Abstract
Background
Sexually transmitted disease (STD) patients are more likely to experience a future STD including HIV.
Purpose
To examine the efficacy of behavioral interventions to reduce sexual risk behavior and incident STDs among patients attending STD clinics in the United States.
Methods
Meta-analysis of 32 studies with 48 separate interventions targeting STD patients (N = 67,538). Independent raters coded study, sample, and intervention characteristics. Effect sizes, using both fixed- and random-effects models, were calculated. Potential moderators of intervention efficacy were assessed.
Results
Relative to controls, intervention participants increased their condom use and had fewer incident STDs, including human immunodeficiency virus (HIV), across assessment intervals (d+s ranging from 0.05 to 0.64). Several sample (e.g., age, ethnicity) and intervention features (e.g., targeting intervention to a specific group) moderated the efficacy of the intervention.
Conclusions
Behavioral interventions targeted to STD clinic patients reduce sexual risk behavior and prevent HIV/STDs. Widespread use of behavioral interventions in STD clinics should be a public health priority.
Keywords: HIV/STD, condom, sex, meta-analysis, behavior, prevention
Sexually transmitted diseases (STDs) remain a major public health concern. The Centers for Disease Control and Prevention (CDC) estimates that 45 million Americans have been infected with genital herpes, 20 million with human papillomavirus (HPV), and more than 1 million with HIV. The annual incidence of HPV exceeds 6 million, trichomoniasis exceeds 7 million, and HIV newly infects more than 56,000 (1). Untreated STDs can result in pelvic inflammatory disease, chronic pelvic pain, ectopic pregnancy, birth complications, and infertility among women, and epididymitis and urethritis among men (2, 3). Moreover, untreated STDs substantially increase the risk of both acquisition and transmission of HIV (3–5). In addition to the health consequences, STDs pose a huge economic burden to the U.S. health care system with an estimated direct cost of $15.3 billion annually (3). To reduce the health and economic burden of STDs, the CDC has called for the expansion of prevention efforts (3). Fundamental to expanded prevention efforts is the identification and evaluation of successful STD prevention and intervention programs in reducing sexual risk behavior, STD acquisition, and HIV transmission.
STD clinics provide an opportune setting for evaluating sexual risk reduction prevention efforts. Patients attending STD clinics are known to engage in risky sexual behavior and other health behaviors (e.g., alcohol and drug use) that facilitate the acquisition of STDs, including HIV (6, 7). Not only do STD clinic patients report riskier sexual behaviors, they are more likely to return with a subsequent STD (8, 9). Moreover, they are at increased risk of HIV infection relative to the general population (10, 11). Compared with uninfected individuals, people with untreated STDs are two to five times more at risk of contracting HIV through sexual contact (3, 4). Because patients at STD clinics are more susceptible to HIV, and STDs increase the risk of transmitting HIV to a sexual partner (3), identifying successful intervention strategies among STD clinic patients is critical in the prevention of HIV and other STDs.
To prevent sexually transmitted infections, the CDC recommends a comprehensive approach to STD prevention that includes early STD diagnosis, treatment, and behavioral intervention (3). Evaluating the efficacy of behavioral interventions to reduce sexual risk among STD clinic patients is essential to improving comprehensive prevention efforts. Several literature (12) and meta-analytic reviews (13, 14) have evaluated the efficacy of behavioral interventions to reduce sexual risk behavior and incident STDs among clinic patients. In general, these reviews found behavioral interventions were successful at increasing condom use among treatment patients relative to controls; however, findings for incident STDs were inconsistent (or could not be determined). For instance, DiClemente et al.’s (12) review of clinic-based sexual risk reduction interventions among adolescents (k = 9) could not investigate the efficacy of behavioral interventions to reduce STDs due to the lack of inclusion of STDs as an outcome, whereas Ward et al. (14) found a reduction in incident STDs among patients in studies reporting clinical diagnoses (k = 3) but not for those studies reporting laboratory confirmed STDs (k = 8). Reduction in incident STDs (k = 13) was found in a meta-analysis of 18 randomized controlled trials (RCTs) focusing on Black and Hispanic STD clinic patients in the United States (13). Although these reviews do provide evidence that behavioral interventions are efficacious at reducing sexual risk behavior (i.e., condom use) and incident STDs among some STD clinic patients (i.e., Blacks and Hispanics in the United States), it is unclear whether incident STDs are improved in broader samples of patients. Furthermore, these reviews did not (or could not) address number of sexual partners, which is associated with the prevalence of STDs. Finally, efficacy of behavioral interventions among STD clinic patients were based on the longest assessment interval, ranging from 3 to 12 months post-intervention, rather than examining the durability of the interventions over time.
The purpose of the current study was to use meta-analytic techniques to systematically evaluate the efficacy of behavioral interventions when implemented with STD clinic patients. We extend prior reviews of behavioral interventions for STD clinic patients by using a larger sample of studies (k = 32) to address the aforementioned limitations. Specifically, we include studies sampling any patients attending U. S. STD clinics, examine both condom use and number of sexual partners, and assess longer-term outcomes (i.e., up to 2-years post-intervention). Intervention success was measured with four outcomes: (a) condom use, (b) number of sexual partners, and (c) incident STDs, including (d) HIV. We hypothesized that STD clinic patients who received a sexual risk reduction intervention would show increases in condom use, report fewer sexual partners, and would be less likely to acquire STDs, including HIV, relative to control participants.
We also examined the durability of intervention improvements and the extent to which efficacy depended upon participant or intervention characteristics. Moderators included (a) age, race, and gender, (b) baseline STD diagnosis, (c) intervention content (tailored or targeted, motivation and skills training), and (d) intervention length. We hypothesized that interventions would be more efficacious when they (a) sampled greater proportions of those who bear the heaviest burden of STDs (3)—namely, young adults (ages 15 to 24), Blacks, and women; (b) sampled patients diagnosed with a STD, as they may be more motivated to initiate sexual risk reduction than uninfected patients; (c) targeted motivation and provided skills training, consistent with the Information-Motivation-Behavioral Skills Model of HIV-prevention (15, 16); (d) tailored content to the individual or targeted content toward a specific group (e.g., gender), thus increasing message relevancy (17); and (e) were of longer duration, providing additional time to develop risk reduction skills (15, 16).
METHODS
Search Strategy and Study Selection
A comprehensive search strategy was used to obtain relevant studies. Studies were retrieved from (a) electronic databases (PsycINFO, PubMed, Dissertation Abstracts, ERIC, CINAHL, and The Cochrane Library) using a Boolean search strategy with the following terms: (HIV OR AIDS OR (human AND immu* AND virus) OR (acquired AND immu* AND deficien* AND syndrome)) AND (prevent* OR interven*) AND (condom* OR sex*) AND ((sexually and transmitted and infection*) OR (sexually and transmitted and disease*) OR STI OR STD)) AND (clinic OR hospital OR healthcare OR center OR infirmary OR dispensary)), (b) reference sections of relevant manuscripts, (c) electronic content of professional journals, and (d) electronic database searches for manuscripts authored by researchers with relevant funding (i.e., list of principal investigators retrieved from the CRISP database [now known as the NIH RePORTER]). To optimize thoroughness, we conducted the database search at study onset (September 2008) and upon completion of the initial coding (February 2009).
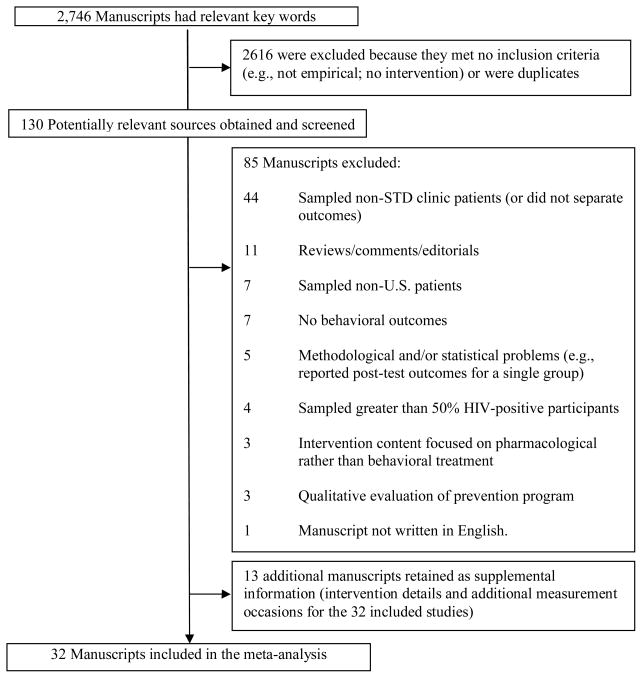
Studies were included if the author(s) (a) examined an individual- or group-level behavioral intervention intended to reduce sexual risk behavior and the risk of STDs, including HIV, (b) sampled patients attending a STD clinic in the United States, (c) used a randomized controlled trial (RCT) or a quasi-experimental design reporting pre-test outcomes (used to evaluate the equivalence of the treatment and control participants) with a comparison condition, (d) assessed sexual risk behavior or STD acquisition, and (e) provided information needed to calculate effect sizes. Studies were excluded if they (a) did not focus on improving individual-level sexual risk behaviors, (b) sampled patients from other locations (e.g., community agency), (c) included samples with greater than 50% HIV-positive patients (because these individuals require more comprehensive care, including interventions that focus on secondary prevention of HIV transmission rather than STD prevention), (d) used a within-subjects design with no comparison condition, or (e) evaluated a strictly structural-level (e.g., mass media) intervention. When authors reported details and/or outcomes in multiple manuscripts, the studies were linked in the database and represented as a single study. If a study reported on more than one comparison condition, the comparison condition with the least contact (e.g., wait-list) was used. When author(s) reported insufficient details, they were contacted for information. Of the three authors contacted, 100% responded resulting in the retention of two studies and the exclusion of one study. Studies that fulfilled the selection criteria and were available by March of 2009 were included. Thus, we included 32 manuscripts with 48 separate interventions (Figure 1).
Figure 1.
Selection process for study inclusion in the meta-analysis
Coding and Reliability
Two independent coders [LAJSS, RLF] rated the study information, sample characteristics (e.g., sex, ethnicity), design and measurement specifics (e.g., number of follow-ups), and details of the control and intervention condition(s) (e.g., number of sessions, STD testing and treatment, provided condoms). Based on the Information-Motivation-Behavioral Skills Model of behavior change (15, 16), intervention content included information (STD or HIV education), motivation (risk feedback such as STD-knowledge scores, risk awareness, assessments of the pros and cons of sexual risk behavior, attitudes toward condom use or partner reduction, and transsituational motivational factors such as life goals, personal and/or community values), and behavioral skills (condom, communication, and self-management [i.e., planning and/or goal setting] skills). Finally, we evaluated whether the intervention content was tailored (content altered for a specific individual, e.g., addressing specific sexual risk reduction knowledge deficits) or targeted (i.e., content altered for a specific sub-group, e.g., focused on women-specific risks associated with STD transmission) based on Kreuter and Wray’s (17) description of tailored and targeted health communication.
Methodological quality for each study was assessed using 12 items (e.g., random assignment) from validated measures (18, 19); scores range from 0 to 17. Twenty studies were randomly selected to assess inter-rater reliability. For the categorical variables, raters agreed on 59% to 100% of the judgments (mean Cohen’s κ = .71). Reliability for the continuous variables (calculated using the intraclass correlation coefficient; ρ) yielded an average ρ = .93 across categories (median = 1.00). Disagreements between coders were resolved through discussion.
Study Outcomes
For each study, effect size estimates were calculated for condom use (or unprotected sex), number of sexual partners, and incident STDs, including HIV. Studies assessed condom use using a variety of measures (e.g., condom use at last sex, proportion of unprotected sexual events) for vaginal and anal sex. Thus, condom use included protected or unprotected vaginal, anal, or unspecified sex. Because none of the investigators measured the number of sexual partners separately by partner type, number of sexual partners refers to the number of any type of sexual partner over a specified interval. (Studies typically did not report on partner concurrency so this could not be coded.) Incident STDs refers to laboratory- or clinically-diagnosed STDs. Laboratory-confirmed new HIV infections comprised Incident HIV.
Effect Size Derivation
Because the majority of the author(s) reported continuous measures, effect sizes (d) were defined as the mean difference between the treatment and control groups divided by the pooled standard deviation (20). When means and standard deviations were not provided, other information (e.g., t or F test) was used (21). If a study reported dichotomous outcomes, we calculated an odds ratio and transformed it to d using the Cox transformation (22). If no statistical information was available (and could not be obtained) and the author(s) reported no significant between-group differences, we estimated that effect size as zero (21). (Of the 184 effect sizes calculated, 8 were estimated as zero.) In calculating d, we controlled for baseline differences when pre-intervention measures were available (23). All effect sizes were corrected for sample size bias (24). Positive effect sizes indicated more risk reduction, that is, participants receiving the intervention increased their condom use, decreased their number of sexual partners, and had fewer incident STDs or HIV infections compared to controls.
Multiple effect sizes were calculated from individual studies when they had more than one outcome, multiple intervention conditions, or when outcomes were separated by sample characteristics (e.g., gender). Effect sizes calculated for each intervention and by sample characteristic were analyzed as a separate study (21). When a study contained multiple measures of the same outcome, the effect sizes were averaged (with corresponding sample sizes averaged). Two authors independently calculated effect sizes using DSTAT 2.0 (25); discrepancies were examined for errors and corrected.
Timing of post-intervention assessments varied with the first assessment occurring between 0 to 64 weeks (k = 46), the second at 13 weeks (k = 1), and a third assessment at 26 weeks (k = 1). To avoid violating the assumption of study independence and as a strategy to examine all study assessments, effect sizes were clustered into three intervals: (a) short-term (4 to 13 weeks; k = 35), (b) intermediate (22 to 39 weeks; k = 29), and (c) long-term (52 to 104 weeks; k =20) on the basis of natural clusters of assessments on a stem-and-leaf plot (available from the authors).
Statistical Analysis
All dependent variables were examined for outliers (26); for each variable and assessment interval, extreme effect sizes (i.e., effect sizes more than 2 standard deviations from the mean) were recoded to be equivalent to the value at 2 standard deviations (i.e., winsorizing) (21). Of the 184 effect sizes, 7 outliers were detected (4% of the total number of effect size estimates). Weighted mean effect sizes, d+, were calculated using fixed- and random-effects procedures (21). The homogeneity statistic, Q, determined whether each set of d+s shared a common effect size. The homogeneity of variance statistic has an approximate chi-square distribution with the number of effect sizes (k) minus 1 degrees of freedom (27); a significant Q indicates a lack of homogeneity. To further assess homogeneity, the I2 index (ranging between 0 and 100%) and its corresponding 95% confidence intervals (CIs) were calculated (28, 29). If the 95% confidence interval around I2 includes zero, the set of effect sizes is considered homogeneous.
To explain variability in effect sizes, the relation between sample, methodological, or intervention characteristics and the magnitude of the effects were examined using modified weighted least squares regression analyses (following fixed-effects assumptions) with weights equivalent to the inverse of the variance for each effect size (21, 30). Univariate regression analyses examined a priori determined moderators of condom use effect sizes at all assessments. Sample characteristics (age group, sex, ethnicity, STD diagnosis), intervention content (provided motivation or behavioral skills including condom distribution), features of the intervention (individually tailored or group targeted content), and intervention dose were examined. To control for Type I error, we used the Bonferroni correction to adjust the P-values, in this case P = .005. Significant univariate moderators were simultaneously entered into multiple regression models to test for unique variance. Multiple regression analyses were conducted only for outcomes with sufficient effect sizes per moderator (i.e., > 5 cases per independent variable). For the multiple regression analyses, continuous variables (e.g., proportion women) were mean-centered to reduce multicolinearity. Analyses were conducted in Stata 10.0 (31) using macros provided by Lipsey and Wilson (21).
Publication Bias
We tested for publication bias (i.e., when studies with significant findings are published, whereas studies with non-significant findings remain unpublished; the file-drawer effect (32)). We examined our data for publication bias by (a) generating and inspecting funnel plots of the weighted mean effect size by standard error (33) and (b) systematically examining funnel plot asymmetry using two methods: non-parametric (estimating the correlation between a standardized effect size estimate and its variance) (34) and linear regression (standardized effect size estimate is regressed against its precision, defined as the inverse of the standard error) (35, 36).
RESULTS
Study, Sample, and Intervention Details
Table 1 provides sample and intervention details for the 32 included studies. Studies appeared between 1991 and early 2009 (median publication year was 1999). Methodological quality (MQ) of the studies ranged from 7 to 15 (mean = 10.52; median = 11; SD = 1.99). Publication year and MQ score were correlated (r = 0.39, P = .03) with newer studies (studies published in or after the year 2000) of higher quality (median score = 11) than older studies (median score = 10).
Table 1.
Study, Sample, and Intervention Characteristics of the 32 Studies Included in the Meta-Analysis
| Intervention Details | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Sample | Location | Control | Delivery | Facilitators | Sessions | Dosea | Incentive | MQ |
| Artz et al. (57, 83, 84) | N = 427 (16%); 100% F; 88% B; M age = 25; 48% STD | Birmingham, AL | INFO | Video + Individual | 1 Para | 1 | 49 | NR | 11 |
| Boyer et al. (37) | N = 395 (28%); 33% F; 46% B | San Francisco, CA | SC | Individual | 1 Para | 4 | 240 | Assessments (US $20–30) | 11 |
| Branson et al. (38) | N = 964 (46%); 44% F; 90% B; 50% STD | Houston, TX | SC | Group | 1 | 4 | 240 | Attendance (US $15 per session) | 10 |
| Carey et al. (67) | N = 60 (0%); 17% F; 67% B; 17% M age = 31.3; STD | Rochester, NY | SC | Individual | 1 Para | 1 | 17.5 | Assessment (US $20) | 11.3 |
| Carey et al. (39) | N = 1497 (26%); 46% F; 64% B; 18% STD | Rochester, NY | INFO | Individual + Group | 1.5 Para/Prof | 2 | 255 | Assessments (US $20–30); | 12 |
| Individual + Group | 1.5 Para/Prof | 2 | 255 | Attendance (US $40); child care, lunch | |||||
| Individual + Group | 1 Para/Prof | 2 | 255 | ||||||
| Individual + Group | 1.5 Para/Prof | 2 | 255 | ||||||
| Individual | 1 Para | 1 | 15 | Assessments (US $20–30) | |||||
| Cohen et al. (59) | N =192 (0%); 41% F; 67% B; 88% STD | Los Angeles, CA | AO | Group | 1 Para | 1 | 12.5 | NR | 10.2 |
| Cohen et al. (60) | N = 903 (0%); 39% F; 72% B; 88% STD | Los Angeles, CA | AO | Group | 1 Para | 1 | 17.5 | Assessment (US $5) | 10 |
| Group | 1 Para | 17.5 | |||||||
| Group | 1 Para | 1 | 17.5 | ||||||
| Cohen et al. (61) | N = 551 (33%); 29% F; 92% B; M age = 28.4; 100% STD | Los Angeles, CA | AO | Group | 1 Para | 1 | 45 | NR | 9 |
| Crosby et al. (40) | N = 266 (26%); 0% F; 100% B; M age = 23.3; 100% STD | Southern US city | SC | Individual | 1 Para | 1 | 47.5 | Assessments (US $40–60) | 12 |
| DeLamater et al. (41) | N = 562 (1%); 0% F; 100% B; M age = 18.3 | Milwaukee, WI | SC | Video | NR | 1 | 14 | Assessment (US $10–20) | 11 |
| Individual | 1 Para | 1 | 14 | ||||||
| Gillmore et al. (42) | N = 226 (36%); 58% F; 54% W; M age = 17.11 | Northwestern US | RCNM | Video + Comic Book | NR | 1 | 43 | Assessments (US $10–40); gift bag | 7 |
| Gollub et al. (62) | N = 1591 (0%); 100% F; 91% B; M age = 27.75; 60% STD | Philadelphia, PA | RCM | Group | 1 Para | 1 | 22.5 | NR | 11 |
| Gollub et al. (43) | N = 292 (38%); 100% F; 91% B; M age = 27.9 | Philadelphia, PA | RCM | Group | 1 Para | 1 | 21.5 | NR | 7 |
| Jenkins et al. (85, 86) | N = 400 (27%); 0% F; 57% B; 69% STD | Fort Bragg, NC | SC | Individual | 1 Para/Prof | 2 | 35 | NR | 9 |
| Individual + Video | 1 Para/Prof | 2 | 35 | ||||||
| Individual | 1 Para/Prof | 2 | 35 | ||||||
| Kalichman et al. (47) | N = 752 (35%); 31% F; 95% B; M age = 35.3 | Milwaukee, WI | INFO | Individual | 1 Para | 1 | 90 | Assessments (US $35–45) | 13 |
| Individual | 1 Para | 1 | 90 | ||||||
| Individual | 1 Para | 1 | 90 | ||||||
| Kalichman et al. (44) | N = 108 (11%); 0% F; 100% B; M age = 33.8 | Southeastern US | RCM | Group | 2 Para | 1 | 180 | Assessments (US $30–35); snacks, lunch | 11 |
| Group | 2 Para | 1 | 180 | ||||||
| Kalichman et al. (45) | N = 117 (18%); 0% F; 100% B; M age = 33.3 | Atlanta, GA | RCM | Group | 2 Para | 2 | 360 | Assessments (US $25–35); snacks, lunch | 11 |
| Kalichman et al. (46) | N = 81 (6%); 100% F; 100% B; M age = 31.5 | Atlanta, GA | ICM | Groups | 2 Para | 1 | 150 | Assessments (US $30–35); snacks, lunch | 9 |
| Kamb et al. (48, 87) | N = 5708 (46%); 43% F; 59% B; 32% STD | Multiple U.S. cities | INFO | Individual | 1 Para | 4 | 200 | Assessments (US $15–25); | 13 |
| Individual | 1 Para | 2 | 40 | Attendance (US $15) | |||||
| Kissinger et al. (49) | N = 977 (21%); 0% F; 96% B; 100% STD | New Orleans, LA | RCM | Individual | 1 Para | 1 | 15 | Assessments (US $10–40) | 8.5 |
| Individual | 1 Para | 1 | 15 | ||||||
| Maher et al. (63) | N = 581 (60%); 0% F; 100% B; M age = 23.6; 36.3% STD | Dade County, FL | SC | Individual | 1 Para | 3 | 152.5 | Attendance ($15) | 9 |
| Metcalf et al. (50, 88) | N = 3297 (13%); 45% F; 51% B; M age = 25.6; 25.4% STD | Multiple U.S. cities | RCNM | Individual | 1 Para | 2 | 40 | Assessments (US $25–50) | 14.5 |
| Metzler et al. (51) | N = 339 (56%); 68% F; 68% W; M age = 17.3; 100% STD | Oregon | SC | Individual | 1 Para | 3.7 | 277.5 | Assessments (US $10–50); Assessments (US $5–20 | 12 |
| NIMH (52) | N = 2426 (21%); 36% F; 74% B; 100% STD | Multiple U.S. cities | INFO | Group | 2 Para | 7 | 735 | Assessments (US $5–40); Attendance (US $10–20); Bonus (US $1–10) | 15 |
| O’Donnell et al. (64, 89–91) | N = 3348 (3%); 40% F; 62% B; M age = 29.8; 54% STD | South Bronx, NY | AO | Video | NR | 1 | 20 | NR | 7 |
| Video + Group | 1 Para | 1 | 40 | ||||||
| O’Leary et al. (53, 92) | N = 659 (28%); 41% F; 91% B; M age = 30.1 | Maryland, Georgia, and New Jersey | SC/ICM | Group | 2 Para/Prof | 7 | 630 | NR | 10 |
| Orr et al. (54) | N = 209 (46%); 100% F; 55% B; M age = 17.9; 100% STD | Indiana | INFO | Individual | 1 Para | 1 | 15 | Free treatment medication | 10.6 |
| Shain et al.(55, 93, 94) | N = 739 (26%); 100% F; 69% B; M age = 21.6; 100% STD | San Antonio, TX | SC | Individual | 1 Para | 3 | 630 | Assessments (US $25–50); Attendance (US $15–25); meals, gifts | 10 |
| Shain et al. (58) | N = 775 (9%); 100% F; 75% B; M age = 20.93; 100% STD | San Antonio, TX | SC | Group | 1 Para | 5.12 | 658.3 | Assessments (US $15–50); | 12 |
| Group | 1 Para | 4 | 557.5 | Attendance (US $5–25); meals, gifts | |||||
| Smith et al. (65) | N = 205 (70%); 100% F; 73% B | Houston, TX | SC | Group | 1 Para | 1 | 37.5 | NR | 8 |
| Warner et al. (66, 95) | N = 38,635 (0%); 30% F; 46% W; 16% STD | Multiple U.S. cities | AO | Individual | NR | 1 | 23 | NR | 12.5 |
| Wenger et al. (56) | N = 256 (27%); 33% F; 88% B; M age = 27 | Los Angeles, CA | RCNM | Group | 1 Prof | 2 | 50 | NR | 9 |
Note. N, number of consenting participants (attrition); F, proportion female; W, proportion White; B, proportion Black; AO, assessment only control; SC, standard STD or HIV counseling; INFO, information-only; RCM, relevant content, time matched; RCNM, relevant content, not time matched; ICM, irrelevant content, time matched; Para, paraprofessionals; Prof, professionals; NR, none/not reported; MQ, methodological quality score.
Estimated number of minutes of intervention content excluding measurement.
All studies were conducted in the United States: 28% Southwest, 25% Southeast, 16% Northeast, 9% Midwest, 6% Northwest and 16% conducted in multiple regions. Of the 67,538 participants sampled (median = 392 participants), 46% were women, 72% Black, and mean age was 25.80 (SD = 5.75; range = 17 to 35; 41% age 24 and under). Several studies targeted women (25%; k =8) or Blacks (19%; k =8). Most studies (72%) restricted participation to patients who had a STD (37.5%; self-reported or diagnosed via a clinical exam and/or laboratory test), were at elevated risk of contracting a STD (16%; e.g., multiple sexual partners, unprotected sex) or both (9%). Of the 23 studies reporting baseline STDs, current STD diagnosis was confirmed via clinical exam or laboratory test in 69% (median = 88%, range = 16 to 100%; 9 studies restricted their samples to only those with current STDs) of participants.
Interventions were typically conducted in one session (56%; range = 1 to 7) lasting a median of 44 minutes (range = 12.5 to 210 minutes). Most interventions (62.5%) occurred during the clinic visit (either simultaneously or immediately after); 37.5% were scheduled for a later time. Facilitators delivered the intervention via individuals (k = 22), small groups (k = 19), or both individually and small groups (k = 7). Many interventions were individually tailored to the patient (42%) and 31% were targeted to a group (e.g., ethnicity, gender). Intervention content included STD testing and counseling (98%), education (85%), skills training (85%; interventions included 75% condom, 56% self-management [i.e., planning and/or goal setting], and 58% communication skills), and motivational components (79%; 60% risk awareness, 33% risk feedback, 31% attitudes toward condom use and/or reducing the number of sexual partners, 23% transsituational motivational factors, and 15% assessing pros and cons of risk behavior). Only 40% of the interventions specifically reported providing condoms to patients. Comparison conditions were most often an active comparison (83%; e.g., STD/HIV education, brief form of the intervention); only 17% used an assessment-only control. Active comparisons met with a facilitator for a single session (range = 1 to 2) of a median of 15 minutes (range = 5 to 180 minutes). Condoms were provided in 36% of the control groups.
Publication Bias
Visual inspection of the funnel plots (Figures A1 – A10, electronic supplementary materials) suggested that the effect sizes of the interventions represented for any outcome by assessment interval did not reveal a publication bias. More formal testing (see Table A1, electronic supplementary materials) using Begg’s adjusted rank correlation (34) did not indicate a significant association between effect size estimates and variance for any outcome by assessment interval (Ps > .14). Results using Egger et al.’s (35) regression asymmetry test indicated that the intercept from the regression analyses did not differ from zero for any outcome by assessment interval (Ps >.18) except for number of sexual partners at short-term assessment (P = .04). To determine the number of non-significant unpublished studies necessary to reduce a significant result to non-significant, we calculated Rosenthal’s fail-safe N (32) for number of sexual partners at short-term assessment. Results indicate that 84 interventions assessing number of sexual partners (within 3 months post-intervention) with non-significant results would be necessary to reverse the significant findings.
Overall Efficacy of the Interventions
Table 2 provides the weighted mean effect sizes, d+, for the 20 studies (k = 34) reporting condom use outcomes (37–56), 15 (k = 28) reporting number of sexual partners (38–42, 45, 47, 48, 50, 51, 53, 55–58), 22 (k = 40) reporting incident STDs (37–40, 47–52, 54, 55, 57–66), and 5 (k = 6) reporting incident HIV (48, 50, 63, 66, 67). Overall, analyses indicate that risk reduction interventions showed small to medium improvements in condom use and reduced the number of sexual partners and incident STDs and HIV compared with controls. At short-term and intermediate assessments, intervention participants increased their condom use (d+s = 0.05 to 0.09, random effects) compared to controls. Participants reduced their number of sexual partners at short-term assessment (d+ = 0.08, random effects) compared to controls. At intermediate (d+ = 0.11, random effects)and long-term (d+ = 0.10, random effects) assessment, incident STDs were significantly reduced among intervention participants versus controls. Insufficient studies were available to examine incident HIV at short-term (39) and intermediate assessment (none); however, the incidence of HIV was significantly reduced among intervention participants at long-term (d+ = 0.64, random effects). We found no difference between the intervention and control participants with respect to condom use at long-term assessment (d+ = 0.04, random effects), number of sexual partners at intermediate and long-term assessment (d+s = 0.05 to 0.09, random effects), and incident STDs at short-term assessment (d+ = −0.08, random effects). The overall pattern of results was consistent using fixed- or random-effects assumptions.1
Table 2.
Weighted mean effect sizes and homogeneity statistics for sexual risk reduction outcomes by assessment interval
| Outcomea | k | d+ (95% CI) | Homogeneity | I2 index (95% CI) | ||
|---|---|---|---|---|---|---|
| Fixed effects | Random effects | Q | p | |||
| Short-Term Assessment (4 to 13 weeks) | ||||||
| Condom use, overall | 31 | 0.10 (0.07, 0.14) | 0.09 (0.03, 0.15) | 73.57 | <.001 | 59% (39, 73) |
| No. of Sexual Partners | 24 | 0.09 (0.06, 0.13) | 0.08 (0.04, 0.12) | 28.17 | .21 | 0 |
| Incident STDs | 8 | 0.01 (−0.06, 0.08) | −0.08 (−0.42, 0.27) | 166.14 | <.001 | 96% (94, 97) |
| Intermediate Assessment (22 to 39 weeks) | ||||||
| Condom use, overall | 26 | 0.05 (0.02, 0.09) | 0.05 (0.01, 0.10) | 38.68 | .04 | 0 |
| No. of Sexual Partners | 22 | 0.06 (0.03, 0.09) | 0.05 (−0.01, 0.10) | 33.87 | .04 | 0 |
| Incident STDs | 21 | 0.13 (0.08, 0.18) | 0.11 (0.01, 0.21) | 79.98 | <.001 | 75% (62, 84) |
| Long-Term Assessment (≥52 weeks) | ||||||
| Condom use, overall | 13 | 0.03 (−0.00, 0.06) | 0.04 (−0.03, 0.10) | 33.44 | <.001 | 64% (35, 80) |
| No. of Sexual Partners | 11 | 0.06 (0.01, 0.11) | 0.09 (−0.01, 0.18) | 31.92 | <.001 | 69% (41, 83) |
| Incident STDs | 23 | 0.10 (0.08, 0.12) | 0.10 (0.05, 0.14) | 72.84 | <.001 | 70% (54, 80) |
| Incident HIV | 5 | 0.56 (0.54, 0.58) | 0.64 (0.29, 0.98) | 595.91 | <.001 | 99% (99, 100) |
Extreme effect size values were recoded (i.e., winsorized) for condom use at short- and long-term assessment, number of sexual partners at short-term and intermediate assessments, and incident STDs at intermediate and long-term assessments. The magnitude and direction of the weighted mean effect sizes including outliers were consistent with the results excluding outliers (except for incident STDs at intermediate assessment, random effects: d+ = 0.10, 95% CI = −0.01, 0.21).
Note. k, number of interventions. d+, weighted mean effect size. CI, confidence interval. Boldface text indicates statistically significant improvements among treatment compared with control patients.
Except for number of sexual partners at short-term assessment, the hypothesis of homogeneity was rejected for each outcome across assessment intervals. Moderator tests were conducted to examine whether a priori determined sample, methodological, or intervention characteristics related to the variability in effect sizes (reported below). Specifically, we examined (a) age group (age ≥ 24 years vs. age < 24 years), race (proportion Black), and gender (proportion women), (b) baseline STD diagnosis (% clinically diagnosed with an STD at baseline), (c) intervention content (motivation and/or skills training component, providing condoms, and tailored or targeted content,), and (d) intervention length (total intervention dose). Due to insufficient sample size (k = 5), moderator tests were not conducted for incidence of HIV.
Moderators of Intervention Impact on Condom Use
Moderators of intervention impact on condom use are reported in Table 3. Interventions increased short-term condom use when (a) sampling younger participants (≤24 years of age), (b) condoms were provided, and (c) the intervention targeted a specific group. When entered simultaneously, targeting a specific group (β = .47, P <.001) remained significant. Intermediate condom use improved when researchers sampled participants diagnosed with a STD. At long-term assessment, interventions were successful in improving condom use when (a) sampling more participants diagnosed with a STD, (b) the intervention targeted a specific group and was not tailored to an individual and (c) was of longer duration. None of the moderators remained significant when entered simultaneously in the regression model.
Table 3.
Moderators of condom use, number of sexual partners, and number of incident STDs by assessment interval*
| Short-Term | Intermediate | Long-Term | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderators | β | P | k | β | P | k | β | P | k |
| Condom Use | |||||||||
| Age group | .36 | .002 | 31 | .25 | .115 | 26 | .05 | .770 | 13 |
| % Black | .00 | .973 | 31 | .11 | .504 | 26 | −.17 | .312 | 13 |
| % Women | −.25 | .035 | 31 | −.08 | .633 | 26 | .33 | .054 | 13 |
| % STD | .34 | .012 | 22 | .68 | .002 | 14 | .78 | <.001 | 13 |
| Motivation | −.00 | .986 | 31 | .24 | .133 | 26 | .11 | .541 | 13 |
| Skills | .08 | .468 | 31 | .24 | .129 | 26 | .17 | .329 | 13 |
| Provided condoms | .37 | .001 | 31 | .24 | .129 | 26 | −.15 | .388 | 13 |
| Tailored | −.07 | .555 | 31 | −.33 | .041 | 26 | −.49 | .005 | 13 |
| Targeted | .36 | .002 | 31 | .39 | .014 | 26 | .82 | <.001 | 13 |
| Total dose | .27 | .022 | 31 | .40 | .012 | 26 | .72 | <.001 | 13 |
| No. of Sexual Partners | |||||||||
| Age group | .55 | .003 | 24 | .43 | .012 | 22 | .87 | <.001 | 11 |
| % Black | −.32 | .088 | 24 | −.41 | .017 | 22 | −.64 | <.001 | 11 |
| % Women | −.07 | .716 | 24 | .20 | .233 | 22 | .87 | <.001 | 11 |
| % STD | −.26 | .207 | 18 | .37 | .078 | 12 | .82 | <.001 | 11 |
| Motivation | −.11 | .574 | 24 | .00 | .977 | 22 | −.14 | .444 | 11 |
| Skills | −.01 | .939 | 24 | .01 | .957 | 22 | .06 | .754 | 11 |
| Provided condoms | .58 | .002 | 24 | .28 | .102 | 22 | . | . | 11 |
| Tailored | .38 | .042 | 24 | .26 | .124 | 22 | −.63 | <.001 | 11 |
| Targeted | −.01 | .978 | 24 | −.31 | .069 | 22 | .87 | <.001 | 11 |
| Total dose | −.25 | .189 | 24 | .14 | .422 | 22 | .78 | <.001 | 11 |
| Incident STDs | |||||||||
| Age group | .95 | <.001 | 8 | .08 | .477 | 21 | .37 | .002 | 23 |
| % Black | .74 | <.001 | 8 | −.03 | .781 | 21 | −.03 | .769 | 23 |
| % Women | −.91 | <.001 | 8 | −.43 | <.001 | 21 | .33 | .005 | 23 |
| % STD | .95 | <.001 | 8 | .19 | .092 | 19 | .23 | .057 | 22 |
| Motivation | −.70 | <.001 | 8 | .40 | <.001 | 21 | −.12 | .316 | 23 |
| Skills | −.52 | <.001 | 8 | .07 | .558 | 21 | −.19 | .107 | 23 |
| Provided condoms | . | . | 8 | −.11 | .328 | 21 | .13 | .272 | 23 |
| Tailored | −.70 | <.001 | 8 | .20 | .071 | 21 | .06 | .632 | 23 |
| Targeted | . | . | 8 | −.13 | .252 | 21 | .14 | .247 | 23 |
| Total dose | −.74 | <.001 | 8 | .20 | .071 | 21 | .07 | .526 | 23 |
Models used the inverse of the variance for each effect size as weights; reported coefficients (β) are standardized. k = number of studies. Bold typeface values are significant at P ≤ .005 level (Bonferroni adjusted P-value). Potential moderators with missing values indicate all observations contained identical values.
Moderators of Intervention Impact on Number of Partners
Moderator tests for number of partners are reported in Table 3. Numbers of sexual partners were reduced at short-term assessment when studies sampled younger participants (≤24 years of age) and provided condoms. When entered simultaneously in a regression model, neither age nor condom provision remained significant. We found no significant moderators of number of sexual partners at intermediate assessment. At long-term assessment, interventions were efficacious in reducing the number of sexual partners when (a) sampling younger participants (≤24 years of age), fewer Blacks, more women, or participants diagnosed with an STD, (b) intervention content was not individually tailored but was targeting a specific group, and (c) interventions lasted longer. All other tests for moderators were non-significant (Ps >.005; Bonferroni adjusted P-value). Multiple moderator tests were not conducted at long-term assessment due to the small sample size (k = 11).
Moderators of Intervention Impact on Incident STDs
Table 3 provides results from the moderator tests of intervention impact on incident STDs. Interventions were successful at reducing the incidence of STDs at short-term when (a) sampling younger participants (≤24 years of age), more Blacks, fewer women, and more participants with a current STD, (b) content did not include motivation or skills training, (c) content was not individually tailored, and (d) interventions were shorter. Due to insufficient sample size (k = 8), multiple moderator tests were not conducted. At intermediate assessment, interventions were successful when fewer women were sampled and the intervention content included a motivational component. When entered simultaneously, both moderators remained significant (proportion women: β = −.33, P <.01; motivational component: β = .29, P =.02) and accounted for 26% of the variance. Interventions reduced the incidence of STDs at long-term when younger participants were sampled.
DISCUSSION
This meta-analytic review examined 32 manuscripts evaluating 48 behavioral interventions to reduce sexual risk behavior among 67,538 STD clinic patients in the United States. Behavioral risk reduction interventions succeeded at increasing condom use, reducing number of sexual partners, and lowering the incidence of STDs; however, the efficacy of these interventions to reduce sexual risk behaviors and incident STDs varied across assessment intervals. Interventions were successful in improving condom use and reducing numbers of sexual partners for durations of up to 40 weeks (average of 19 and 12 weeks for condom use and number of sexual partners, respectively). Moreover, intervention success for incident STDs was sustained over 104 weeks (average of 26 weeks) with an effect size of small magnitude (d+ = 0.10). In the current meta-analysis, the sexual risk reduction interventions were most often compared with an active comparison rather than an assessment-only, wait-list, or no-treatment control. Prior research indicates that between-groups effect sizes are generally smaller when comparing an intervention to an active comparison relative to a no-treatment control (68). Nonetheless, the magnitude of effects for condom use, number of sexual partners, and incident STDs corroborates effects reported in previous meta-analyses (13, 69).
The incidence of HIV over 64 weeks (average of 54 weeks) was significantly lower among intervention compared to control participants (d+ = 0.64), an effect size of medium magnitude. Consistent with prior research documenting the increased risk of HIV among STD clinic patients (10, 11), overall incidence of HIV was 0.06% (27 out of 46,571 U.S. STD clinic patients) compared with 0.02% in the general population (estimated incidence of HIV in 2006 was 56,300) (70). Moreover, STD incidence was significantly lower among the same intervention participants compared to control patients at long-term assessment (d+ random = 0.11, 95% CI = 0.07, 0.14, k = 5). To our knowledge, this meta-analysis is the first to demonstrate the efficacy of behavioral interventions among STD clinic patients as measured with HIV incidence. Overall, these findings demonstrate that behavioral interventions reduce sexual risk among STD clinic patients.
Several sample and intervention characteristics moderated the impact of the intervention on condom use, number of sexual partners, and incident STDs. First, consistent with our hypothesis, interventions were more successful at improving condom use, reducing the number of sexual partners, and lowering incident infections at short-term follow-up when sampling younger rather than older patients. Reductions in number of sexual partners and incident STDs among younger patients were also observed at long-term follow-up. Because younger patients may have had fewer life experiences, they may be more amenable to health-related attitudinal and behavioral changes relative to older patients whose behavior patterns are better established (71, 72). Our findings suggest that sexual risk reduction behavioral interventions for younger patients should be routinely implemented in clinic settings.
Second, short-term reductions in incident STDs were found among studies sampling more men rather than women, contrary to our hypothesis. Compared to women, men often have greater relationship power and, as a consequence, men have greater control over sexual decision-making (73, 74). To alleviate symptoms of STDs, male STD patients exposed to a sexual risk reduction intervention may be particularly motivated to engage in risk-reducing strategies with their female partners. (In the current study, more than two-thirds of the participants had an STD at baseline [49% of studies assessing incident STDs at short-term assessment]). Future research should explore motivational factors associated with STD diagnoses among men.
Third, interventions sampling more Blacks were less efficacious in reducing number of sexual partners at long-term follow-up but were more efficacious at reducing incident STDs at short-term follow-up, partially supporting our hypothesis. Explanations for these findings are necessarily speculative. One possible explanation relates to the targeting of the intervention based on race/ethnicity. Among the interventions assessing number of partners at long-term follow-up, only 3 (27%) of these interventions were specifically targeted to race. None of the interventions assessing incident STDs at short-term were targeted by race. Thus, these findings are difficult to interpret and warrant further investigation.
Fourth, consistent with our hypothesis, interventions were typically more successful when studies sampled patients diagnosed with a STD. Compared to uninfected individuals, patients with a current STD may be particularly motivated to change their sexual behavior (16). However, research examining the effects of STD diagnosis alone has found little change in sexual risk behavior compared with individuals not diagnosed with an STD (75, 76). Thus, STD diagnosis by itself appears to be insufficient in changing sexual risk behavior. Future research might examine the interactive effects of STD diagnosis and intervention efficacy.
Fifth, as we expected, interventions were more successful at promoting condom use when the intervention content was targeted to specific subgroups. Theory (16, 77), as well as previous research (13, 14, 78), suggest that targeting intervention content facilitates behavior change. We also found interventions that included a motivational component were less successful at reducing incident STDs at short-term but were more successful in reducing incident STDs at intermediate assessment. One possible explanation for this finding is the benefits of motivational enhancement hinged on interpersonal and condom use skill acquisition, which takes more time to emerge, consistent with the Information-Motivation-Behavioral Skills Model (15, 79). The current findings suggest that interventionists developing HIV-prevention programs should conduct formative research to identify the specific needs of the population of interest.
Finally, interventions were more successful at improving sexual risk behaviors (condom use, number of sexual partners) at long-term follow-up when delivered in longer doses. Lengthier doses of intervention content may be necessary in the development of skills needed to enact and maintain behavioral change. A paradoxical finding was that intervention content delivered in shorter doses was more successful in improving incident STDs at short-term. It is possible that immediate change in incident STDs, rather than long-term maintenance, may be easier to achieve with briefer interventions. Furthermore, similar findings were found in a recent synthesis of meta-analyses examining behavioral interventions across multiple health behaviors (80). Nonetheless, this finding is difficult to explain and warrants further investigation.
Limitations
Several limitations should be considered when interpreting these findings. First, many outcomes involve self-reports, which are vulnerable to cognitive (e.g., memory) and social (e.g., self-presentation) biases (81, 82). Self-report is imperfect, but most researchers employed methods designed to optimize the quality of these data. Furthermore, laboratory- and/or clinically-diagnosed STDs corroborate self-reported results. Second, the small number of studies available at each assessment interval could not support multivariate moderator tests for all outcomes. Moreover, our analyses are based largely on different sets of studies at each assessment and are not directly comparable across intervals. Thus, our moderator analyses should be considered preliminary.
CONCLUSION
Behavioral interventions implemented with STD clinic patients succeed at reducing sexual risk behaviors and incident STDs. These behavioral interventions reduce risk most among those who bear the heaviest burden of HIV (i.e., young adults and Blacks). If widely implemented, these interventions can help to lower long-term risk of incident STDs including HIV among patients, potentially reducing the health and economic burden of STDs. To increase their efficacy, researchers should consider including patients at most risk, targeting the content specifically for STD patients, including specific subgroups (e.g., young adults), and delivering intervention content in longer doses. Translating, enhancing, and implementing efficacious behavioral intervention among STD clinic patients should be a high priority.
Acknowledgments
This research was facilitated by National Institute of Health Grant R01-MH068171 to Michael P. Carey. We thank the study authors who provided additional intervention details or data for this study.
Footnotes
Insufficient variability in type of control condition for most dependent variables meant that comparisons between active comparisons and assessment-only controls were not possible. Among the dependent variables with sufficient variability for type of control, no significant differences were found.
References
References marked with an asterisk indicate studies included in the meta-analysis.
References marked with a dagger indicate supplemental manuscripts providing intervention details and additional measurement occasions for the 32 included studies.
- 1.Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. 2006 Disease Profile. 2008. [inclusive page numbers] [Google Scholar]
- 2.World Health Organization (WHO) . Global prevalence and incidence of selected curable sexually transmitted infections: Overview and estimates. Retrieved June 18, 2009; from http://www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf.
- 3.Centers for Disease Control and Prevention (CDC). . Trends in reportable sexually transmitted diseases in the United States, 2007. Retrieved May 7, 2010, from http://www.cdc.gov/std/stats07/trends.pdf.
- 4.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) . Global strategy for the prevention and control of sexually transmitted infections: 2006–2015. Retrieved June 18, 2009, from http://www.who.int/reproductivehealth/publications/rtis/RHR_06_10/en/
- 6.Cook RL, Comer DM, Wiesenfeld HC, et al. Alcohol and drug use and related disorders: An underrecognized health issue among adolescents and young adults attending sexually transmitted disease clinics. Sex Transm Dis. 2006;33:565–570. doi: 10.1097/01.olq.0000206422.40319.54. [DOI] [PubMed] [Google Scholar]
- 7.Howards PP, Thomas JC, Earp JA. Do clinic-based STD data reflect community patterns? Int J STD AIDS. 2002;13:775–780. doi: 10.1258/095646202320753745. [DOI] [PubMed] [Google Scholar]
- 8.Leichliter JS, Ellen JM, Gunn RA. STD repeaters: Implications for the individual and STD transmission in a population. In: Aral SO, Douglas JM, Lipshultz JA, editors. Behavioral interventions for prevention and control of sexually transmitted diseases. New York: Springer; 2007. pp. 354–373. [Google Scholar]
- 9.Newman LM, Warner L, Weinstock HS. Predicting subsequent infection in patients attending sexually transmitted disease clinics. Sex Transm Dis. 2006;33:737–742. doi: 10.1097/01.olq.0000218865.37084.f6. [DOI] [PubMed] [Google Scholar]
- 10.Weinstock HS, Sidhu J, Gwinn M, Karon J, Petersen LR. Trends in HIV seroprevalence among persons attending sexually transmitted disease clinics in the United States, 1988–1992. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:514–522. [PubMed] [Google Scholar]
- 11.Weinstock H, Sweeney S, Satten GA, Gwinn M. HIV seroincidence and risk factors among patients repeatedly tested for HIV attending sexually transmitted disease clinics in the United States, 1991 to 1996. STD Clinic HIV Seroincidence Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:506–512. doi: 10.1097/00042560-199812150-00010. [DOI] [PubMed] [Google Scholar]
- 12.DiClemente RJ, Milhausen R, Sales JM, Salazar LF, Crosby RA. A programmatic and methodologic review and synthesis of clinic-based risk-reduction interventions for sexually transmitted infections: Research and practical implications. Seminars in Pediatric Infectious Diseases. 2004;16:199–218. doi: 10.1053/j.spid.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Crepaz N, Horn AK, Rama SM, et al. The efficacy of behavioral interventions in reducing HIV risk sex behaviors and incident sexually transmitted disease in black and Hispanic sexually transmitted disease clinic patients in the United States: a meta-analytic review. Sex Transm Dis. 2007;34:319–332. doi: 10.1097/01.olq.0000240342.12960.73. [DOI] [PubMed] [Google Scholar]
- 14.Ward DJ, Rowe B, Pattison H, Taylor RS, Radcliffe KW. Reducing the risk of sexually transmitted infections in genitourinary medicine clinic patients: a systematic review and meta-analysis of behavioural interventions. Sex Transm Infect. 2005;81:386–393. doi: 10.1136/sti.2004.013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychological Bulletin. 1992;111:455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 16.Fisher WA, Fisher JD, Harman J, Suls J, Wallston KA. Social psychological foundations of health and illness. Malden, MA US: Blackwell Publishing; 2003. The information-motivation-behavioral skills model: A general social psychological approach to understanding and promoting health behavior; pp. 82–106. [Google Scholar]
- 17.Kreuter MW, Wray RJ. Tailored and targeted health communication: strategies for enhancing information relevance. Am J Health Behav. 2003;27(Suppl 3):S227–232. doi: 10.5993/ajhb.27.1.s3.6. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Miller WR, Brown JM, Simpson TL, et al. Handbook of alcoholism treatment approaches: Effective alternatives. 2. Needham Heights, MA US: Allyn & Bacon; 1995. What works? A methodological analysis of the alcohol treatment outcome literature; pp. 12–44. [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. New York: Erlbaum; 1988. [Google Scholar]
- 21.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 22.Sanchez-Meca J, Marin-Martinez F, Chacon-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8:448–467. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- 23.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6:107–128. [Google Scholar]
- 25.Johnson BT, Wood T. DSTAT 2.0: Software for Meta-Analysis. 2006. [Google Scholar]
- 26.Emerson JD, Strenio J. Boxplots and batch comparisons. In: Hoaglin DC, Mosteller F, Tukey JW, editors. Understanding robust and exploratory data analysis. New York: Wiley; 1983. pp. 58–96. [Google Scholar]
- 27.Hedges LV, Olkin L. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 30.Hedges LV. Fixed effects models. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 285–299. [Google Scholar]
- 31.StataCorp. Stata. College Station, TX: StataCorp; 2007. [Google Scholar]
- 32.Rosenthal R. The “file-drawer” problem and tolerance for null results. Psyc Bull. 1979;86:638–641. [Google Scholar]
- 33.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenhouse JB, Iyengar S. Sensitivity analysis and diagnosis. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 383–398. [Google Scholar]
- *37.Boyer CB, Barrett DC, Peterman TA, Bolan G. Sexually transmitted disease (STD) and HIV risk in heterosexual adults attending a public STD clinic: evaluation of a randomized controlled behavioral risk-reduction intervention trial. AIDS. 1997;11:359–367. doi: 10.1097/00002030-199703110-00014. [DOI] [PubMed] [Google Scholar]
- *38.Branson BM, Peterman TA, Cannon RO, Ransom R, Zaidi AA. Group counseling to prevent sexually transmitted disease and HIV: a randomized controlled trial. Sex Transm Dis. 1998;25:553–560. doi: 10.1097/00007435-199811000-00011. [DOI] [PubMed] [Google Scholar]
- *39.Carey MP, Senn TE, Vanable PA, Coury-Doniger P, Urban MA. Brief and intensive interventions to promote sexual risk reduction among STD clinic patients: A randomized controlled trial. AIDS & Behav. 2009 doi: 10.1007/s10461-009-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *40.Crosby RA, DiClemente R, Charnigo R, Snow G, Troutman A. Evaluation of a lay health advisor model risk-reduction intervention for promoting safer sex among heterosexual African American men newly diagnosed with an STD: A randomized controlled trial. Am J Public Health. 2008 doi: 10.2105/AJPH.2007.123893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.DeLamater J, Wagstaff DA, Havens KK. The impact of a culturally appropriate STD/AIDS education intervention on black male adolescents’ sexual and condom use behavior. Health Educ Behav. 2000;27:454–470. doi: 10.1177/109019810002700408. [DOI] [PubMed] [Google Scholar]
- *42.Gillmore MR, Morrison DM, Richey CA, et al. Effects of a skill-based intervention to encourage condom use among high risk heterosexually active adolescents. AIDS Educ Prev. 1997;9:22–43. [PubMed] [Google Scholar]
- *43.Gollub EL, French P, Latka M, Rogers C, Stein Z. Achieving safer sex with choice: studying a women’s sexual risk reduction hierarchy in an STD clinic. J Womens Health Gend Based Med. 2001;10:771–783. doi: 10.1089/15246090152636532. [DOI] [PubMed] [Google Scholar]
- *44.Kalichman SC, Cherry C. Male polyurethane condoms do not enhance brief HIV-STD risk reduction interventions for heterosexually active men: results from a randomized test of concept. Int J STD AIDS. 1999;10:548–553. [PubMed] [Google Scholar]
- *45.Kalichman SC, Cherry C, Browne-Sperling F. Effectiveness of a video-based motivational skills-building HIV risk-reduction intervention for inner-city African American men. J Consult Clin Psychol. 1999;67:959–966. doi: 10.1037//0022-006x.67.6.959. [DOI] [PubMed] [Google Scholar]
- *46.Kalichman SC, Williams E, Nachimson D. Brief behavioural skills building intervention for female controlled methods of STD-HIV prevention: outcomes of a randomized clinical field trial. Int J STD AIDS. 1999:10–174. doi: 10.1258/0956462991913844. [DOI] [PubMed] [Google Scholar]
- *47.Kalichman SC, Cain D, Weinhardt L, et al. Experimental components analysis of brief theory-based HIV/AIDS risk-reduction counseling for sexually transmitted infection patients. Health Psychol. 2005:24–198. doi: 10.1037/0278-6133.24.2.198. [DOI] [PubMed] [Google Scholar]
- *48.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. Project RESPECT Study Group. JAMA. 1998:280–1161. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- *49.Kissinger P, Mohammed H, Richardson-Alston G, et al. Patient-delivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis. 2005:41–623. doi: 10.1086/432476. [DOI] [PubMed] [Google Scholar]
- *50.Metcalf CA, Douglas JM, Jr, Malotte CK, et al. Relative efficacy of prevention counseling with rapid and standard HIV testing: a randomized, controlled trial (RESPECT-2) Sex Transm Dis. 2005:32–130. doi: 10.1097/01.olq.0000151421.97004.c0. [DOI] [PubMed] [Google Scholar]
- *51.Metzler CW, Biglan A, Noell J, Ary DV, Ochs L. A randomized controlled trial of a behavioral intervention to reduce high-risk sexual behavior among adolescents in STD clinics. Behav Ther. 2000:31–27. [Google Scholar]
- *52.The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. NIMH> The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. Science. 1998:280–1889. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- *53.O’Leary A, Ambrose TK, Raffaelli M, et al. Effects of an HIV risk reduction project on sexual risk behavior of low-income STD patients. AIDS Educ Prev. 1998:10–483. [PubMed] [Google Scholar]
- *54.Orr DP, Langefeld CD, Katz BP, Caine VA. Behavioral intervention to increase condom use among high-risk female adolescents. J Pediatr. 1996:128–288. doi: 10.1016/s0022-3476(96)70413-4. [DOI] [PubMed] [Google Scholar]
- *55.Shain RN, Piper JM, Newton ER, et al. A randomized, controlled trial of a behavioral intervention to prevent sexually transmitted disease among minority women. N Engl J Med. 1999:340–93. doi: 10.1056/NEJM199901143400203. [DOI] [PubMed] [Google Scholar]
- *56.Wenger NS, Linn LS, Epstein M, Shapiro MF. Reduction of high-risk sexual behavior among heterosexuals undergoing HIV antibody testing: a randomized clinical trial. Am J Public Health. 1991:81–1580. doi: 10.2105/ajph.81.12.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *57.Artz L, Macaluso M, Meinzen-Derr J, et al. A randomized trial of clinician-delivered interventions promoting barrier contraception for sexually transmitted disease prevention. Sex Transm Dis. 2005:32–672. doi: 10.1097/01.olq.0000175404.18098.dd. [DOI] [PubMed] [Google Scholar]
- *58.Shain RN, Piper JM, Holden AE, et al. Prevention of gonorrhea and Chlamydia through behavioral intervention: results of a two-year controlled randomized trial in minority women. Sex Transm Dis. 2004:31–401. doi: 10.1097/01.olq.0000135301.97350.84. [DOI] [PubMed] [Google Scholar]
- *59.Cohen DA, Dent C, MacKinnon D. Condom skills education and sexually transmitted disease reinfection. Journal of Sex Research. 1991:28–139. [Google Scholar]
- *60.Cohen DA, Dent C, MacKinnon D, Hahn G. Condoms for men, not women. Results of brief promotion programs. Sex Transm Dis. 1992:19–245. doi: 10.1097/00007435-199209000-00002. [DOI] [PubMed] [Google Scholar]
- *61.Cohen DA, MacKinnon DP, Dent C, Mason HR, Sullivan E. Group counseling at STD clinics to promote use of condoms. Public Health Rep. 1992:107–727. [PMC free article] [PubMed] [Google Scholar]
- *62.Gollub EL, French P, Loundou A, et al. A randomized trial of hierarchical counseling in a short, clinic-based intervention to reduce the risk of sexually transmitted diseases in women. AIDS. 2000:14–1249. doi: 10.1097/00002030-200006160-00023. [DOI] [PubMed] [Google Scholar]
- *63.Maher JE, Peterman TA, Osewe PL, Odusanya S, Scerba JR. Evaluation of a community-based organization’s intervention to reduce the incidence of sexually transmitted diseases: a randomized, controlled trial. South Med J. 2003:96–248. doi: 10.1097/01.SMJ.0000054605.31081.07. [DOI] [PubMed] [Google Scholar]
- *64.O’Donnell LN, Doval AS, Duran R, O’Donnell C. Video-based sexually transmitted disease patient education: its impact on condom acquisition. Am J Public Health. 1995:85–817. doi: 10.2105/ajph.85.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *65.Smith PB, Weinman ML, Parrilli J. The role of condom motivation education in the reduction of new and reinfection rates of sexually transmitted diseases among inner-city female adolescents. Patient Educ Couns. 1997:31–77. doi: 10.1016/s0738-3991(97)01009-4. [DOI] [PubMed] [Google Scholar]
- *66.Warner L, Klausner JD, Rietmeijer CA, et al. Effect of a brief video intervention on incident infection among patients attending sexually transmitted disease clinics. PLoS Med. 2008;5:e135. doi: 10.1371/journal.pmed.0050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Carey MP, Coury-Doniger P, Senn TE, Vanable PA, Urban MA. Improving HIV rapid testing rates among STD clinic patients: a randomized controlled trial. Health Psychol. 2008:27–833. doi: 10.1037/0278-6133.27.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grissom RJ. The magical number .7 +/− .2: meta-meta-analysis of the probability of superior outcome in comparisons involving therapy, placebo, and control. J Consult Clin Psychol. 1996;64:973–982. doi: 10.1037//0022-006x.64.5.973. [DOI] [PubMed] [Google Scholar]
- 69.Neumann MS, Johnson WD, Semaan S, et al. Review and meta-analysis of HIV prevention intervention research for heterosexual adult populations in the United States. J Acquir Immune Defic Syndr. 2002;30(Suppl 1):S106–117. [PubMed] [Google Scholar]
- 70.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krosnick JA, Alwin DF. Aging and susceptibility to attitude change. Journal of Personality and Social Psychology. 1989;57:416–425. doi: 10.1037//0022-3514.57.3.416. [DOI] [PubMed] [Google Scholar]
- 72.Visser PS, Krosnick JA. Development of attitude strength over the life cycle: Surge and decline. Journal of Personality and Social Psychology. 1998;75:1389–1410. doi: 10.1037//0022-3514.75.6.1389. [DOI] [PubMed] [Google Scholar]
- 73.Amaro H. Love, sex, and power. Considering women’s realities in HIV prevention. Am Psychol. 1995;50:437–447. doi: 10.1037//0003-066x.50.6.437. [DOI] [PubMed] [Google Scholar]
- 74.Pearson J. Personal control, self-efficacy in sexual negotiation, and contraceptive risk among adolescents: The role of gender. Sex Roles. 2006;54:615–625. [Google Scholar]
- 75.Kershaw TS, Ickovics JR, Lewis JB, et al. Sexual risk following a sexually transmitted disease diagnosis: the more things change the more they stay the same. J Behav Med. 2004;27:445–461. doi: 10.1023/b:jobm.0000047609.75395.62. [DOI] [PubMed] [Google Scholar]
- 76.Wilson TE, Jaccard J, Levinson RA, Minkoff H, Endias R. Testing for HIV and other sexually transmitted diseases: implications for risk behavior in women. Health Psychol. 1996;15:252–260. doi: 10.1037//0278-6133.15.4.252. [DOI] [PubMed] [Google Scholar]
- 77.Prochaska JO, DiClemente CC. Transtheoretical therapy: Toward a more integrative model of change. Psychother Theor Res. 1982;19:276–288. [Google Scholar]
- 78.Lyles CM, Kay LS, Crepaz N, et al. Best-evidence interventions: findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. Am J Public Health. 2007;97:133–143. doi: 10.2105/AJPH.2005.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fisher JD, Fisher WA, Shuper PA. The information-motivation-behavioral skills model of HIV preventive behavior. In: DiClemente RJ, Crosby RA, Kegler MC, editors. Emerging theories in health promotion practice and research. San Francisco, CA: Jossey-Bass; 2009. [Google Scholar]
- 80.Johnson BT, Scott-Sheldon LA, Carey MP. Meta-Synthesis of Health Behavior Change Meta-Analyses. Am J Public Health. doi: 10.2105/AJPH.2008.155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: II. Accuracy of self-reports. Ann Behav Med. 2003;26:104–123. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weinhardt LS, Forsyth AD, Carey MP, Jaworski BC, Durant LE. Reliability and validity of self-report measures of HIV-related sexual behavior: progress since 1990 and recommendations for research and practice. Arch Sex Behav. 1998;27:155–180. doi: 10.1023/a:1018682530519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- †83.Artz L, Macaluso M, Kelaghan J, et al. An intervention to promote the female condom to sexually transmitted disease clinic patients. Behav Modif. 2005:29–318. doi: 10.1177/0145445504272605. [DOI] [PubMed] [Google Scholar]
- †84.Legardy JK, Macaluso M, Artz L, Brill I. Do participant characteristics influence the effectiveness of behavioral interventions? Promoting condom use to women. Sex Transm Dis. 2005:32–665. doi: 10.1097/01.olq.0000175392.84989.ec. [DOI] [PubMed] [Google Scholar]
- †85.Jenkins PR, Jenkins RA, Nannis ED, McKee KT, Jr, Temoshok LR. Reducing risk of sexually transmitted disease (STD) and human immunodeficiency virus infection in a military STD clinic: evaluation of a randomized preventive intervention trial. Clin Infect Dis. 2000:30–730. doi: 10.1086/313743. [DOI] [PubMed] [Google Scholar]
- †86.McKee KT, Jr, Jenkins PR, Garner R, et al. Features of urethritis in a cohort of male soldiers. Clin Infect Dis. 2000:30–736. doi: 10.1086/313745. [DOI] [PubMed] [Google Scholar]
- †87.Kamb ML, Dillon BA, Fishbein M, Willis KL. Quality assurance of HIV prevention counseling in a multi-center randomized controlled trial. Project RESPECT Study Group. Public Health Rep. 1996;111(Suppl 1):99–107. [PMC free article] [PubMed] [Google Scholar]
- †88.Metcalf CA, Malotte CK, Douglas JM, Jr, et al. Efficacy of a booster counseling session 6 months after HIV testing and counseling: a randomized, controlled trial (RESPECT-2) Sex Transm Dis. 2005:32–123. doi: 10.1097/01.olq.0000151420.92624.c0. [DOI] [PubMed] [Google Scholar]
- †89.O’Donnell L, San Doval A, Duran R, O’Donnell CR. The effectiveness of video-based interventions in promoting condom acquisition among STD clinic patients. Sex Transm Dis. 1995:22–97. doi: 10.1097/00007435-199503000-00004. [DOI] [PubMed] [Google Scholar]
- †90.O’Donnell CR, O’Donnell L, San Doval A, Duran R, Labes K. Reductions in STD infections subsequent to an STD clinic visit. Using video-based patient education to supplement provider interactions. Sex Transm Dis. 1998:25–161. doi: 10.1097/00007435-199803000-00010. [DOI] [PubMed] [Google Scholar]
- †91.O’Donnell L, San Doval A, Vornfett R, O’Donnell CR. STD prevention and the challenge of gender and cultural diversity: knowledge, attitudes, and risk behaviors among black and Hispanic inner-city STD clinic patients. Sex Transm Dis. 1994:21–137. doi: 10.1097/00007435-199405000-00003. [DOI] [PubMed] [Google Scholar]
- †92.O’Leary A, Maibach E, Ambrose TK, Jemmott JB, 3rd, Celentano D. Social cognitive predictors of sexual risk behavior change among STD clinic patients. AIDS Behav. 2000:4–309. [Google Scholar]
- †93.Thurman AR, Holden AE, Shain RN, Perdue S, Piper JM. Preventing recurrent sexually transmitted diseases in minority adolescents: a randomized controlled trial. Obstet Gynecol. 2008:111–1417. doi: 10.1097/AOG.0b013e318177143a. [DOI] [PubMed] [Google Scholar]
- †94.Shain RN, Perdue ST, Piper JM, et al. Behaviors changed by intervention are associated with reduced STD recurrence: the importance of context in measurement. Sex Transm Dis. 2002:29–520. doi: 10.1097/00007435-200209000-00005. [DOI] [PubMed] [Google Scholar]
- †95.Myint UA, Bull S, Greenwood GL, et al. Safe in the City: Developing an Effective Video-Based Intervention for STD Clinic Waiting Rooms. Health Promot Pract. 2008 doi: 10.1177/1524839908318830. [DOI] [PubMed] [Google Scholar]