Abstract
Purpose
This study was designed to identify factors associated with persistent delirium in an older medical ICU population.
Materials and Methods
Prospective cohort study of 309 consecutive medical ICU patients age ≥60. Persistent delirium was defined as delirium occurring in the ICU and continuing upon discharge to the ward. The Confusion Assessment Method (CAM) was used to assess for delirium. Patient demographics, severity of illness, and medication data were collected. Univariate and multivariate analysis were used to assess factors associated with persistent delirium.
Results
Of 309 consecutive admissions to the ICU, 173 patients had ICU delirium, survived the ICU stay, and provided ward data. One-hundred patients (58%) had persistent delirium. In a multivariable logistic regression model, factors significantly associated with persistent delirium included age >75 years (OR, 2.52, 95% CI, 1.23–5.16), opioid (morphine equivalent) dose >54 mg/day (OR, 2.90, 95% CI, 1.15–7.28), and haloperidol (OR, 2.88, 95% CI, 1.38–6.02); change in code status to ‘Do Not Resuscitate’ (DNR) (OR, 2.62, 95% CI 0.95–7.35) and dementia (OR, 1.93, 95% CI 0.95–3.93) had less precise associations.
Conclusions
Age, use of opioids and haloperidol were associated with persistent delirium. Further research is needed regarding the use of haloperidol and opioids on persistent delirium.
Keywords: persistent delirium, critical care, aged
Introduction
Delirium in the ICU is increasingly recognized as an important indicator of acute brain dysfunction and a reliable predictor of poor outcomes 1. The prevalence of delirium in the ICU ranges from 50–87% depending on the patient population and screening instrument 2,3. ICU and non-ICU studies have shown that the presence of delirium contributes to adverse outcomes, increased mortality, nursing home placement, longer ICU stays, and costlier hospitalizations 4–6. It is well documented that patients with delirium are at increased risk for adverse events such as falls and inadvertent removal of lines and catheters 7. They are also at risk for pneumonia and pressure ulcers, as well as for adverse drug reactions related to the treatment of agitation or insomnia 8.
In a mechanically ventilated ICU population, the presence of delirium in the ICU was the only predictor of prolonged hospital stay after controlling for relevant confounders 9. Although it was not examined in the prior study the prolonged hospital stay may have been related to delirium with persisted after ICU discharge. Patients with longer ICU and hospital stays have increased risks for nosocomial infections and other hospital related complications.
While there is no literature regarding nursing intensity in delirious ICU patients, there is data to support the need for greater intensity of nursing care for non-ICU patients with delirium 10,11. Often ICU physicians and nurses are reluctant to discharge patients with delirium from the ICU even though their other critical care issues have resolved due to concerns about patient safety on a floor that has a higher patient to nurse ratio. It is documented that the cost of caring for ICU patients is increased in those with delirium, likely related to ICU length of stay 6. This expenditure of ICU resources for monitoring delirious patients whose critical illness has resolved is sub-optimal.
Our primary aim is to identify both baseline patient and ICU-care related factors associated with persistent delirium after ICU discharge. We are interested in identifying ICU factors, including psychoactive medication use, which are amenable to intervention. In addition, identifying patients at risk for persistent delirium after ICU discharge can help inform decisions regarding efficient use of hospital resources.
Methods
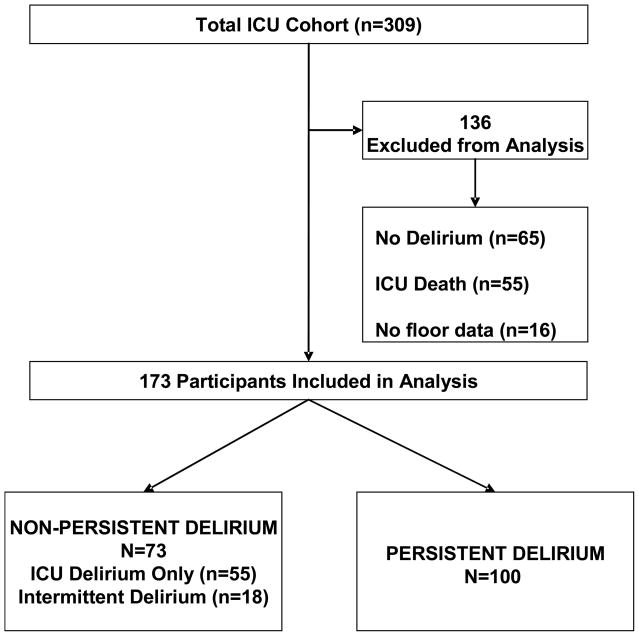
Study participants were 173 patients ≥60 years admitted to the medical ICU at Yale-New Haven Hospital, a 900-bed university hospital with a 14-bed ICU, from September 5, 2002 through September 30, 2004, that had ICU delirium and survived their ICU admission (See Figure 1). These patients were drawn from a cohort collected to examine outcomes in older ICU patients that has been described previously 12. Informed consent was obtained in accord with the IRB of Yale University.
Figure 1.
ICU death includes 5 patients who had persistent stupor/coma. Intermittent delirium is delirium which occurred in the ICU but was not continuous upon discharge to the ward. There was greater than 48 hours between the end of the ICU delirium episode and the beginning of the ward delirium episode.
Data Collection
As previously described 13, proxy respondents were the primary source of baseline information due to patients critical illness. To evaluate the prevalence of dementia, we used the short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) 14. This instrument is designed for proxy assessment of dementia and has been used in prior ICU studies 13.
Medication Data
We recorded use of opioids (fentanyl and morphine), benzodiazepines (lorazepam and midazolam) and propofol on a daily basis. These are the medications available on our formulary for pain control and sedation. All administration of medication was recorded, including continuous intravenous (IV), intermittent IV and oral dosing. For opioids we converted all medication to morphine equivalents 15. For benzodiazepines we converted all medications to lorazepam equivalents 15. Use of other psychoactive medications, including typical and atypical antipsychotics, anticonvulsants, and antidepressants, were recorded. A research nurse blinded to the delirium assessment performed the medication abstraction.
Delirium Assessment
Delirium was assessed at least once a day on Monday through Friday in the ICU using the Confusion Assessment Method-ICU (CAM-ICU) 16. After discharge to the ward delirium was assessed once a day on Monday, Wednesday and Friday using the CAM algorithm 17. We supplemented the interviews using a validated chart review method on a daily basis 18,19. The chart review method has a positive predictive accuracy of 87% and a 60% negative predictive accuracy for delirium detection 19. Chart review findings were used on days when a research delirium assessment was unavailable. Research nurses conducted study assessments after extensive training and inter-rater reliability testing.
Outcomes
Our primary outcome was persistent delirium. Persistent delirium was defined as an episode of delirium which began in the ICU and continued after the patient was discharged to the ward. An episode of delirium consisted of consecutive days of delirium. The delirium episode was considered ended when there were 48 consecutive hours without delirium. The comparison group consists of patients experiencing delirium in the ICU only and patients experiencing delirium both in the ICU and on the ward, but with at least 48 hours of no delirium between the episodes occurring in the ICU and ward.
Definition of Variables
Variables were considered as potential factors associated with persistent delirium based on prior literature and clinical experience. Baseline variables include age, dementia defined as an IQCODE score >3.3 13, depression determined from either the proxy or chart review and the Charlson Comorbidity Index 20. We also examined individual diagnoses which we felt might clinically be associated with persistent delirium. These included history of diabetes, chronic liver, renal or respiratory diseases. At ICU admission we used the APACHE II score minus the Glasgow Coma Scale to assess illness severity 1,5.
Factors related to ICU stay included intubation, days of mechanical ventilation, non-invasive ventilation, Hemodialysis, length of ICU stay and pain within 24 hours of ICU discharge. Psychoactive medications were examined in several ways. We looked at use of a benzodiazepine or opioid during ICU stay. We then examined total ICU benzodiazepine and opioid use adjusted for days of drug receipt. Low dose of benzodiazepines was defined as ≤4.76 mg/day and high dose as >4.76 mg/day. Low dose of opioids was defined as ≤54 mg/day and high dose as >54 mg/day. We also examined use of propofol, haloperidol, steroids and medium to high potency anticholinergic medications (diphenhydramine, atropine, dicyclomine, paroxetine, amitryiptyline, imipramine, olanzapine, promethazine, meclizine).
Statistical Analysis
Descriptive statistics include counts and percentages, means and standard deviations. We examined the unadjusted associations between variables and persistent delirium with the likelihood ratio chi-square statistic. We examined correlations among the variables using Kendall’s tau-b statistic. Logistic regression was employed for multivariable modeling. Variables were considered for inclusion in the multivariable model if they had a prevalence of ≥10% and an unadjusted likelihood ratio chi-square statistic >1.64 (p-value <0.20). Variables were retained in the multivariable model if they had a likelihood ratio chi-square value >2.70 (p-value<0.10). We forced the APACHE score into the model to control for severity of illness on ICU admission.
Model goodness of fit was verified by examination of residuals and the Hosmer-Lemeshow statistic. We assessed model discrimination by using a C statistic. Bootstrapping was used to confirm the robustness of the significant associations. All statistical tests were two-tailed with P<0.05 indicating significance. All statistical analyses were performed with SAS statistical software, version 9.1.3.
Results
Of the 309 patients enrolled in our original cohort study, the 173 who had ICU delirium, survived their ICU stay and provided ward data were at risk for persistent delirium (Figure 1). One hundred participants (58%) had delirium which persisted beyond their ICU stay. The median duration of delirium that persisted post-ICU discharge was 9 days (Range 2–52) and for delirium which did not persist the median duration was 3 days (Range 1–16). Table 1 presents unadjusted analyses where age >75, race, dementia, depression, change of code status, pain within 24 hours of ICU discharge, restraint use, higher opioid doses and haloperidol use were associated with persistent delirium.
Table 1.
Unadjusted Analyses of Participant Characteristics and Persistent Delirium Outcome (n=173)*
| Persistent Delirium (n=100) | Non-Persistent Delirium (n=73) | LR P-value | |
|---|---|---|---|
|
Demographics |
|||
| Age greater than 75 | 49 (49) | 24 (33) | 0.033§ |
| Non-white race |
15 (15) | 20 (27) | 0.046§ |
|
Baseline Health Measures |
|||
| Dementia by IQCODE (>3.3)† | 45 (46) | 22 (31) | 0.047§ |
| History of depression (surrogate or chart) | 39 (39) | 18 (25) | 0.046§ |
| Charlson Comorbidity Index, mean ± sd† | 1.84 ± 1.83 | 1.88 ± 1.79 | 0.89 |
| Diabetes† | 12 (12) | 10 (14) | 0.76 |
| Chronic Liver Disease† | 4 (4) | 3 (4) | 0.98 |
| Chronic Renal Disease† | 3 (3) | 2 (3) | 0.91 |
| Chronic Respiratory Disease† | 30 (30) | 32 (44) | 0.068§ |
| Do Not Resuscitate on ICU admission | 14 (14) | 8 (11) | 0.55 |
| Change in Code Status to ‘DNR’ |
22 (22) | 6 (8) | 0.012§ |
|
Physiologic Measurements |
|||
| APACHE II (minus the Glasgow Coma Scale), mean ± sd | 22.13 ± 5.62 | 22.3 ± 4.89 | 0.83 |
| PaO2/FiO2 < 300 | 61 (61) | 53 (73) | 0.20 |
| Serum creatinine > 2 mg/dl | 35 (35) | 26 (36) | 0.93 |
| Blood urea nitrogen/creatinine> 18 | 66 (66) | 52 (71) | 0.46 |
| Arterial pH <7.35† | 59 (67) | 41 (60) | 0.38 |
| Alanine aminotransferase > 40, U/L† |
24 (29) | 17 (28) | 0.92 |
|
ICU Factors |
|||
| Intubation | 62 (62) | 42 (58) | 0.55 |
| Number of days on mechanical ventilation, median (range) | 6 (2–43) | 4.5 (1–47) | 0.88 |
| Non-invasive ventilation | 29 (29) | 18 (25) | 0.52 |
| Hemodialysis | 8 (8) | 5 (7) | 0.77 |
| Pulmonary Artery catheterization | 11 (11) | 5 (7) | 0.34 |
| Pain within 24 hours of ICU discharge† | 43 (43) | 20 (28) | 0.039§ |
| Restraint Use | 67 (67) | 36 (49) | 0.019 |
| Length of ICU stay, mean ± sd | 8.3 ± 7.92 | 7.7 ± 9.7 | 0.65 |
| Length of ICU stay, median (range) |
5 (2–45) | 5 (2–51) | |
|
Medications ‡ |
|||
| No benzodiazepines | 22 (22) | 21 (29) | NA |
| Benzodiazepine adjusted: Low total dose up to 4.76 mg | 50 (50) | 33 (45) | 0.33 |
| Benzodiazepine adjusted: High total dose greater than 4.76 mg | 28 (28) | 19 (26) | 0.42 |
| No opioids | 20 (20) | 27 (37) | NA |
| Opioid adjusted: Low total dose up to 54 mg | 38 (38) | 25 (34) | 0.064§ |
| Opioid adjusted: High total dose greater than 54 mg | 42 (42) | 21 (29) | 0.011§ |
| Propofol use | 9 (9) | 4 (6) | 0.37 |
| Benzodiazepine or opioid use | 88 (88) | 57 (78) | 0.082§ |
| Haloperidol use | 48 (48) | 16 (22) | 0.000§ |
| Steroid use | 53 (53) | 40 (55) | 0.81 |
| Medium to High Potency anticholinergic medication use | 31 (31) | 19 (26) | 0.47 |
Abbreviations: LR, Likelihood ratio chi-square statistic; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; APACHE, Acute Physiology and Chronic Health Evaluation; ICU, Intensive Care Unit; NA, Not applicable.
Data are presented as the number and the percentage (%) with the characteristic except where indicated.
Data were missing for some variables: dementia 2; Charlson Comorbidity Index 1; diabetes 1; chronic respiratory disease 1; chronic liver disease 1; chronic renal disease 1; alanine aminotransferase 28; arterial pH 17; PaO2/FiO2 19; pain with 24 hours of ICU discharge 1.
For opioids we converted all medication to morphine equivalents. For benzodiazepines we converted all medications to lorazepam equivalents 22. Medium to High Potency anticholinergic medication included: diphenhydramine, atropine, dicyclomine, paroxetine, amitryiptyline, imipramine, olanzapine, promethazine, and meclizine
This indicates variables included in the multivariable model selection process.
Supplemental analysis expanded on several of the Table 1 findings. Of the sixty-three participants reporting pain within 24 hours of ICU discharge, 30 (48%) received opioids within 48 hours of discharge with no significant difference in the rate of persistent delirium among those with untreated pain relative to those treated with opioids (Risk Ratio =1.05 (CI 0.75 –1.47)). Among the 64 patients receiving haloperidol, 49 (76%) had agitation on the first day they received the drug. Of the twenty-eight participants receiving haloperidol on day of ICU discharge, 27 (96%) exhibited persistent delirium. Six of the 64 patients (9%) received their first dose of haloperidol on the day of ICU discharge. Finally, of the 17 deaths on the ward, 13 (76%) had persistent delirium.
The results of the multivariable model are presented in Table 2. Age >75, opioid dose >54 mg/day and haloperidol were significantly associated with persistent delirium. Dementia and change in code status were not significantly associated with persistent delirium. None of the tested interactions of variables in the multivariable model were statistically significant. Bootstrapping results confirmed the robustness of the significant associations while yielding a mean C-statistic of 0.75.
Table 2.
Data on Sedation Assessments
| RASS | Patient days in the ICU N=1398 |
|---|---|
| Agitated | 250 (18%) |
| Alert and calm | 358 (26%) |
| Lethargic | 550 (39%) |
| Stupor/coma | 218 (16%) |
| Missing | 22 (1%) |
Abbreviations: RASS, Richmond Agitation Sedation Scale; ICU, Intensive Care Unit.
Discussion
We have identified admission and ICU-related factors associated with persistent delirium among older ICU patients. Age >75 and dementia both have been shown to be important in the development of delirium 21. Dementia is a risk factor for delirium in multiple subgroups of patients, and it was associated with persistent delirium, although not statistically significant in multivariate analysis, in our cohort. It is likely that there were too few participants with dementia to demonstrate a statistically significant difference. Another possibility is that patients with dementia had higher ICU mortality and thus would not have persistent delirium. In our cohort, the ICU mortality in patients with dementia was 19%, compared to 17% for patients without dementia.
The association of haloperidol with persistent delirium presents additional interpretive challenges. Our decision to include haloperidol in our analyses was motivated by the practice in our ICU during this study where physicians tended to use haloperidol in older patients with agitation to avoid excessive use of benzodiazepines. While we don’t have documentation of why the physicians prescribed haloperidol, we did ensure that it was given at or after the onset of delirium and our model demonstrates a significant association between haloperidol and persistent delirium. We offer some possible scenarios for this significant association. Delirium exists along a spectrum, and patients with more severe delirium may have been more likely to have their delirium recognized and hence treated with haloperidol. It is also possible that the more severe the delirium the more likely it is to persist after ICU discharge. We do not have a measure of delirium severity in the ICU and hence are unable to evaluation this scenario. The other possible scenario is that haloperidol was prescribed to treat agitation associated with delirium and converted a hyperactive delirium into a hypoactive delirium. We speculate that haloperidol may treat some delirium symptoms (agitation) while exacerbating others (lethargy). To explore this, we reviewed use of haloperidol and sedation status in our cohort and found that 76% had agitation on the first day they received haloperidol, on the day of ICU discharge 21/28 (75%) of patients had hypoactive delirium and 7/28 (25%) had hyperactive delirium. Delirium is a syndrome and in the ICU is often identified through patient agitation 22. Studies now show that the majority of ICU delirium is hypoactive and unrecognized 23. Given the results of this study and recent literature concerning haloperidol safety we believe it is important to perform randomized controlled trials of haloperidol for delirium treatment 24.
Reported associations between opioid use and delirium are inconsistent. Several studies have examined this relationship, but have been underpowered and not identified an association 25,26. A study in cancer patients demonstrated a significant impact of opioid use with delirium 27. Two ICU studies examining opioids and delirium found no significant association 12,28. In this study we have demonstrated a significant association between use of opioids at doses > 54mg/day and persistent delirium.
Several studies have suggested that untreated pain is a risk factor for delirium development 1,29. In our cohort, pain within 24 hours of ICU discharge had a significant unadjusted association with persistent delirium. This association was not significant in multivariable analysis. It is conceivable that patient pain reported by the ICU nursing staff was adequately treated. Of the 63 patients with documented pain at ICU discharge, 30 (48%) also received opioids within 48 hours of ICU discharge. Correlation between pain within 24-hours of ICU discharge and use of opioids was not significant.
Benzodiazepines have been associated with delirium in other studies 28,30. We recently demonstrated that use of benzodiazepines prior to ICU admission was significantly associated with the development of delirium 12, and lorazepam has been shown to be a risk factor for transitioning to delirium 28. This study did not find a significant association between benzodiazepines and persistent delirium which may be related to our definition of persistent delirium as delirium occurring in the ICU and continuing upon discharge to the floor. It is possible that participants receiving benzodiazepines may have remained in the ICU until their delirium resolved. During this study our ICU did not use a sedation protocol and it is possible that the lack of association of benzodiazepines with persistent delirium is related to the prescribing practices in our ICU.
In our study population severity of illness as measured by the APACHE II score on ICU admission and intubation were not significantly associated with persistent delirium. Both of these factors have been shown to be associated with occurrence of ICU delirium in other studies 12. In this study our outcome is qualitatively different from the other studies referenced, i.e. the occurrence of delirium in the ICU that persists versus incident or prevalent ICU delirium.
Another interesting finding of our study was that a change in a patient’s code status to ‘DNR’ was marginally associated with persistent delirium in multivariable analysis. We do not know why the code status was changed in these patients, but it is possible that their ICU delirium may have played a role. Six of the 28 patients with change of code status died, 5 (83%) of these patients had persistent delirium.
Chief strengths of this study are the high participation rate with a screen to enrollment ratio of 98% and minimal loss to follow-up after ICU discharge. We used previously validated measures to detect delirium in our patient population. In addition, we collected a detailed, clinically rich prospective data set for multiple potential risk factors using validated instruments. This data set represents the largest collection of data on delirium among older ICU patients to date and is the first to examine factors associated with persistent delirium.
Because these factors were identified in an older medical ICU population, they cannot be generalized to younger populations or other settings. Another limitation is that we did not examine the individual opioids or benzodiazepines but instead combined the drugs in each class using dosing equivalents. It may be possible certain drugs in each class may be more likely than others to be associated with persistent delirium. The importance of individual drugs should be evaluated in further studies. Data on events occurring after ICU discharge that may have contributed to prolongation of the ICU delirium were not available.
Interventions, such as using targeted sedation goals that may reduce doses of medication, or trials examining the impact of haloperidol on delirium may help to reduce persistent delirium. Physicians caring for older patients on hospital wards after discharge from an ICU should recognize the risk for persistent delirium. Reducing persistent delirium may decrease morbidity including hospital-acquired infections and adverse medication reactions as well as reducing hospital length of stay. The goal of hospitals should be to ensure that morbidity related to delirium is reduced and ICU resources are maximally utilized.
Table 3.
Multivariable Model for Post-ICU Persistent Delirium
| Factor | Odds Ratio 95% Confidence Interval | LR P-value |
|---|---|---|
| Age > 75 years | 2.60 (1.26–5.39) | 0.01 |
| APACHE II Score (minus the Glasgow Coma Scale) | 0.98 (0.92–1.04) | 0.51 |
| Opioid dose adjusted for number of days of use: High | ||
| total dose > 54 mg* | 2.90 (1.19–7.30) | 0.02 |
| Opioid dose adjusted for number of days of use: Low | ||
| total dose ≤ 54 mg* | 1.86 (0.79–4.36) | 0.15 |
| Use of Haloperidol during the ICU stay | 2.85 (1.37–5.96) | 0.005 |
| Change in Code Status to ‘DNR’ | 2.75 (0.98–7.33) | 0.05 |
| Dementia | 1.90 (0.93–3.88) | 0.07 |
Abbreviations: LR, Likelihood ratio chi-square statistic; ICU, Intensive Care Unit; DNR, Do Not Resuscitate.
Referent dose is no opioid medications during ICU stay
Acknowledgments
Grant Support: This work was supported in part by the American Lung Association and Connecticut Thoracic Society (ID# CG-002-N), Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (2P30AG021342-06), the T. Franklin Williams Geriatric Development Initiative through The CHEST Foundation, ASP, Hartford Foundation, and grants through the National Institute on Aging [K23AG23023].
The authors acknowledge the contributions of Peter Charpentier for database development, Wanda Carr for data entry; Karen Wu and Andrea Benjamin for enrolling participants and interviewing family members. We thank the families, nurses, and physicians in the Yale Medical Intensive Care Unit, whose cooperation and participation made this study possible.
Footnotes
Author Contributions: Margaret A. Pisani: Study concept and design, acquisition of subjects, data analysis, interpretation of data, preparation of manuscript
Terrence E. Murphy: Data analysis, interpretation of data, preparation of manuscript
Katy L.B. Araujo: Study concept and design, data analysis, interpretation of data, preparation of manuscript
Peter H. Van Ness: Data analysis, interpretation of data, preparation of manuscript
Sponsors Role: None
Dr. Pisani has no financial or other potential conflict of interest.
Dr. Murphy has no financial or other potential conflict of interest.
Ms. Araujo has no financial or other potential conflict of interest.
Dr. Van Ness has no financial or other potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 4.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 6.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 7.Dharmarajan TS. Falls and fractures linked to anemia, delirium, osteomalacia, medications, and more: the path to success is strewn with obstacles! J Am Med Dir Assoc. 2007;8:549–550. doi: 10.1016/j.jamda.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Mecocci P, von Strauss E, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20:262–269. doi: 10.1159/000087440. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brannstrom B, Gustafson Y, Norberg A, et al. Problems of basic nursing care in acutely confused and non-confused hip-fracture patients. Scand J Caring Sci. 1989;3:27–34. doi: 10.1111/j.1471-6712.1989.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 11.Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics. 2002;43:183–194. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 12.Pisani MA, Murphy TE, Van Ness PH, et al. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 13.Pisani MA, Inouye SK, McNicoll L, et al. Screening for preexisting cognitive impairment in older intensive care unit patients: use of proxy assessment. J Am Geriatr Soc. 2003;51:689–693. doi: 10.1034/j.1600-0579.2003.00215.x. [DOI] [PubMed] [Google Scholar]
- 14.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 15.Cammarano WB, Drasner K, Katz JA. Pain control, sedation and use of muscle relaxants. In: Hall JB, Schmidt GA, Wood LDH, editors. Principles of Critical Care. New York: McGraw-Hill; 1998. pp. 87–109. [Google Scholar]
- 16.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 17.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 18.Inouye SK, Leo-Summers L, Zhang Y, et al. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 19.Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121. doi: 10.1186/cc5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51:591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 22.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32:106–112. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 23.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: A study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 24.FDA. FDA - MedWatch - Haloperidol Marketed As Haldol, Haldol Decanoate, And Haldol Lactate Get New Warnings And Revised Prescription Information. US Food and Drug Administration Center for Drug Evaluation and Research Department of Health and Human Services; 2007. [Google Scholar]
- 25.Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–785. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Martin NJ, Stones MJ, Young JE, et al. Development of delirium: a prospective cohort study in a community hospital. Int Psychogeriatr. 2000;12:117–127. doi: 10.1017/s1041610200006244. [DOI] [PubMed] [Google Scholar]
- 27.Gaudreau JD, Gagnon P, Roy MA, et al. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109:2365–2373. doi: 10.1002/cncr.22665. [DOI] [PubMed] [Google Scholar]
- 28.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam Is an Independent Risk Factor for Transitioning to Delirium in Intensive Care Unit Patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 30.Gray SL, Lai KV, Larson EB. Drug-induced cognition disorders in the elderly: incidence, prevention and management. Drug Saf. 1999;21:101–122. doi: 10.2165/00002018-199921020-00004. [DOI] [PubMed] [Google Scholar]