Abstract
The primary objective of the present study was to examine whether a combination of parent-child DRD4 genotypes results in more informative prognostic biomarkers of oppositional, separation anxiety, and repetitive behaviors in children with autism spectrum disorder (ASD). Based on prior research indicating the 7-repeat allele as a potential risk variant, participants were sorted into one of four combinations of parent-child genotypes. Owing to the possibility of parent-of-origin effects, analyses were conducted separately for mother-child (MC) and father-child (FC) dyads. Mothers completed a validated DSM-IV-referenced rating scale. Partial eta-squared (ηp2) was used to determine the magnitude of group differences: 0.01–0.06=small, 0.06–0.14=moderate, and >0.14=large. Analyses indicated that children in MC dyads with matched genotypes had the least (7−/7−) and most (7+/7+) severe mother-rated oppositional-defiant (ηp2=0.11) and separation anxiety (ηp2=0.19) symptoms. Conversely, youths in FC dyads with matched genotypes had the least (7−/7−) and most (7+/7+) severe obsessive-compulsive behaviors (ηp2=0.19) and tics (ηp2=0.18). Youths whose parents were both noncarriers had less severe tics than peers with at least one parental carrier, and the effect size was large (ηp2=0.16). There was little evidence that noncarrier children were rated more severely by mothers who were carriers versus noncarriers. Transmission Disequilibrium Test analyses provided preliminary evidence for undertransmission of the 2-repeat allele in youths with more severe tics (p=0.02). Parent genotype may be helpful in constructing prognostic biomarkers for behavioral disturbances in ASD; however, findings are tentative pending replication with larger, independent samples.
Keywords: autism spectrum disorder, DRD4, oppositional defiant disorder, separation anxiety disorder, obsessions-compulsions, tic disorder
1. Introduction
Decades of research have clearly shown that raising a child with autism spectrum disorder (ASD) can be a profoundly stressful experience for parents, a task that is seriously exacerbated by co-occurring irritability, noncompliance, temper tantrums and repetitive behaviors (e.g., Benson, 2006; Hastings et al., 2005; Herring et al., 2006; Lecavalier et al., 2006; Pottie et al., 2009), and may even impact maternal cortisol levels (Seltzer et al., 2009). Although the clinical implications of dysfunctional interpersonal interactions are self-evident to experienced clinicians (e.g., Rao & Beidel, 2009), less well appreciated are their relevance for molecular biology as maternal report is often the primary or sole basis for diagnosing or characterizing neurobehavioral (endo-) phenotypes and co-occurring symptomatology. In other words, maternal genotype influences mother-child interactions, child disruptive behavior, as well as perceptions and therefore reports of behavior problems.
For a variety of reasons, few investigators have explored candidate genes for behavioral disturbances in children with ASD, but preliminary findings suggest that some common susceptibility alleles may be potential biomarkers for severity of co-occurring symptomatology in this clinical population (e.g., Brune et al., 2006; Cohen et al., 2003; Gadow et al., 2008b, 2009; Roohi et al., 2009). One example is a common 48bp variable number tandem repeat (VNTR) polymorphism within exon 3 of the D4 receptor gene (DRD4) located on chromosome 11. The actual number of repeats varies from 2 to 11, with the 2-, 4-, and 7-repeats being the most common. The 7-repeat allele purportedly results in less D4 receptor responsiveness (reduced dopamine binding efficiency) (Asghari et al., 1995; Cravchik et al., 2000; van Tol et al., 1992) and shows an association with response to pharmacotherapy (McGough 2005) and behavioral intervention (Bakermans-Kranenburg et al., 2008) for disruptive behaviors. Previously, we found that youths with ASD and who had at least one copy of the 7-repeat allele obtained significantly more severe maternal ratings of oppositional defiant disorder (ODD) (ηp2=0.10), obsessive-compulsive behavior (OCB) (ηp2=0.08), and tics (ηp2=0.07) than youths with two shorter alleles (Gadow and co-workers, unpublished results). In addition, there was tentative evidence (p=0.08) that 7-repeat allele carriers had more severe symptoms of separation anxiety disorder (SAD) (ηp2=0.05), a syndrome that appears to have considerable overlap with ODD (Foley et al., 2004; Gadow et al., 2008a).
These results are particularly interesting in light of recent research with typically developing, non-ASD samples linking maternal stress, parenting, and child behavior problems. For example, there is evidence that mothers who are DRD4 7-repeat allele carriers (7+) are more reactive to stress and engage in less sensitive parenting than noncarriers (7−) (van Ijzendoorn et al., 2008). Moreover, it has been reported that child DRD4 7-repeat allele carriers are differentially more responsive to certain types of parenting behavior (e.g., Bakermans-Kranenburg & van Ijzendoorn, 2006, 2007; Bakermans-Kranenburg et al., 2008; Gervai et al., 2007; Sheese et al., 2007), have more problems with peer aggression (DiLalla et al., 2009), and are more reactive to negativistic parenting behavior, which likely contributes to the ontogeny of child aggression (Bakermans-Kranenburg & van Ijzendoorn, 2006; DiLalla et al., 2009). To date, however, no studies have examined whether parental DRD4 genotype is associated with challenging child behaviors or if knowledge of parent genotype can be used to enhance the clinical utility of gene-behavior biomarkers in children with ASD.
Owing to the complexities of both gene-behavior associations and the biologic mechanisms that underlie behavioral variation, we examined mother-child (MC) and father-child (FC) dyads separately. Based on the aforementioned research, we predicted á priori that children in MC7− dyads would evidence significantly less severe symptoms than dyads with two 7-repeat allele carriers (MC7+). Planned comparisons were also conducted to determine if children who were noncarriers were rated differently by mothers who were carriers versus noncarriers (e.g., maternal genotype might directly influence or alter perceptions of mother-child interactions). To explore whether DRD4 alleles might be involved in symptom modulation, family-based allelic transmission analyses were also conducted.
2. Materials and methods
2.1 Participants
Participants in this study were recruited from referrals to a university hospital developmental disabilities specialty clinic located on Long Island, New York. All families with at least one child with a confirmed diagnosis of ASD were contacted by mail for participation in genetic research. A total of 92 individuals were initially recruited, but to maximize homogeneity, the study sample (N=64) was limited to individuals who were children (4–14 years old) when the diagnostic and behavioral evaluations were conducted. The study protocol stipulated that children would be excluded if a Rett MECP2 or a Fragile X mutation was discovered; however, none were found. Demographic characteristics were as follows: age (M=6.9; SD=2.6), gender (87% male), ethnicity (96% Caucasian), IQ (M=79.2; SD=23.2), socioeconomic status (SES) assessed with Hollingshead’s (1975) index of occupational and educational social status (M=42.4; SD=11.4), single-parent household (1%), and psychotropic medication use (24%). DNA samples and maternal ratings were available for 59 mother-child dyads, and DNA samples were available for 53 father-child dyads. This study was approved by a university Institutional Review Board; informed consent was obtained; and appropriate measures were taken to protect patient (and rater) confidentiality.
2.2 Procedure
Diagnoses of ASD were confirmed by an expert diagnostician and based on five sources of information about ASD symptoms to verify DSM-IV criteria: (a) comprehensive developmental history, (b) clinician interview with child and caregiver(s), (c) direct observations of the child, (d) review of validated ASD rating scale data including the Child Symptom Inventory-4 (CSI-4) (De Vincent & Gadow, 2009; Gadow et al., 2008c), (e) prior evaluations, and additionally (n=49) with (f) the Autism Diagnostic Observation Schedule (Lord et al., 2000) and/or Autism Diagnostic Interview-Revised (Rutter et al., 2003).
Prior to scheduling their initial clinic evaluation, the parents of potential participants were mailed a packet of materials including behavior rating scales, background information questionnaire, and permission for release of school reports, psycho-educational, and special education evaluation records. Rating scales included the parent version of the CSI-4, which was completed by the child's mother. Genotype status was determined using DNA isolated from peripheral blood cells and polymerase chain reaction.
2.3 Genotyping
Polymerase chain reaction was carried out in a total volume of 20 µl with forward (5’-GCGACTACGTGGTCTACTCG -3’) and reverse primers (5’- AGGACCCTCATGGCCTTG). Each amplification contained 20 ng of genomic DNA, 1 × multiplex master mix (Qiagen, Valencia, CA) and 1 µM each of the primers. Reaction conditions began with an initial denaturation at 98° C for 15 minutes, followed by 40 cycles of 98° C for 30 seconds, 60° C for 90 seconds, and 72° C for 60 seconds, with a final extension step of 10 minutes at 72° C. Products were analyzed on a QIAxcel System (Qiagen, Valencia, CA) and genotype analysis conducted by an investigator (D.O. and V.P.) who were blind to the behavioral characteristics of the study sample.
2.4 Measures
The CSI-4 (Gadow & Sprafkin 1986, 2002) is a behavior rating scale that assesses the behavioral symptoms of a broad range of psychiatric syndromes. Individual items bear one-to-one correspondence with DSM-IV symptoms (i.e., high content validity). To assess symptom severity, items are scored (never=0, sometimes=1, often=2, and very often=3) and summed separately for each disorder’s symptoms. In the present study, analyses pertained to two behavioral domains: oppositionality (ODD, SAD) and repetitive behaviors (OCB, tics). The findings of numerous studies indicate that the CSI-4 demonstrates satisfactory psychometric properties in community-based normative, clinic-referred non-ASD, and ASD samples (see Gadow & Sprafkin, 2009). Moreover, confirmatory factor analysis in a large (N=730) sample of children with diagnosed ASD supports the construct validity of DSM-IV syndromes (Lecavalier et al., 2009).
2.5 Statistical analyses
Prior to conducting our planned analyses, dependent variables (i.e., ODD, SAD, OCB, tics) were examined for outliers, skewness, and kurtosis. Variables not normally distributed were transformed using the square root function. Covariates to be included in subsequent analyses were identified by examining potential maternal and paternal genotype group differences in demographic characteristics, as well as associations between these demographic variables and the dependent variables. Chi-square tests (categorical variables), correlations (continuous variables), and ANOVAs (combined categorical and continuous variables) were used to test these relations.
The first step in the analyses was to conduct separate MANOVAs to determine which child behaviors from the two domains of interest were associated with maternal genotype. Because evidence points to the 7-repeat allele as a potential risk variant in both children (Smith, 2010) and adults (Congdon et al., 2008; van IJzendoorn et al., 2008), we adopted a widely used procedure of comparing 7-repeat allele carriers versus non-carriers. This also reduced the number of potential genotype groups, which had important statistical advantages. We limited examination of subsequent univariate analyses to situations where the multivariate F was significant, thereby reducing the risk of Type 1 error for multiple, related variables.
Next we constructed four groups of mother-child dyads based on whether individuals were 7-repeat allele carriers (7+) or noncarriers (7−): MC7−, MC7+, M7+/C7−, and M7−/C7+. In order to maximize our ability to detect group differences, all á priori pairwise comparisons included the largest group (MC7− dyads). With these comparisons we sought to determine if (a) mother-child genotype was associated with symptom severity (MC7−<MC7+), and (b) mothers’ genotype was associated with ratings of misbehavior in noncarrier offspring (MC7−<M7+/C7−). We repeated the aforementioned analyses using father-child dyads and parent-child triads to determine whether these configurations would also be more informative as biomarkers of symptom severity. There were two situations where mother, father, and child were all 7-repeat allele carriers.
We calculated partial eta-squared (ηp2) to gauge the magnitude of group differences and to address in part the inherent limitations of significance testing (Cohen, 1994; Feise, 2002; Perneger, 1998; Rothman, 1990; Zhang et al., 1997). A rule of thumb for determining the magnitude of ηp2 suggests the following: 0.01–0.06=small, 0.06–0.14=moderate, and >0.14 = large (Cohen, 1988).
Family-based analyses were carried out using the Transmission Disequilibrium Test (TDTae; ae=allowance for errors) program 2.0 (Gordon et al., 2001, 2004; Yang et al., 2008), which is available online (http://linkage.rockefeller.edu/pawe). TDTae allows for both Mendelian inconsistencies resulting from random genotyping errors and missing parental genotype data. Children were separated into two groups comprised of youngsters with more and less symptom severity (median split). Variables not normally distributed were transformed with the square root function. Owing to the fact that these analyses were exploratory and symptom variables were not independent, no corrections were made for multiple comparisons.
3. Results
Mothers’ DRD4 allelic frequencies were as follows: allele 2 (12%), allele 3 (2.5%), allele 4 (66%), allele 5 (2.5%), allele 6 (1%), and allele 7 (16%); fathers’ allelic frequencies showed a similar distribution: allele 2 (12%), allele 3 (2%), allele 4 (64%), and allele 7 (22%). Neither mothers’ nor fathers’ allele distributions deviated from Hardy-Weinberg equilibrium (Χ2 =0.58, p=0.45; Χ2=1.71, p=.19, respectively). The distribution of mothers’/fathers’ genotype groups were 7-repeat allele carriers (n=18, 31%/ n=18, 34%) and noncarriers (n=41, 69%/ n=35, 66%), respectively.
Importantly, mothers’ and fathers’ genotype groups did not significantly differ with regard to child’s IQ or severity of the three core domains of ASD symptomatology (communication and social deficits, perseverative behaviors). Neither did the genotype groups differ in demographic characteristics (i.e., child’s age, gender, ethnicity, psychotropic medication, special education, mothers’ level of education, family’s SES, or whether the mother was a single-parent).
3.1 Mothers’ genotype
MANOVAs did not indicate multivariate effects of mothers’ genotype for either ODD/SAD (F=2.72, p=0.08) or repetitive behavior (F=1.15, p=0.33); therefore, follow-up univariate analyses were not conducted for these variables.
3.2 Mother-child dyads
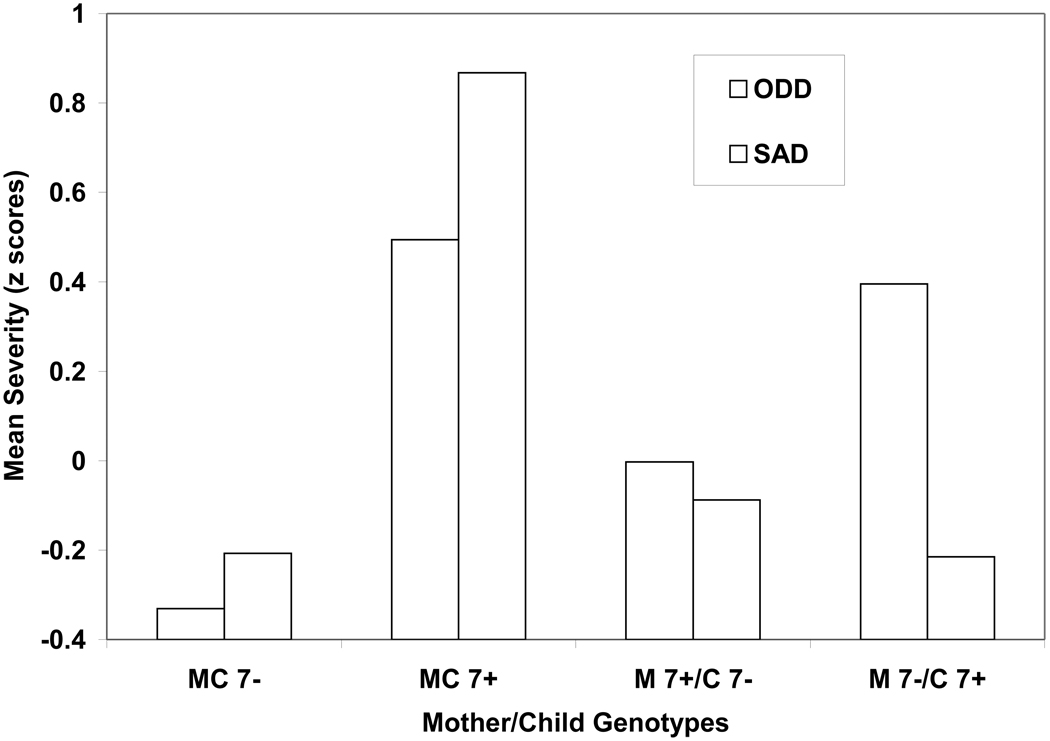
Mother-child dyads were as follows: MC7− (n=32), MC7+ (n= 10), M7+/C7− (n=8), and M7−/C7+ (n=9). The multivariate effect was significant for ODD/SAD (F=2.81, p=0.02) as were the univariate analyses for both ODD (F=2.83, p=0.05, ηp2=0.15) and SAD (F=3.75, p=0.02, ηp2=0.19). For ODD symptoms, planned comparisons indicated that the MC7− dyad had less severe symptoms than the MC7+ dyad (p=0.02, ηp2=0.11) (Figure 1). There was no statistically significant evidence that mothers’ genotype may have influenced ratings of ODD symptoms. In other words, noncarrier children were rated similarly by noncarrier (MC7−) and 7-repeat allele carrier (M7+/C7−) mothers.
Figure 1.
Mother-child dyads (MC), DRD4 7-repeat allele carriers (7+) and noncarriers (7−), and maternal ratings of oppositional defiant disorder (ODD) and separation anxiety disorder (SAD) symptom severity (z scores).
For SAD (Figure 1), planned comparisons indicated that children in the MC7− dyad had less severe SAD than youngsters in the MC7+ dyad (p<0.01; ηp2=0.19). Moreover, exploratory analyses indicated significant differences between the MC7+ dyad and the M7+/C7− (p=0.01; ηp2=0.15) and M7−/C7+ (p=0.02; ηp2=0.23) dyads suggesting that the MC7+ genotype configuration may be a potential biomarker for SAD severity.
The multivariate effect of mother-child dyads was not significant for repetitive behaviors (F=1.38, p=0.23); therefore, follow-up univariate analyses were not conducted.
3.3 Fathers’ genotype
For analyses involving fathers’ genotype, it is helpful to recall that ratings of child behavior are based on maternal report. MANOVA was not significant for ODD/SAD (F=2.14, p=0.13), but there was a significant multivariate effect for repetitive behaviors (F=5.15, p<0.01). Univariate analyses were significant for both OCB (F=5.40, p=0.03, ηp2=0.11) and tics (F=7.36, p<0.01, ηp2=0.14). Planned comparisons indicated that children whose fathers were 7-repeate allele carriers were rated as having more severe OCB (p=0.03, ηp2=0.11) and tics (p<0.01, ηp2=0.14) than youths whose fathers were noncarriers. This suggests that paternal DRD4 genotype may be a potential biomarker for repetitive behaviors regardless of child genotype.
3.4 Father-child dyads
Father-child dyads were as follows: FC7− (n=28), FC7+ (n=11), F7+/C7− (n=7), and F7−/C7+ (n=7). Unlike the findings for mother-child dyads, the MANOVA for father-child dyads was not significant for ODD/SAD (F=1.85, p=0.10). The MANOVA was, however, significant for repetitive behaviors (F=2.95, p=0.01) as was the univariate test of tic severity (F=3.80, p=0.02, ηp2=0.21). OCB ratings were marginally significant (F=2.92, p=0.05, ηp2=0.17).
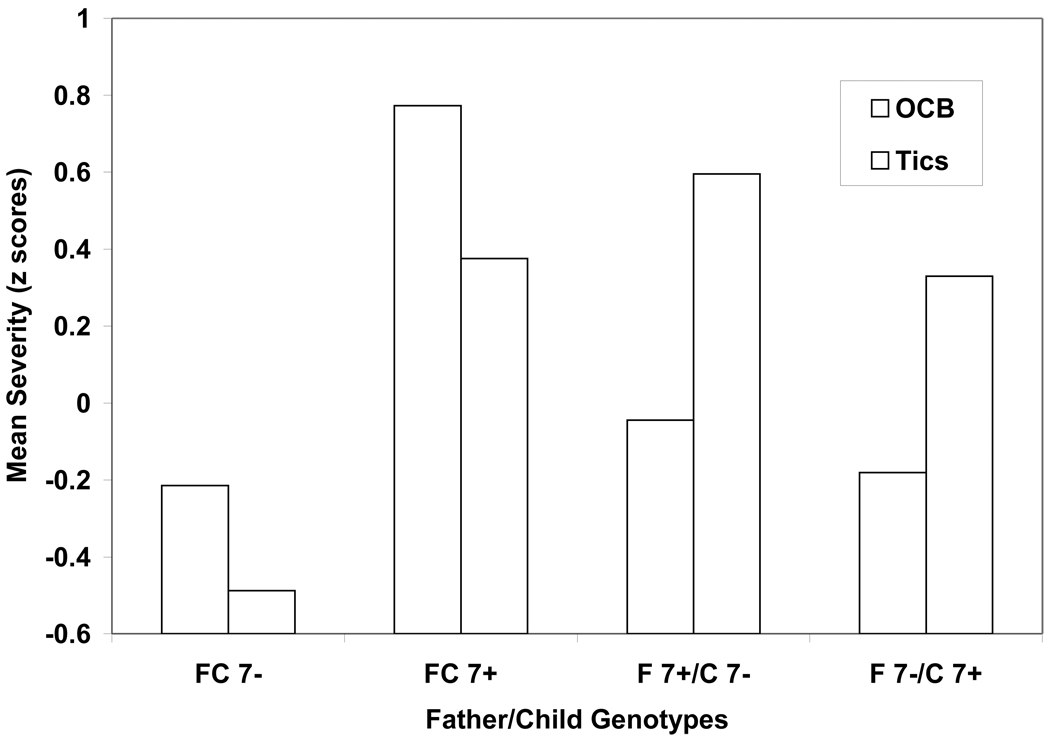
Children in the FC7− dyads were rated as having less severe tics than youngsters in the FC7+ (p=0.02, ηp2=0.18) and F7+/C7− (p=0.01, ηp2=0. 21) dyads (Figure 2). In addition, exploratory analyses also indicated the difference between the FC7− and F7−/C7+ dyads was marginally significant (p=0.07). Collectively, these results suggest that having at least one parent who is a DRD4 7-repeat allele carrier may be a potential biomarker for tic severity, regardless of the child’s 7-repeat allele status. This hypothesis is examined in the following section.
Figure 2.
Father-child dyads (FC), DRD4 7-repeat allele carriers (7+) and noncarriers (7−), and maternal ratings of obsessive-compulsive behavior (OCB) and tic severity (z scores).
Planned comparisons indicated children in FC7− dyads were rated as having less severe OCB than youths in FC7+ dyads (p<0.01, ηp2=0.19) (Figure 2). Moreover, exploratory analyses indicated marginally significant differences between the FC7+ dyad and the F7+/C7− (p=0.08) and F7−/C7+ (p=0.05) dyads suggesting that the FC7+ genotype configuration may be a potential biomarker for OCB severity.
3.5 Parent-child triads
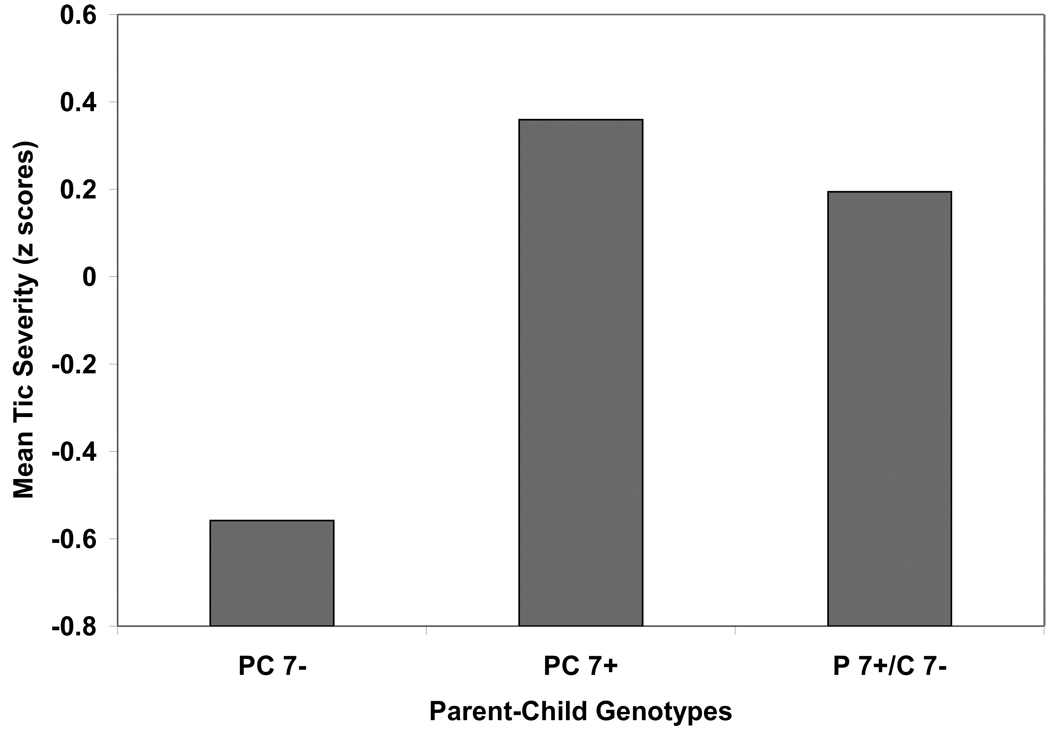
We also compared families with the following three genotype configurations: child and at least one parent was a 7-repeat allele carrier (PC7+; n=17), child was a noncarrier but at least one parent was a 7-repeat allele carrier (P7+/C7−; n=13), and child plus both parents were noncarriers (PC7−; n=19). The MANOVA was significant for repetitive behaviors (F=2.73, p=0.03) but not ODD/SAD (F=1.94, p=0.11). Subsequent univariate analyses indicated genotype groups differed for tic (F=4.42, p=0.02, ηp2=0.16) but not OCB (F=2.22, p=0.12, ηp2=0.09) severity. Children in PC7− triads were rated as having less severe tics than youths in either PC7+ (p<0.01, ηp2=0.20) or P7+/C7− (p=0.04, ηp2=0.14) genotype groups (Figure 3).
Figure 3.
Parent-child triads (PC), DRD4 7-repeat allele carriers (7+) and noncarriers (7−) and maternal ratings of tic severity (z scores).
3.6 Allelic transmission
TDTae analyses indicated that estimated genotyping error rates were uniformly zero (i.e., no instance of parent-child incompatibility). When the sample was dichotomized into more and less severe groups using a median split procedure, analyses indicated undertransmission of the 2 allele in the group of youths with more severe tics (Χ2=8.25, p=0.02), which was still significant when corrected for multiple comparisons (p=0.03). For OCB, there was marginally significant evidence for the undertransmission of the 7 allele (Χ2=5.78, p=0.06).
4. Discussion
The results of this study suggest that knowledge of parental genotype, in conjunction with child genotype, may be helpful in identifying prognostic biomarkers for co-occurring behavioral disturbances in children with ASD. Although optimal DRD4 genotype configuration varied as a function of parent (mother, father) and symptom dimension, obtained effect sizes for particular subgroup comparisons were generally larger than for gene-behavior associations based solely on child genotype. Specifically, mother-child and father-child 7-repeat genotypes were associated with ODD and SAD severity and with OCB and tic severity, respectively. In addition, youths who did not have a parent who was a 7-repeat carrier (which comprised 39% of the study sample) were less likely to have severe tic-like behaviors than the 61% who did (i.e., regardless of the child’s genotype), and the magnitude of this group difference was large (p=0.005, ηp2=0.16). Nevertheless, effect sizes for tic severity were even larger for some subgroup comparisons of father-child dyads. Because there are seemingly countless biopsychosocial variables that contribute to variation in parent and child behavior, to include gene-gene and gene × environment interactions, parent-of-origin effects, and allelic and locus heterogeneity, the search for clinically useful prognostic biomarkers is a dynamic process that will need to accommodate to ever more complex models of disease and prevention, requiring ever larger samples. Moreover, this diversity in sources of variation and mechanisms of pathogenesis may also explain the less than perfect alignment with specific findings for allelic transmission.
Studies examining the relation of parental genotype with variables associated with child behavior problems are limited, and all pertain to non-ASD samples. In one study of toddlers at risk for disruptive-aggressive behavior problems, van IJzendoorn et al. (2008) compared mothers who were and were not carriers of genes associated with less efficient dopaminergic system functioning (i.e., DRD4 7-repeat allele carriers, COMT Val+ genotype). Mothers with a combination of both “risk” genotypes engaged in less than optimal parenting behavior if they were also confronted with higher levels of daily hassles versus similar parents with lower levels of daily hassles. Moreover, the latter were actually more sensitive parents than the comparison genotype groups. Gervai et al. (2007), however, failed to detect an association between maternal DRD4 genotype and level of disrupted maternal affective communication with infants, but a recently reported study by Kaitz et al. (2010) found that mothers who were DRD4 7-repeat carriers behaved more sensitively toward fussy than less fussy infants compared with mothers who were not 7-repeat allele carriers. Lastly, Lee et al. (2008) reported an association between a maternal dopamine transporter gene (DAT1, SL6A3) polymorphism and negative parenting in a sample of children with ADHD and controls, which was significantly stronger for children who were highly disruptive during a mother-child interaction task.
Given the (a) well-documented stress involved in raising a child with ASD and (b) evidence in adults associating the 7-repeat allele with impulsivity (Congdon et al., 2008) and less than optimal parenting in stressful situations (van IJzendoorn et al., 2008)), we explored whether mothers’ DRD4 genotype would influence ratings of symptom severity, either directly through reciprocal interaction with the child or indirectly though altered perceptions of misbehavior. Although noncarrier children in our study were not rated more severely by mothers who were carriers versus noncarriers, mean ratings for ODD and SAD symptom severity were in the predicted direction (Figure 1). However, the modest size of the study sample and concerns about Type 2 error restrict inferences to suggestive evidence. Conversely, it is possible that susceptibility alleles are differentially more problematic for children with certain types of central nervous system disease than for their parents (who do not have the disorder). Alternatively, if 7-repeat allele carriers do engage in less effective parenting (which was not assessed in this study), their noncarrier offspring may be buffered from the experience by the behavior of a noncarrier spouse or partner. Regardless, further study with larger samples is warranted.
We have found associations of repetitive behaviors with both impulsive-disruptive behaviors and anxiety in this same sample for other candidate genes that influence dopaminergic system function or development (Gadow et al., 2008b, 2009; Roohi et al., 2009) as have others for non-ASD samples (Comings et al., 1996; Rowe et al., 1998). This is consistent with research findings from diverse disciplines to include the following: (a) co-occurrence of these behaviors in animals (Hutt & Hutt, 1965; Koolhaas et al., 1999; Sih et al., 2004) and many different neurodevelopmental syndromes in humans, (b) phylogeny of seemingly purposeless movements in emotional expression of animals and humans (Darwin, 1890, Sherrington, 1900) and their phenomenological similarities (Eilam et al., 2006; Tinbergen & Tinbergen, 1976), (c) both phenotypic and genotypic evidence of routines or repetitive behaviors in proactive animal personality (Koolhaas et al., 1999; Sih et al., 2004), which may include high levels of exploratory behavior, activity, and aggression but low levels of flexibility, arguably a characteristic of ODD and SAD, (e) likely role of repetitive behaviors in anxiety/stress reduction (Eilam et al., 2006; Hutt & Hutt, 1965; Koolhaas et al., 1999), and (f) involvement of the dopaminergic system in repetitive behaviors in animals (Eilam et al., 2006; Koolhaas et al., 1999) and humans (see below). Based on the aforementioned research, it also seems reasonable to speculate that for some children with ASD, repetitive behaviors may compensate for a seemingly diminished capacity for exploratory behavior (Hutt, 1969; Tinbergen & Tinbergen, 1976). Although evidence supporting the heritability of certain types of parenting behaviors is compelling (McGuire, 2003) and findings supporting an association of the 7-repeat DRD4 allele with child and parent behaviors is growing, the biologic substrates of these gene-behavior relations, both individually and interactively, are largely unknown, but progress is being made in this area as well (see McCormack et al., 2009; Meaney & Szyf, 2005; Swain et al., 2007).
Because children with ASD have seemingly high rates of OCB, tics, and SAD (e.g., Gadow & DeVincent, 2005; Gadow et al., 2005; Zandt et al., 2007) as do their relatives (Bolton et al., 1998; Micali et al., 2004; Piven & Palmer, 1999), it is possible DRD4 exon 3 VNTR variants may actually be implicated in the pathogenesis of behavioral disturbances. In the case of repetitive behaviors, TDTae analyses indicated that greater versus lesser tic severity (categorical model) was associated with the 2-repeat allele (undertransmission) with marginally significant evidence for the 7-repeat allele (undertransmission) and OCB. The relation of DRD4 alleles with obsessive-compulsive disorder, tic disorder, or both in non-ASD samples has been examined with both case-control and within-family transmission analyses in studies from North America (Billett et al., 1998; Camarena et al., 2007; Cruz et al., 1997; Díaz-Anzaldúa et al., 2004; Grice et al., 1996), Europe (Millet et al., 2003; Tarnock et al., 2007; Walitzia et al., 2008), Isreal (Frisch et al., 2000) and South Africa (Hemmings et al., 2004). Of the studies that examined tics, several provide at least tentative evidence supporting an association with DRD4 (Billet et al., 1998; Camarena et al., 2007; Cruz et al., 1997; Díaz-Anzaldúa et al., 2004; Grice et al., 1996; Walitzia et al., 2008), but results vary as a function of methodology, risk/protective allele, and patient characteristics, and at least two studies were negative (Millet et al., 2003; Tarnock et al., 2007). Although linkage disequilibrium may explain some discrepancies (Lin et al., 2007), replication drift, especially in view of the diversity in assessment strategies for measuring the phenotype, is also a concern as are trait heterogeneity and gene × environment interactions (Moffitt et al., 2005) to name but a few. For these and other reasons it is nevertheless remarkable that this particular locus has been implicated in so many different studies.
4.1 Limitations and directions for future research
Our results are subject to at least several qualifications. We used a dimensional strategy to measure the severity of co-occurring symptoms in children with diagnosed ASD as compared with categorical psychiatric diagnoses, so obtained findings may not apply to the latter. The modest size of the study sample increases the probability of spurious findings, decreases our ability to detect valid gene-behavior associations, and prevents more detailed analyses of parent-of-origin effects. It also precludes comparison of various combinations of parent-child genotypes to include the 2-repeat allele, which may be functionally intermediate between the 4- and 7-repeat alleles (Armbruster et al., 2009; Kang et al., 2008; Wang et al., 2004), and heterozygotes (Comings & MacMurray, 2000). Other variables of concern in terms of informing etiology include referral bias, linkage disequilibrium, and in the case of the MANOVA analyses, population structure (Cardon & Palmer, 2003), although meta-analyses of gene-disease research (Bamshad, 2005; Goldstein & Hirschorn, 2004; Ioannidis et al., 2004) and review of the extant literature (Hutchison et al., 2004) suggest this potential threat to internal validity may be overstated. Moreover, in the present study, because controls were ASD children with less severe symptoms from the same primarily Caucasian sample and were recruited and genotyped in identical fashion and at the same time, it is less likely that population structure or genotyping error confounded obtained results. Nevertheless, this remains a possibility. Lastly, we conceptualized the 7-repeat allele as a “risk genotype” when in fact interactions with favorable environmental experiences may facilitate more desirable outcome for some children or their parents (Bakermans-Kranenburg & van IJzendoorn, 2007; Belsky et al., 2009; van IJzendoorn et al., 2008), which if true may have led to the under-representation of 7+/7+ dyads with exceptionally non-conflicted interactions in our sample.
We did not investigate whether parental 7-repeat allele was actually associated with differentially higher levels of stress or less effective parenting, both of which may be important in explaining the observed association between parents’ DRD4 genotype and child behavior (e.g., Gervai et al., 2007; van IJzendoorn et al., 2008). The particular pattern of behaviors (ODD, SAD) associated with DRD4 genotype share similarities with disorganized attachment, which may play a role in the pathogenesis of disruptive behavior disorder (see Kochanska et al., 2009). Because polygeny and epistasis are part of the genetic architecture of behavioral characteristics (Flint & Mackay, 2009; Moore, 2003), it is reasonable to expect that multiple genes are involved in parenting behavior as well. For example, there is another variation in the DRD4 gene, a single nucleotide polymorphism, -521 C/T, also shown to reduce transciptional activity (Okuyama et al., 1999) that may be associated with mother-child interactions (see Bakermans-Kranenburg & van Ijzendoorn, 2007).
Although the present study obtained fairly detailed accounts of child behavior problems, this was not the case for the mental health status of the children’s mothers or fathers. For example, there is some indication that ODD is a viable clinical phenotype in adults (Gadow et al., 2007), and it would be informative to determine if parents’ DRD4 genotype is associated with behavioral characteristics that might influence parenting. Moreover, there is some research indicating that child misbehavior appears to have relatively less significance for paternal than maternal stress (e.g., Hastings et al., 2005; Herring et al., 2006), possibly as a function of differentially less involvement in child care, which also warrants closer examination.
For all the aforementioned reasons, our reported findings must be considered tentative pending replication in larger independent samples. Moreover, they are hypothesis generating and not hypothesis confirming and as such are presented here as indications for further study in what has to date been a relatively ignored topic within the ASD clinical phenotype.
4.2 Clinical implications
Co-occurring behavioral disturbances in children with ASD pose serious challenges to intervention efforts, function as critical impediments to social integration with peers and later life adjustment; and typically result in considerable stress in the home. The identification and validation of potential prognostic biomarkers is a necessary first step in the formulation of genomic profiles that can be ascertained at the point of diagnosis and later used to inform treatment and long-term clinical management decisions. The findings of the present study suggest one possible strategy for enhancing the predictive power of common gene variants as possible biomarkers of behavioral disturbances in children with ASD and indicate directions for further study.
Acknowledgement
This study was supported, in part, by grants from the National Institutes of Health, M01RR10710 (GCRC) and MH071523 (Dr. Mendell, PI) and the National Alliance for Autism Research (Dr. Hatchwell, PI) and charitable donations to the Matt and Debra Cody Center for Autism and Developmental Disorders. The authors wish to thank Dr. John Pomeroy for supervising the ASD diagnoses, Mrs. Elizabeth Luchsinger for facilitating the genotyping, and anonymous reviewers for providing helpful comments.
Abbreviations
- ADHD
attention-deficit hyperactivity disorder
- ASD
autism spectrum disorder
- CSI-4
Child Symptom Inventory-4
- DAT1
dopamine transporter gene
- DRD4
dopamine receptor D4 gene
- ηp2
partial eta-squared
- F
father
- FC
father-child
- M
mother
- MC
mother-child
- OCB
obsessive-compulsive behavior
- ODD
oppositional defiant disorder
- SAD
separation anxiety disorder
- SES
socioeconomic status
- TDT
transmission disequilibrium test
- VNTR
variable number tandem repeat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Kenneth D. Gadow: shareholder in Checkmate Plus, publisher of the Child Symptom Inventory-4; Carla J. DeVincent : none; Victoria Pisarevskaya: none; Doreen Olvet: none; Wenjie Xu: none; Nancy Mendell: none; Steven Finch: none; Eli Hatchwell: none
Contributor Information
Kenneth D. Gadow, Department of Psychiatry, Director of Research, Division of Child and Adolescent Psychiatry, Director of Clinical Research, Cody Center for Autism and Developmental Disabilities (Pediatrics), Putnam Hall, South Campus, Stony Brook University, Stony Brook, NY 11794-8790, Phone: (631) 632-8858, FAX: (631) 632-8953, kenneth.gadow@stonybrook.edu
Carla J. DeVincent, Department of Pediatrics, State University of New York at Stony Brook, Stony Brook, NY 11794-8788, Phone: (631) 632-3042, FAX: (631) 632-3021, carla.devincent@sunysb.edu
Victoria Pisarevskaya, Department of Pathology, Stony Brook University, Stony Brook, NY 11794-8088, Victoria.Pisarevskaya@stonybrook.edu.
Doreen M. Olvet, Zucker Hillside Hospital, Psychiatry Research, North Shore – Long Island Jewish Health System, Glen Oaks, NY 11004, dolvet@nshs.edu
Wenjie Xu, Department of Applied mathematics and Statistics, Stony Brook University, Stony Brook, NY 11794-8088, tasu@163.com.
Nancy Mendell, Department of Applied mathematics and Statistics, Stony Brook University, Stony Brook, NY 11794-8088, nancy.mendell@stonybrook.edu.
Stephen J. Finch, Department of Applied mathematics and Statistics, Stony Brook University, Stony Brook, NY 11794-8088, steven.finch@stonybrook.edu
Eli Hatchwell, Department of Pathology, Director of the Genomics Core Facility and Associate Professor, HSC-T8, Room 053, Stony Brook University, Stony Brook, NY 11794-8088, Phone: 631-444-1206, FAX: 631-444-3129, eli.hatchwell@stonybrook.edu.
References
- Armbruster D, Mueller A, Moser DA, Lesch KP, Brocke B, Kirschbaum C. Interaction effect of D4 dopamine receptor gene and serotonin transporter promoter polymorphism in the cortisol stress response. Behav Neurosci. 2009;123:1288–1295. doi: 10.1037/a0017615. [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and onserveed maternal insensitivity predicting externalizing behavior in preschoolers. Dev Psychobiol. 2006;49:619–632. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Research review: Genetic vulnerability or differential susceptibility in child development: The case of attachment. J Child Psychol Psychiatry. 2007;48:1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Pijlman FTA, Mesman J, Juffer F. Experimental evidence for differential sensitivity: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Dev Psychol. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Bamshad M. Genetic influences on health. JAMA. 2005;294:937–946. doi: 10.1001/jama.294.8.937. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes. Mol Genet. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson PR. The impact of child symptom severity on depressed mood among parents of children with ASD. The mediating role of stress proliferation. J Autism Dev Disord. 2006;36:685–695. doi: 10.1007/s10803-006-0112-3. [DOI] [PubMed] [Google Scholar]
- Billet EA, Richter MA, Sam F, Swinson RP, Dai X-Y, King N, et al. Investigation of dopamine system genes in obsessive-compulsive disorder. Psychiatr Genet. 1998;8:163–169. doi: 10.1097/00041444-199800830-00005. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and oth4er psychiatric disorders: Patterns of familial aggregation. Psychol Med. 1998;28:385–395. doi: 10.1017/s0033291797006004. [DOI] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163:2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Camarena B, Loyzaga C, Aguilar A, Weissbecker K, Nicolini H. Association study between the dopamine receptor D4 gene and obsessive-compulsive disorder. Eur Neuuropsychopharmacol. 2007;17:406–409. doi: 10.1016/j.euroneuro.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Schultz C, White BN, Jenkins EC, Brown WT, et al. Association of autism severity with a monoamine oxidase A functional polymorphism. Clin Genet. 2003;64:190–197. doi: 10.1034/j.1399-0004.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Cohen J. The earth is round (p<.05) Am Psychol. 1994;49:997–1003. [Google Scholar]
- Comings DE, MacMurray JP. Molecular heterosis: A review. Mol Genet Metabolism. 2000;71:19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- Comings DE, Wu S, Chiu C, Ring RH, Gade R, Ahn C, et al. Polygenetic inheritance of Tourette syndrome, stuttering, attention deficit hyperactivity, conduct, and oppositional defiant disorder. Am J Med Genet Part B. 1996;67B:264–288. doi: 10.1002/(SICI)1096-8628(19960531)67:3<264::AID-AJMG4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. Am J Med Genetics Part B. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Cravchik A, Goldman D. Neurochemical individuality: genetic diversity among human dopamine and serotonin receptors and transporters. Arch Gen Psychiatry. 2000;57:1105–1114. doi: 10.1001/archpsyc.57.12.1105. [DOI] [PubMed] [Google Scholar]
- Cruz C, Camarena B, King N, Páez F, Sidenberg D, Ramón J, et al. Increased prevalence of the seven-repeat variant of the dopamine D4 receptor gene in patients with obsessive-compulsive disorder with tics. Neurosci Lett. 1997;231:1–4. doi: 10.1016/s0304-3940(97)00523-5. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of emotions in man and animals. 2nd edn. London: John Murray; 1890. (originally published 1872) [Google Scholar]
- DeVincent CJ, Gadow KD. Relative clinical utility of three Child Symptom Inventory-4 scoring algorithms for differentiating children with autism spectrum disorder versus attention-deficit hyperactivity disorder. Autism Res. 2009;2:312–321. doi: 10.1002/aur.106. [DOI] [PubMed] [Google Scholar]
- Díaz-Anzaldúa A, Joober R, Rivière J-B, Dion Y, Lespérance P, Richer F, et al. Tourette syndrome and dopaminergic genes: A family-based association study in the French Canadian founder population. Mol Psychiatr. 2004;9:272–277. doi: 10.1038/sj.mp.4001411. [DOI] [PubMed] [Google Scholar]
- DiLalla LF, Elam KK, Smolen A. Genetic and gene-environment interaction effects on preschoolers’ social behaviors. Dev Psychobiol. 2009;51:451–464. doi: 10.1002/dev.20384. [DOI] [PubMed] [Google Scholar]
- Eilam D, Zor R, Szechtman H, Hermesh H. Rituals, stereotypy and compulsive behavior in animals and humans. Neurosci Behav Rev. 2006;30:456–471. doi: 10.1016/j.neubiorev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodology. 2002;2 doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Mackay TFC. Genetic architecture of quantitative traits in mice, flies, and humans. Genome Res. 2009;19:723–733. doi: 10.1101/gr.086660.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch A, Michaelovsky E, Rockah R, Amir I, Hermesh H, Laor N, et al. Association between obsessive-compulsive disorder and polymorphisms of genes encoding components of the serotonergic and dopaminergic pathways. Eur Neuropsychopharm. 2000;10:205–209. doi: 10.1016/s0924-977x(00)00071-7. [DOI] [PubMed] [Google Scholar]
- Foley DL, Pickles A, Maes HM, Silberg JL, Eaves LJ. Course and short-term outcomes of separation anxiety disorder in a community sample of twins. J Am Acad Child Adolesc Psychiatry. 2004;43:1107–1114. doi: 10.1097/01.chi.0000131138.16734.f4. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ. Clinical significance of tics and attention-deficit hyperactivity disorder (ADHD) in children with pervasive developmental disorder. J Child Neurol. 2005;20:481–488. doi: 10.1177/08830738050200060301. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Drabick DAG. Oppositional defiant disorder as a clinical phenotype in children with autism spectrum disorder. J Autism Dev Disord. 2008a;38:1302–1310. doi: 10.1007/s10803-007-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-aged children with PDD versus clinic and community samples. Autism. 2005;9:392–415. doi: 10.1177/1362361305056079. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Roohi J, DeVincent CJ, Hatchwell E. Association of ADHD, tics, and anxiety with dopamine transporter (DAT1) genotype in autism spectrum disorder. J Child Psychol Psychiatry. 2008b;49:1331–1338. doi: 10.1111/j.1469-7610.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Roohi J, DeVincent CJ, Kirsch S, Hatchwell E. Association of COMT (Val158Met) and BDNF (Val66Met) gene polymorphisms with anxiety, ADHD and tics in children with autism spectrum disorder. J Autism Dev Disord. 2009;39:67–74. doi: 10.1007/s10803-009-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Schwartz J, DeVincent C, Strong G, Cuva S. Clinical utility of autism spectrum disorder scoring algorithms for the Child Symptom Inventory. J Autism Dev Disord. 2008c;38:419–427. doi: 10.1007/s10803-007-0408-y. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Department of Psychiatry. Stony Brook: State University of New York; 1986. Stony Brook Child Psychiatric Checklist-3. [Google Scholar]
- Gadow KD, Sprafkin J. Child Symptom Inventory-4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- Gadow KD, Sprafkin J. The Symptom Inventories: An Annotated Bibliography [On-line] Stony Brook, NY: Checkmate Plus; 2009. (Available: www.checkmateplus.com.) [Google Scholar]
- Gadow KD, Sprafkin J, Schneider J, Nolan EE, Schwartz J, Weiss MD. ODD, ADHD, versus ODD+ADHD in clinic and community adults. J Attention Disord. 2007;11:374–383. doi: 10.1177/1087054706295609. [DOI] [PubMed] [Google Scholar]
- Gervai J, Novak A, Lakatos K, Toth I, Danis I, Ronai Z, et al. Infant genotype may moderate sensitivity to mater affective communications. Social Neurosci. 2007;2:307–319. doi: 10.1080/17470910701391893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Hirschorn JN. In genetic control of disease, does ‘race’ matter? Nature Genet. 2004;36:1243–1244. doi: 10.1038/ng1204-1243. [DOI] [PubMed] [Google Scholar]
- Gordon D, Haynes C, Johnnidis C, Patel S, Bowcock AM, Ott J. A transmission disequilibrium test for general pedigrees to the presence of random genotyping errors and any number of untyped parents. Eur J Hum Genet. 2004;12:752–761. doi: 10.1038/sj.ejhg.5201219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Heath SC, Liu X, Ott J. A transmission/disequilibrium test that allows for genotyping errors in the analysis of single-nucleotide polymorphism data. Am J Hum Genet. 2001;69:371–380. doi: 10.1086/321981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice DE, Leckman JF, Pauls DL, Kurlan R, Kidd KK, Pakstis AJ, et al. Linkage disequilibrium between an allele at the dopamine D4 receptor locus and Tourette syndrome, by the transmission-disequilibrium test. Am J Hum Genet. 1996;59:644–652. [PMC free article] [PubMed] [Google Scholar]
- Hastings RP, Kovshoff H, Ward NJ, degli Espinosa F, Brown T, Remington B. Systems analysis of stress and positive perceptions in mothers and fathers of preschool children with autism. J Autism Dev Disord. 2005;35:635–644. doi: 10.1007/s10803-005-0007-8. [DOI] [PubMed] [Google Scholar]
- Hemmings SMJ, Kinnear CJ, Lochner C, Niehaus DJH, Knowles JA, Moolman-Smook JC, et al. Early- versus late-onset obsessive-compulsive disorder: Investigating genetic and clinical correlates. Psychiatr Res. 2004;128:175–182. doi: 10.1016/j.psychres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Herring S, Gray K, Taffe J, Tonge B, Sweeney D, Einfeld S. Behaviour and emotional problems in toddlers with pervasive developmental delay. J Intellect Dis Res. 2006;50:874–882. doi: 10.1111/j.1365-2788.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Department of Sociology. New Haven, CT: Yale University; 1975. Four Factor Index of Social Status. [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: Fatal threat of red herring? Psych Bull. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Hutt C. Exploration, arousal and autism. Psychol Forsch. 1969;33:1–8. doi: 10.1007/BF00424612. [DOI] [PubMed] [Google Scholar]
- Hutt C, Hutt JS. Effects of environmental complexity on stereotyped behaviours of children. Anim Behav. 1965;13:1–4. [Google Scholar]
- Ioannidis JPA, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nature Genet. 2004;36:1312–1318. doi: 10.1038/ng1474. [DOI] [PubMed] [Google Scholar]
- Kaitz M, Shalev I, Sapir N, Devor N, Samet Y, Mankuta D, et al. Mothers’ dopamine receptor polymorphism modulates the relation between infant fussiness and sensitive parenting. Dev Psychobiol. 2010;52:149–157. doi: 10.1002/dev.20423. [DOI] [PubMed] [Google Scholar]
- Kang JI, Namkoong K, Kim SJ. The association of 5-HTTLPR and DRD4 VNTR polymorphisms with affective temperamental traits in healthy volunteers. J Affective Disord. 2008;109:157–163. doi: 10.1016/j.jad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Barry RA, Stellern SA, O’Bleness JJ. Early attachment organization moderates the parent-child mutually coercive pathway to children’s antisocial conduct. Child Dev. 2009;80:1288–1300. doi: 10.1111/j.1467-8624.2009.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behavior and stress-physiology. Neuroisci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Gadow KD, DeVincent CJ, Edwards MC. Validation of DSM-IV model of psychiatric syndromes in children with autism spectrum disorder. J Autism Dev Disord. 2009;39:278–289. doi: 10.1007/s10803-008-0622-2. [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J Intellect Dis Res. 2006;50:172–183. doi: 10.1111/j.1365-2788.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- Lee SS, Chronis-Tuscano A, Keenan K, Pelham WE, Loney L, Van Hulle CA, et al. Association of maternal dopamine transporter genotype with negative parenting: evidence for a gene x environment interaction with child disruptive behavior. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.102. 10.1038/mp.2008.102. [DOI] [PubMed] [Google Scholar]
- Lin P-I, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: The flip-flop phenomenon. Am J Hum Genet. 2007;80:531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin trasnsporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough JJ. Attention-deficit/hyperactivity disorder pharmacogenomics. Biol Psychiatry. 2005;57:1367–1373. doi: 10.1016/j.biopsych.2004.10.021. [DOI] [PubMed] [Google Scholar]
- McGuire S. The heritability of parenting. Parenting: Science and Practice. 2003;3:73–94. [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? TRENDS Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Micali N, Chakrabarti S, Fombonne E. The broad autism phenotype. Autism. 2004;8:21–37. doi: 10.1177/1362361304040636. [DOI] [PubMed] [Google Scholar]
- Millet B, Chabane N, Delorme R, Leboyer M, Leroy S, Poirier M-F, et al. Association between the dopamine receptor D4 (DRD4) gene and onsessive-compulsive disorder. Am J Med Genetics Part B 2003. 2003;116B:55–59. doi: 10.1002/ajmg.b.10034. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Moore JH. The ubiquitous nature of epistasis in determining susceptibility to common human diseases. Hum Heredity. 2003;56:73–82. doi: 10.1159/000073735. [DOI] [PubMed] [Google Scholar]
- Okuyama Y, Ishiguro H, Toru M, Arinami T. A genetic polymorphism in the promoter region of DRD4 assoicated with expression and schizophrenia. Biochemical Biophysical Res Communications. 1999;258:292–295. doi: 10.1006/bbrc.1999.0630. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. Am J Psychiatry. 1999;156:557–563. doi: 10.1176/ajp.156.4.557. [DOI] [PubMed] [Google Scholar]
- Pottie CG, Cohen J, Ingram KM. Parenting a child with autism: Contextual factors associates with enhanced daily parental mood. J Pediatr Psychol. 2009;34:419–429. doi: 10.1093/jpepsy/jsn094. [DOI] [PubMed] [Google Scholar]
- Rao PA, Beidel DC. The impact of children with high-functioning autism on parental stress, sibling adjustment, and family functioning. Behavior Modification. 2009;33:437–451. doi: 10.1177/0145445509336427. [DOI] [PubMed] [Google Scholar]
- Roohi J, DeVincent CJ, Hatchwell E, Gadow KD. Association of a monoamine oxidase-A gene promoter polymorphism with ADHD and anxiety in boys with autism spectrum disorder. J Autism Dev Disord. 2009;39:67–74. doi: 10.1007/s10803-008-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Rowe DC, Stever C, Gard JMC, Cleveland HH, Sanders ML, Abramowitz A, et al. The relation of the dopamine transporter gene (DAT1) to symptoms of internalizing disorders in children. Behav Genet. 1998;28:215–225. doi: 10.1023/a:1021427314941. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord. 2009 doi: 10.1007/s10803-009-0887-0. doi:10.1007/s1083-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Dev Psychpathol. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Experimentation in emotion. Nature. 1900;62(1065):328–331. [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. TRENDS Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Smith TF. Meta-analysis of the heterogeneity in association of DRD4 7-repeat allele and AD/HD: Stronger association with AD/HD combine type. Am J Med Genet Part B. 2010 doi: 10.1002/ajmg.b.31090. Published online 28 April, 2010. [DOI] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnock Z, Ronai Z, Gervai J, Kereszturi E, Gardoros J, Sasvari-Szekely M, et al. Dopaminergic candidate genes in Tourette syndrome. Am J Med Genet Part B. 2007;144B:900–905. doi: 10.1002/ajmg.b.30517. [DOI] [PubMed] [Google Scholar]
- Tinbergen EA, Tinbergen N. The aetiology of childhood autism: a criticism of the Tinbergens’ theory: a rejoinder. Psychol Med. 1976;6:545–549. doi: 10.1017/s003329170001816x. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J. Dopamine system genes associated with parenting in the context of daily hassles. Genes Brain Behav. 2008;7:403–410. doi: 10.1111/j.1601-183X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- van Tol HMH, Wu CM, Guan H-C, Ohara K, Bunzow JR, Civelli O, et al. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- Walitza S, Scherag A, Renner TJ, Hinney A, Remschmidt H, Herpertz-Dahlmann B, et al. Transmission disequilibrium studies in early onset of obsessive-compulsive disorder for polymorphisms in genes of the dopaminergic system. J Neural Transm. 2008;115:1071–1078. doi: 10.1007/s00702-008-0051-6. [DOI] [PubMed] [Google Scholar]
- Wang E, Ding YC, Flodman P, Kidd JR, Kidd KK, Grady DL, et al. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. Am J Hum Genet. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wise CA, Gordon D, Finch SJ. A family-based likelihood ratio test for general pedigree structures that allows for genotyping error and missing data. Hum Heredity. 2008;66:99–110. doi: 10.1159/000119109. [DOI] [PubMed] [Google Scholar]
- Zandt F, Prior M, Kyrios M. Repetitive behaviour in children with high functioning autism and obsessive compulsive disorder. J Autism Dev Disord. 2007;37:251–259. doi: 10.1007/s10803-006-0158-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Quan H, Ng J, Stepanavage ME. Some statistical methods for multiple endpoints in clinical trials. Control Clin Trials. 1997;18:204–221. doi: 10.1016/s0197-2456(96)00129-8. [DOI] [PubMed] [Google Scholar]