1381–1393. doi: 10.1093/intimm/11.9.1381. [DOI] [PubMed] [Google Scholar]
- McMullen MR, Cocuzzi E, Hatzoglou M, Nagy LE. Chronic ethanol exposure increases the binding of HuR of the TNF alpha 3′untranslated region in macrophages. J Biol Chem. 2003;278:38333–38341. doi: 10.1074/jbc.M304566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, Nance DM. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun. 2004;8:262–273. doi: 10.1016/j.bbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ming XF, Stoecklin G, Lu M, Looser R, Moroni C. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol Cell Biol. 2001;21:5778–5789. doi: 10.1128/MCB.21.17.5778-5789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesic V, Plecas-Solarovic B, Radojevic K, Kosec D, Pilipovic I, Perisic M, Leposavic G. Long-term b-adrenergic receptor blockade increases levels of the most mature thymocyte subsets in aged rats. Int Immunopharmacol. 2007;7:674–686. doi: 10.1016/j.intimp.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri I, Wegmueller D, Gross B, Certa U, Moroni C. Roles of AUF1 isoforms, HuR, and BRF1 in ARE-dependent mRNA turnover studied by RNA interference. Nucleic Acids Res. 2004;32:1279–1288. doi: 10.1093/nar/gkh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta. 2006a;1763:991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006b;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, Hagood JS. Thy-1 promoter hypermethylation: a novel pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:610–618. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekut L, Champion BR, Page K, Menius JA, Jr, Connolly KM. Anti-inflammatory activity of salmeterol: down regulation of cytokine production. Clin Exp Immunol. 1995;99:461–466. doi: 10.1111/j.1365-2249.1995.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabb JB. Physiological substrates of cAMP-dependent protein kinase. Chem Rev. 2001;101:2381–2411. doi: 10.1021/cr000236l. [DOI] [PubMed] [Google Scholar]
- Stoecklin G, Lu M, Rattenbacher B, Moroni C. A constitutive decay element promotes tumor necrosis factor alpha mRNA degradation via an AU-rich element-independent pathway. Mol Cell Biol. 2003;23:3506–3515. doi: 10.1128/MCB.23.10.3506-3515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolnay M, Juang YT, Tsokos GC. Protein kinase A enhances, whereas glycogen synthase kinase-3β inhibits, the activity of the exon 2-encoded transactivation domain of heterogenous nuclear ribonucleoprotein D in an hierarchical fashion. Biochem J. 2002;363:127–136. doi: 10.1042/0264-6021:3630127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajeman-Chao SA, Lancaster SA, Graf LH, Jr, Chambers DA. Mechanism of Catecholamine-mediated destabilization of messenger RNA encoding Thy-1 protein in T-lineage cells. J Immunol. 1998;161:4825–4833. [PubMed] [Google Scholar]
- Wang W, Fan J, Yang X, Furer-Galban S, Lopez de Silanes I, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GM, Lu J, Sutphen K, Sun Y, Huynh Y, Brewer G. Regulation of A+U-rich element-directed mRNA turnover involving reversible phosphorylation of AUF1. J Biol Chem. 2003;278:33029–33038. doi: 10.1074/jbc.M305772200. [DOI] [PubMed] [Google Scholar]
- Wilson GM, Sutphen K, Bolikal S, Chuang KY, Brewer G. Thermodynamics and kinetics of Hsp70 Association with A+U-rich mRNA-destabilizing sequences. J Biol Chem. 2001a;276:44450–44456. doi: 10.1074/jbc.M108521200. [DOI] [PubMed] [Google Scholar]
- Wilson GM, Sutphen K, Chuang KY, Brewer G. Folding of A+U-rich RNA elements modulates AUF1 binding. Potential roles in regulation of mRNA turnover. J Biol Chem. 2001b;276:8695–8704. doi: 10.1074/jbc.M009848200. [DOI] [PubMed] [Google Scholar]
- Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- Xue GP, Calvert RA, Morris RJ. Expression of the neuronal surface glycoprotein Thy-1 is under post-transcriptional control, and is spatially regulated, in the developing olfactory system. Development. 1990;109:851–864. doi: 10.1242/dev.109.4.851. [DOI] [PubMed] [Google Scholar]
- Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, Insel PA. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci U S A. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Kruys V, Huez G, Gueydan C. AU-rich element-mediated translational control: complexity and multiple activities of trans-acting factors. Biochem Soc Trans. 2002;30:952–958. doi: 10.1042/bst0300952. [DOI] [PubMed] [Google Scholar]