Abstract
Purpose
Radiotherapy for head and neck cancer consists of fractionated radiation treatments that cause significant damage to salivary glands leading to chronic salivary gland dysfunction with only limited prevention and treatment options currently available.
Methods and materials
Mouse models were exposed to fractionated radiation and salivary gland function and histological analyses of structure, apoptosis, and proliferation were evaluated.
Results
In this study, we report that treatment with fractionated doses of radiation results in a significant level of apoptotic cells in FVB mice after each fraction, which is significantly decreased in transgenic mice expressing a constitutively active mutant of Akt1 (myr-Akt1). Salivary gland function is significantly reduced in FVB mice exposed to fractionated radiation; however myr-Akt1 transgenic mice maintain salivary function under the same treatment conditions. Injection of recombinant IGF-1 into FVB mice, which activates endogenous Akt, suppresses acute apoptosis and preserves salivary gland function following fractionated doses of radiation 30–90 days after treatment. FVB mice exposed to fractionated radiation have significantly lower levels of PCNA positive salivary acinar cells 90 days after treatment which correlates with a chronic loss of function. In contrast, FVB mice injected with IGF-1 prior to each radiation exhibit acinar cell proliferation rates similar to untreated controls.
Conclusion
These studies suggest that activation of IGF-1 mediated pathways prior to head and neck radiation could modulate radiation-induced salivary gland dysfunction and maintain glandular homeostasis.
Keywords: IGF-1, Akt, radiation, salivary gland dysfunction, apoptosis, xerostomia
INTRODUCTION
Radiation therapy for head and neck cancer results in significant side effects including xerostomia, mucositis, and malnutrition in most patients (1). During therapy, these patients are exposed to consecutive days (5 days/week over a total period of 35–50 days) of radiation therapy which takes a significant toll on the normal salivary gland (2–4). The ensuing salivary gland hypofunction results in significant morbidity, diminishes the effectiveness of anti-cancer therapies, and often permanently decreases the quality of life for these patients (5). Clinically, radiation exposure of parotid salivary glands is kept below 2 Gy/day and a cumulative dose of 24–26 Gy to allow recovery of salivary function (3;6). Although improvements have been made in targeting radiation treatment to the tumor, the salivary glands are often in close proximity to the treatment site and consequently receive enough radiation exposure to affect physiologic functions.
Previous studies in different animal models have identified discrepancies in the contribution of apoptosis to radiation-induced salivary gland dysfunction (reviewed in (7)). In the rat, induction of apoptosis is variable with reports ranging from minimal levels to significantly higher levels (8–10). In the mouse, significant levels of apoptosis can be detected, which increases with radiation dose (11–16). Transgenic mice expressing a constitutively activated Akt (myr-Akt1) suppressed radiation-induced apoptosis in vitro and in vivo by regulating the activation of p53 (14). As a means to translate these studies, a preclinical model was developed using an intravenous injection of recombinant IGF-1 (insulin-like growth factor-1) to activate endogenous Akt in the salivary glands (11). Most importantly, modulation of the apoptotic response via activation of Akt (myr-Akt1 or IGF-1 injection) or loss of p53 expression results in preserved salivary gland function following a single dose of therapeutic radiation (11;12).
In this study, we report that treatment with radiation (1 or 2 Gy/day) over a fractionated scheme (1–5 days) results in a significant increase in apoptotic cells (as determined by activated caspase-3) in FVB mice after each fraction. Similar to previous studies with single doses of radiation (11;14), myr-Akt1 transgenic mice exhibit significant decreases in the presence of apoptotic cells at each fraction. The peak of apoptotic cells was detected 24 hours after the final radiation treatment and dropped significantly by 48 hours. The induction of apoptosis in FVB mice correlated with a significant decrease in physiologic function of the salivary gland following fractionated radiation dose treatment. In contrast, the reduction of apoptotic cells in myr-Akt1 transgenic mice correlated with preserved salivary flow following fractionated radiation. Based on these observations, the pre-clinical model of IGF-1 injection described previously for single dose radiation (11) was modified to the fractionated radiation treatment. Pre-treatment of FVB mice with IGF-1 prior to each radiation treatment preserves salivary function in a fractionated radiation treatment protocol. These data support the hypothesis that modulation of the radiation-induced stress program could significantly benefit salivary gland function.
MATERIALS AND METHODS
Mice
Myr-Akt1 transgenic mice under the control of the mouse mammary tumor virus promoter and maintained on an FVB background, were generated at the University of Colorado Health Sciences Center and the salivary gland phenotype was described previously (14). Primers used for genotyping were obtained from Integrated DNA Technologies (Coralville, IA).
Radiation Treatment
Four-five week-old female FVB, myr-Akt1, or IGF-1 injected mice were anesthetized with avertin (0.4 to 0.6 mg/kg, intraperitoneally [i.p.]), and the head and neck region was exposed to radiation [Cobalt-60 Teletherapy unit from Atomic Energy of Canada Ltd Theratron-80] while the rest of the body was shielded with lead as previously described (12). For IGF-1 injections (GroPrep, Adelaide, Australia), FVB mice were injected with 5µg of IGF-1 diluted in sterile PBS with 10mg/ml BSA (total volume of 100µl) immediately prior to each radiation treatment as previously described (11). Animals were maintained and treated in accordance with protocols approved by the University of Arizona IACUC.
Histology
Tissues were fixed in 10% neutral buffered formalin for 24 hours, transferred to 70% ethanol, and embedded in paraffin. Tissue sections were cut to 4 µm and processed for standard staining with hematoxylin and eosin by the Histology Service Laboratory in the Department of Cell Biology and Anatomy at the University of Arizona.
Induction and quantification of apoptosis
Salivary glands were removed 24, 48, 72, or 96 hours after the last radiation dose and processed for histology. Unstained tissue sections were processed for anti-activated caspase-3 immunohistochemistry as previously described (14). Tissue sections were observed by standard light microscopy, and photomicrographs were taken with a Leica DM5500 with a 4 megapixel Pursuit camera. Individual means for quantification of caspase-3 positive cells were determined by averaging the number of positive cells/total number of cells from a minimum of three fields of view/animal (3 mice per treatment; total cells counted ranged from 2,400 to 3,400 per mouse).
Saliva Collection
Mice were injected i.p. with 0.25 mg carbachol/kg body weight as previously described (14) on days 30, 60, and 90 after radiation treatments. Saliva was collected from 10–17 mice/genotype/treatment on ice immediately following carbachol injection for 5 min into pre-weighed tubes.
Quantification of proliferation
For evaluation of acinar cell proliferation, unstained tissue sections were processed for anti-PCNA (proliferating cell nuclear antigen, Santa Cruz Biotechnology Inc., Santa Cruz, CA) immunohistochemistry 90 days after treatment. Briefly, slides were heated to 37°C for 30 min. and rehydrated in histoclear, graded alcohols, and distilled water washes. Nonspecific peroxidase activity was quenched with 0.3% H2O2. For antigen retrieval, slides were placed in citrate buffer (pH 6.0) and heated in a microwave oven twice for 5 min, and allowed to cool for 20 min. After washes, the slides were treated according to manufacturer’s instructions (Vectastain Elite ABC kit, PK-6101, Vector Laboratories Inc., Burlingame, CA). Color development was achieved with Biogenex DAB incubation for 6 to 8 minutes as previously described (14). Slides were lightly counterstained with Gill's hematoxylin, dehydrated, and mounted in Permount. Tissue sections were observed by standard light microscopy and photomicrographs were taken with a Leica DM5500 with a 4 megapixel Pursuit camera. Individual means for quantification of PCNA positive acinar and ductal cells were determined by averaging the number of positive cells/total number of cells from a minimum of three fields of view/animal (8–11 mice per group; total cells counted ranged from 8,000 to 10,000 per mouse).
Statistics
Comparison of caspase-3 data was accomplished by a one-way ANOVA followed by a Bonferroni test with adjustment for the number of pairwise comparisons between FVB and Akt groups exposed to the same amount of radiation or at the same time points. Saliva flow rates standardized to the respective untreated group and PCNA data were analyzed using a one-way ANOVA followed by Student-Newman Keul’s multiple comparison test. Statistical analysis and graphical generation of data were done using GraphPad Prism software (version 5.0, San Diego, CA).
RESULTS
Apoptosis is induced in FVB mice following each dose of radiation
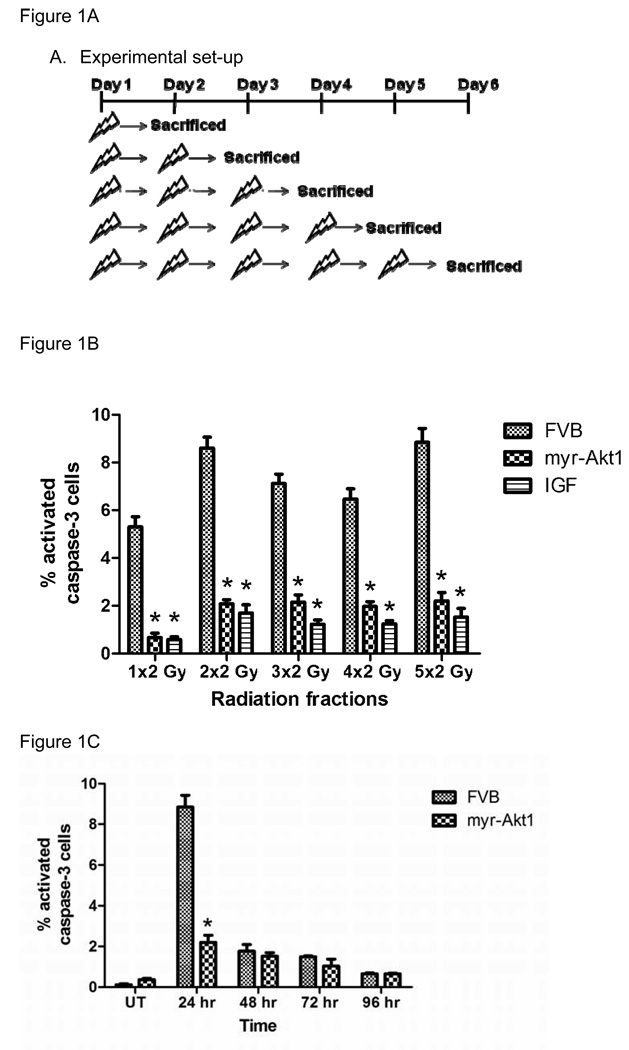
Previous studies have reported that apoptosis is induced in the salivary glands within the first 24 hours after exposure to a single dose of ionizing radiation (11–14). To investigate the contribution of apoptosis under fractionated radiation treatment, we quantified activated caspase-3 positive cells 24 hours after a varying number of daily treatments targeted to the head and neck region of mice (figure 1A). Analysis focused on the parotid gland due to previously reported apoptotic levels following a single dose of radiation (12) and its enhanced radiosensitivity in humans when compared to the submandibular glands (6;17;18). Treatment of FVB mice with 2Gy/day results in a significant level of apoptotic cells, as determined by activated caspase-3 staining, after each fraction of radiation (figure 1B). In contrast, expression of myr-Akt1 or FVB mice injected with IGF-1 significantly reduced the number of apoptotic cells following each radiation dose. We observe a similar induction of apoptosis in FVB mice following treatment with 1Gy/day that was also suppressed in myr-Akt1 transgenic mice (data not shown).
Figure 1. Induction of apoptosis in FVB or myr-Akt1 transgenic mice following 2Gy/day for five consecutive days.
In (A), the experimental set-up for figure 1B is diagramed. In (B), the head and neck region of FVB, myr-Akt1, and FVB mice injected with IGF-1 was exposed to 2 Gy radiation for increasing number of days and the parotid salivary glands were removed 24 hours after the last radiation. Tissues were processed for activated caspase-3 immunohistochemistry as described in the materials and methods section and the number of caspase-3 positive cells is graphed as a percentage of the total number of cells per field of view. In (C), female FVB and myr-Akt1 mice were exposed to 2 Gy radiation per day for five consecutive days as described in (A) and parotid salivary glands were removed at 24, 48, 72, and 96 hours. Tissues were processed for activated caspase-3 immunohistochemistry as described in B. The graph represents all data from three mice per group. Significant differences (p≤0.05) were determined using an ANOVA followed by a pairwise Bonferroni test adjusting for the number of comparisons. (*) indicates significant difference between genotypes of the same treatment.
Due to the similar percentage of apoptotic cells after each radiation treatment, we were interested in determining if these apoptotic cells persisted following the cessation of radiation treatment. FVB and myr-Akt1 transgenic mice were treated with 2Gy/day for five consecutive days (cumulative dose 10 Gy) and activated caspase-3 positive cells were evaluated at various time points following the final radiation dose. In FVB mice, the peak number of apoptotic cells is observed at 24 hours with a significant decline at the later time points (48–96 hour) indicating that these cells may be cleared by phagocytosis. The percentage of apoptotic cells in FVB mice at these later time points is not significantly different from myr-Akt1 transgenic mice. These data suggest that a significant number of apoptotic cells detected after each radiation dose (figure 1B) are new apoptotic cells. Consistent with previously published studies (11;12), a majority (>95%) of the apoptotic cells counted for figure 1 are acinar cells.
Expression of myr-Akt1 and injections with IGF-1 preserve stimulated salivary flow rates following fractionated radiation
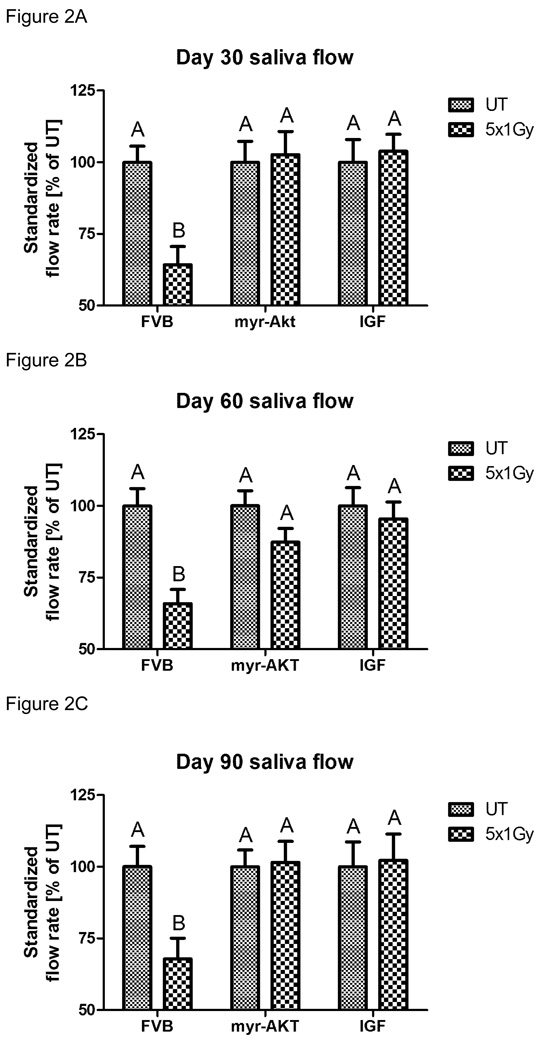
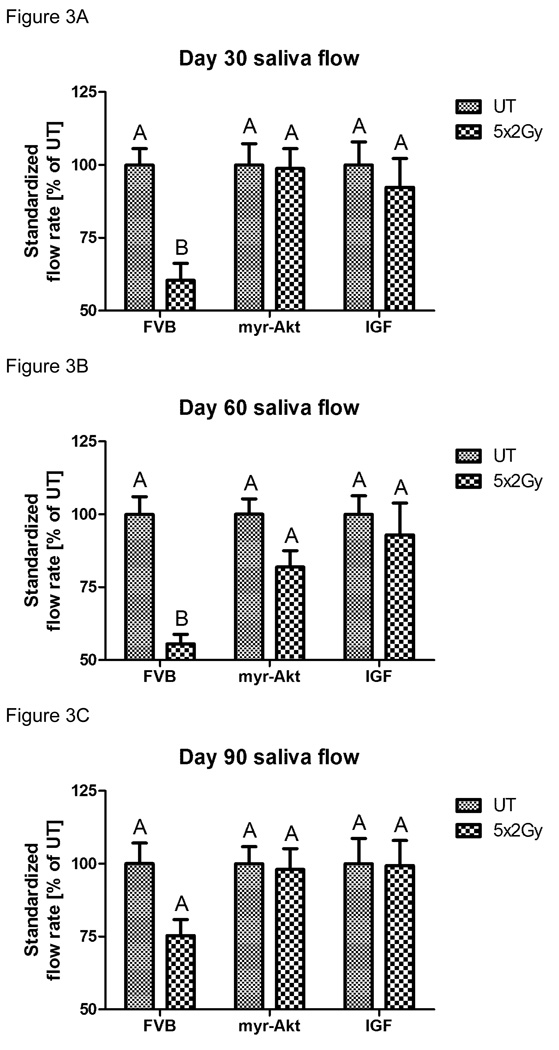
A pre-clinical model has recently been reported using an injection of IGF-1 to activate endogenous Akt and preserve salivary gland function following a single dose of radiation (11). In order to extend the translational relevance of this finding, we were interested in determining the feasibility of IGF-1 in preserving salivary gland function following a fractionated radiation treatment regimen. Due to the presence of apoptotic cells after each dose of radiation observed in figure 1B, we decided to inject IGF-1 immediately prior to each radiation treatment for functional studies. FVB, myr-Akt1, or IGF-1 injected mice were exposed to 1Gy/day for five consecutive days (cumulative dose 5 Gy; figure 2) and stimulated salivary flow rates were determined following carbachol injection on days 30, 60, and 90 post treatment. FVB mice exposed to fractionated radiation exhibit significant reductions in salivary function throughout the time course consistent with previously published studies in other animal models (rats treated with 20–60 Gy and miniature pigs treated with 70 Gy) (19–21). In contrast, myr-Akt1 or injection of IGF-1 into FVB mice preserves salivary function with no statistical difference from the corresponding unirradiated controls at all time points examined. We also evaluated stimulated salivary flow rates in FVB, myr-Akt1, or IGF-1 injected mice exposed to 2Gy/day (cumulative dose 10 Gy; figure 3) for five consecutive days on days 30, 60, and 90 post treatment. Thirty days post-irradiation, FVB mice treated with 2Gy/day for five days had similar reductions in standardized salivary flow rate (40%) as mice treated with 1Gy/day for five days (41%). In FVB mice treated with 2Gy/day for five days, there is an improvement in salivary flow rates 90 days post treatment (figure 3C), albeit the levels are 25% below untreated controls. In myr-Akt1 transgenic mice or IGF-1 injected mice treated with 2Gy/day for five days, we observed no statistical difference from the corresponding unirradiated controls at all time points examined. These data suggest that IGF-1 and myr-Akt1 can modulate the salivary gland response of FVB mice to acute radiation damage leading to preservation of function.
Figure 2. Salivary gland dysfunction in FVB, myr-Akt1 or IGF-1 injected mice following fractionated radiation (1 Gy × 5 days).
The head and neck region of FVB, myr-Akt1, and FVB mice injected with IGF-1 was exposed to 1 Gy radiation per day for five consecutive days and stimulated salivary flow rates were determined as described in the materials and methods section. Irradiated flow rates were normalized to corresponding unirradiated controls on day 30 (A), 60 (B) and 90 (C) after the final radiation treatment. (A) represents all data from at least thirteen mice/group, graph (B) represents all data from at least eleven mice/group, and graph (C) represents all data from at least ten mice/group. Significant differences (p≤0.05) were determined using an ANOVA followed by Student-Newman-Keul’s multiple comparison test. Treatment groups with the same letters are not significantly different from each other.
Figure 3. Salivary gland dysfunction in FVB, myr-Akt1 or IGF-1 injected mice following fractionated radiation (2 Gy × 5 days).
The head and neck region of FVB, myr-Akt1, and FVB mice injected with IGF-1 was exposed to 2 Gy radiation per day for five consecutive days and stimulated salivary flow rates were determined as described in figure 2. Irradiated flow rates were normalized to corresponding unirradiated controls on day 30 (A), 60 (B) and 90 (C) after the final radiation treatment. (A) represents all data from at least twelve mice/group, graph (B) represents all data from at least eleven mice/group, and graph (C) represents all data from at least ten mice/group. Significant differences (p≤0.05) were determined using an ANOVA followed by Student-Newman-Keul’s multiple comparison test. Treatment groups with the same letters are not significantly different from each other.
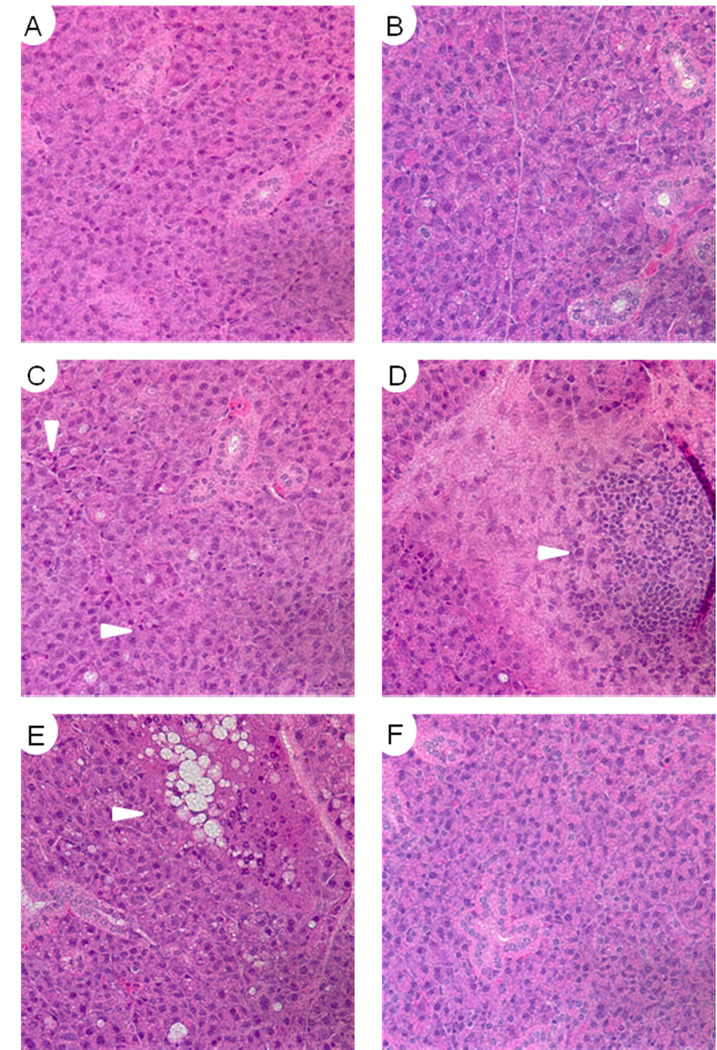
Histological analysis of salivary glands following fractionated radiation
Previously published studies on the affect of fractionated radiation in other animal models have reported increased fibrosis and inflammation six months after treatment (19–21). Hematoxylin and eosin stained parotid salivary gland sections were evaluated for structural abnormalities 90 days after fractionated radiation (2Gy/day for five consecutive days; figure 4). In all irradiated animals there was little evidence for atrophy, fibrosis, or sclerosis, which is typically reported in clinical cases of radiation-induced xerostomia (22;23). In all irradiated FVB mice there were areas of dispersed inflammatory cells (figure 4C), acinar cells containing enlarged nuclei (data not shown), and only one focal infiltrate in six animals evaluated (figure 4D). The irradiated myr-Akt1 mice demonstrated the most structural changes with the presence of large vacuoles within certain regions of the parotid gland of all treated animals (figure 4E). Interestingly, FVB mice pre-treated with IGF1 prior to radiation had no detectable histological differences from unirradiated controls (compare figures 4A and F).
Figure 4. Histological analysis of parotid salivary gland structure 90 days after fractionated radiation.
Parotid salivary glands from mice treated in figure 3 were paraffin embedded and sections were stained with hematoxylin and eosin. A) Untreated FVB, B) Untreated myr-Akt1, C) Irradiated FVB, D) Irradiated FVB, E) Irradiated myr-Akt1, F) Irradiated FVB with IGF-1 injections. Images A, B, C, and F are representative of the overall tissue architecture.
Alterations in acinar cell proliferation following fractionated radiation
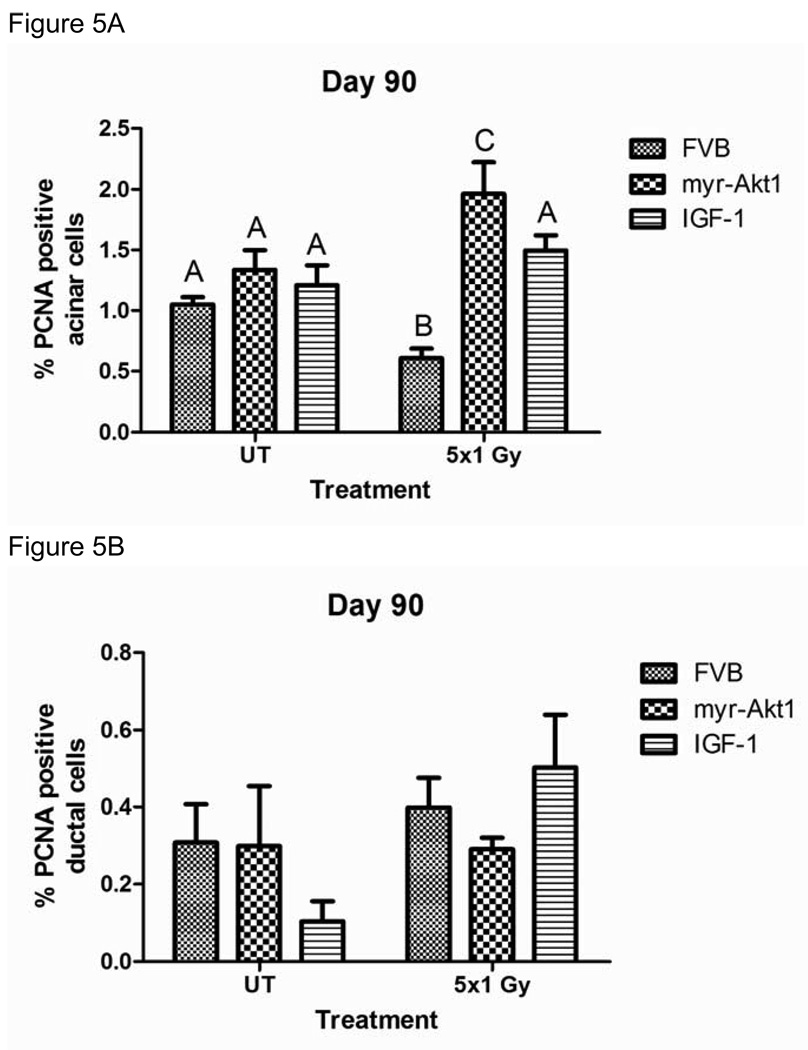
Tissue homeostasis requires the balance of cell death and cell growth (24); therefore, we were interested in determining if radiation-induced damage alters proliferation rates in vivo. Proliferation rates were determined 90 days post treatment by immunohistochemistry for proliferating cell nuclear antigen (PCNA) and positive acinar cells were counted separately from positive ductal cells due to the putative presence of progenitor cells within the ductal cell network (25). FVB mice exposed to 1 Gy/day for five consecutive days have significantly lower levels of PCNA positive salivary acinar cells when compared to untreated controls (figure 5A). In contrast, the number of PCNA positive acinar cells in FVB mice injected with IGF-1 did not change significantly following fractionated radiation (figure 5A). In contrast to FVB mice exposed to fractionated radiation with or without IGF-1, myr-Akt1 transgenic mice treated with fractionated radiation have significantly higher PCNA positive acinar cells. No significant differences in the percentage of PCNA positive ductal cells are observed in any of the treatment groups (figure 5B). Serial sectioned slides were also stained for activated caspase-3, which did not detect any significant increase in apoptotic cells 90 days after treatment in any of the mouse groups (data not shown). These data suggest that alterations in salivary acinar cell proliferation rates may influence the overall tissue homeostasis and correlate with a chronic loss of function.
Figure 5. Proliferation of parotid acinar cells following fractionated radiation (1 Gy × 5 days) in FVB, myr-Akt1 or IGF-1 injected mice.
The head and neck region of FVB, myr-Akt1, and FVB mice injected with IGF-1 was exposed to 1 Gy radiation per day for five consecutive days and parotid glands were removed 90 days after the final radiation treatment. Tissues were embedded into paraffin as described in figure 1 and immunohistochemistry was performed using an antibody against PCNA. Graph (A) represents the number of acinar cells with positive PCNA staining as a percentage of the total number of acinar cells in the field of view. Graph (B) represents the number of ductal cells with positive PCNA staining as a percentage of the total number of ductal cells in the field of view. The graph represents all data from at least eight mice per group. Significant differences (p≤0.05) were determined using an ANOVA followed by Student-Newman-Keul’s multiple comparison test. Treatment groups with the same letters are not significantly different from each other.
DISCUSSION
Radiation-induced damage to the salivary glands is a significant clinical problem for which there are currently few therapies available that significantly improve the quality of life for head and neck cancer patients. We report that injections of IGF-1 immediately prior to radiation preserve salivary gland function in a fractionated treatment protocol. Transgenic mice expressing a constitutively activated mutant of Akt1 (myr-Akt1) also exhibit significantly higher salivary flow rates when compared to irradiated mice. Interestingly, the initial (day 30) radiation-induced salivary gland dysfunction in FVB mice was similar between animals receiving 1Gy/day and 2Gy/day. Previous studies using single doses of radiation (1–5Gy) have also reported little differences in salivary function between radiation doses (11;12). This may indicate the activation of a stress response program after a critical threshold of damage that culminates in chronic loss of function. In patients, depending on whether the caudal or cranial portion of the salivary gland is irradiated, there is significant loss of function at lower mean doses (10–15Gy) (26).
The role of apoptosis in radiation-induced damage to the salivary gland has been debated; studies in the mouse have reported significant levels of apoptotic cells (11–14) while the presence of apoptosis in irradiated rats have been more variable (8–10). Our results indicate that a significant number of apoptotic cells could be detected in FVB mice following each fraction of radiation with little differences between the number of doses. Expression of myr-Akt1 suppressed the induction of apoptosis and preserved salivary gland function, which extends previously reported correlations between radiation-induced apoptosis and loss of salivary gland function (11;12) to a fractionated radiation treatment. Intriguingly, the kinetics of apoptotic cell clearance is similar between single (11) and fractionated doses of radiation. This could suggest that a significant number of the apoptotic cells detected after each radiation treatment are newly activated. One of the most consistent phenotypes in the salivary gland response to radiation is atrophy and loss of glandular weight, and our study implicates a continued apoptotic response contributes to these observations. Alternatively, the process of apoptotic cell clearance may not be activated until the cessation of radiation exposure. This persistence of apoptotic cells in the tissue could contribute to an inflammatory response; however, rodent models of radiation-induced salivary gland damage have not consistently reported the infiltration of inflammatory cells (27;28). In our analysis of H&E stained tissue sections 90 days after treatment, we were able to detect dispersed infiltrates of inflammatory cells in all irradiated FVB mice; however only one mouse parotid gland had evidence of focal inflammation. Most importantly, FVB injected with IGF-1 prior to radiation had no structural alteration when compared to unirradiated controls. Interestingly, salivary gland sections from irradiated myr-Akt1 mice contained vacuoles in ~20% of the total parotid area. Since the function of myr-Akt1 mice is preserved after radiation, it is possible that these vacuoles represent an active autophagy process. It has been previously demonstrated in salivary acinar cells that autophagy is a survival mechanism to hypoxia-induced stress (29).
Maintenance of tissue homeostasis requires a balance between cell growth and cell death (24). Unlike classically radiosensitive tissues which have a high level of proliferation (summarized in (30)), salivary glands have a low percentage of proliferating cells and a high degree of differentiation. In our study, we observe a significant reduction in PCNA positive acinar cells in FVB mice exposed to fractionated radiation compared to untreated controls. This indicates that a subset of salivary acinar cells may induce a permanent arrest phenotype following fractionated radiation treatment that may correlate with a chronic loss of function. FVB mice receiving an injection of IGF-1 prior to each radiation have salivary acinar cell proliferation rates similar to untreated controls suggesting tissue homeostasis is maintained. Expression of myr-Akt1 results in an increase in the number of PCNA positive salivary acinar cells following fractionated radiation, which is significantly different from irradiated FVB mice with or without IGF-1. This difference between myr-Akt1 and IGF-1 injected mice may be due to the transgene being continually expressed versus IGF-1 which has a short biological half-life and can be cleared. Alternatively, IGF-1 and Akt may influence the regulation of proliferation through distinct downstream signaling pathways.
Despite the multiple injections of IGF-1, salivary gland tumors were not detected in any of the mice evaluated. However, one limitation of this study would be the potential adverse effect of IGF-1 on tumor clearance. Future studies are aimed at characterizing the molecular mechanisms responsible for the phenotypic effect of IGF-1 in the preservation of salivary function in order to develop small molecule therapeutics to specifically target the salivary gland. A second limitation involves the use of anesthesia to irradiate mice which is not conducted clinically. These studies are an initial step to validate the feasibility of preventing radiation-induced loss of salivary function in order to improve the quality of life during the treatment for head and neck cancer.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Randy Burd (University of Arizona) for critical review of this manuscript and Dr. Sean Limesand for the use of the Leica DM5500 microscope for imaging. This work was supported in part by the NIH DE17918, DE16096, and DE18888.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification. Conflicts of interest do not exist for any of the authors
Reference List
- 1.Valdez IH. Radiation-induced salivary dysfunction: clinical course and significance. Spec Care Dentist. 1991;11(6):252–255. doi: 10.1111/j.1754-4505.1991.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 2.Henson BS, Eisbruch A, D'Hondt E, Ship JA. Two-year longitudinal study of parotid salivary flow rates in head and neck cancer patients receiving unilateral neck parotid-sparing radiotherapy treatment. Oral Oncol. 1999;35(3):234–241. doi: 10.1016/s1368-8375(98)00104-3. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67(3):660–669. doi: 10.1016/j.ijrobp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braam PM, Terhaard CH, Roesink JM, Raaijmakers CP. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(4):975–980. doi: 10.1016/j.ijrobp.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Fox PC, van der Ven PF, Sonies BC, Weiffenbach JM, Baum BJ. Xerostomia: evaluation of a symptom with increasing significance. J Am Dent Assoc. 1985;110(4):519–525. doi: 10.14219/jada.archive.1985.0384. [DOI] [PubMed] [Google Scholar]
- 6.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 7.Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: From animal models to therapies. J Dent Res. doi: 10.1177/0022034509343143. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paardekooper GM, Cammelli S, Zeilstra LJ, Coppes RP, Konings AW. Radiation-induced apoptosis in relation to acute impairment of rat salivary gland function. Int J Radiat Biol. 1998;73(6):641–648. doi: 10.1080/095530098141898. [DOI] [PubMed] [Google Scholar]
- 9.Boraks G, Tampelini FS, Pereira KF, Chopard RP. Effect of ionizing radiation on rat parotid gland. Braz Dent J. 2008;19(1):73–76. doi: 10.1590/s0103-64402008000100013. [DOI] [PubMed] [Google Scholar]
- 10.Thula TT, Schultz G, Tran-Son-Tay R, Batich C. Effects of EGF and bFGF on irradiated parotid glands. Ann Biomed Eng. 2005;33(5):685–695. doi: 10.1007/s10956-005-1853-z. [DOI] [PubMed] [Google Scholar]
- 11.Limesand KH, Said S, Anderson SM. Suppression of radiation-induced salivary gland dysfunction by IGF-1. PLoS ONE. 2009;4(3):e4663. doi: 10.1371/journal.pone.0004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avila JL, Grundmann O, Burd R, Limesand KH. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys. 2009;73(2):523–529. doi: 10.1016/j.ijrobp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the PKCdelta null mouse in vivo. J Biol Chem. 2006;281(14):9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- 14.Limesand KH, Schwertfeger KL, Anderson SM. MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol. 2006;26:8840–8856. doi: 10.1128/MCB.01846-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bralic M, Muhvic-Urek M, Stemberga V, Golemac M, Jurkovic S, Borcic J, et al. Cell death and cell proliferation in mouse submandibular gland during early post-irradiation phase. Acta Med Okayama. 2005;59(4):153–159. doi: 10.18926/AMO/31948. [DOI] [PubMed] [Google Scholar]
- 16.Muhvic-Urek M, Bralic M, Curic S, Pezelj-Ribaric S, Borcic J, Tomac J. Imbalance between apoptosis and proliferation causes late radiation damage of salivary gland in mouse. Physiol Res. 2006;55(1):89–95. doi: 10.33549/physiolres.930739. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67(3):660–669. doi: 10.1016/j.ijrobp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murdoch-Kinch CA, Kim HM, Vineberg KA, Ship JA, Eisbruch A. Dose-Effect Relationships for the Submandibular Salivary Glands and Implications for Their Sparing by Intensity Modulated Radiotherapy. Int J Radiat Oncol Biol Phys. 2008 doi: 10.1016/j.ijrobp.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich RE, Bartel-Friedrich S, Holzhausen HJ, Lautenschlager C. The effect of external fractionated irradiation on the distribution pattern of extracellular matrix proteins in submandibular salivary glands of the rat. J Craniomaxillofac Surg. 2002;30(4):246–254. doi: 10.1054/jcms.2002.0318. [DOI] [PubMed] [Google Scholar]
- 20.Sagowski C, Wenzel S, Tesche S, Jenicke L, Jaehne M. Investigation of radiosialadenitis during fractioned irradiation: sialoscintigraphical and histomorphological findings in rats. Eur Arch Otorhinolaryngol. 2003;260(9):513–517. doi: 10.1007/s00405-003-0631-x. [DOI] [PubMed] [Google Scholar]
- 21.Radfar L, Sirois DA. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(3):267–274. doi: 10.1016/s1079-2104(03)00369-x. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JS, Fu K, Marks J, Silverman S. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31(5):1141–1164. doi: 10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]
- 23.Radfar L, Sirois DA. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(3):267–274. doi: 10.1016/s1079-2104(03)00369-x. [DOI] [PubMed] [Google Scholar]
- 24.Clarke PR, Allan LA. Cell-cycle control in the face of damage--a matter of life or death. Trends Cell Biol. 2009;19(3):89–98. doi: 10.1016/j.tcb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3(4):e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bussels B, Maes A, Flamen P, Lambin P, Erven K, Hermans R, et al. Dose-response relationships within the parotid gland after radiotherapy for head and neck cancer. Radiother Oncol. 2004;73(3):297–306. doi: 10.1016/j.radonc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Muhvic-Urek M, Bralic M, Curic S, Pezelj-Ribaric S, Borcic J, Tomac J. Imbalance between apoptosis and proliferation causes late radiation damage of salivary gland in mouse. Physiol Res. 2006;55(1):89–95. doi: 10.33549/physiolres.930739. [DOI] [PubMed] [Google Scholar]
- 28.O'Connell AC, Redman RS, Evans RL, Ambudkar IS. Radiation-induced progressive decrease in fluid secretion in rat submandibular glands is related to decreased acinar volume and not impaired calcium signaling. Radiat Res. 1999;151(2):150–158. [PubMed] [Google Scholar]
- 29.Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK. Novel roles for protein kinase Cdelta-dependent signaling pathways in acute hypoxic stress-induced autophagy. J Biol Chem. 2008;283(49):34432–34444. doi: 10.1074/jbc.M804239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall E. Radiobiology for the Radiologist. 5 ed. Philadelphia: Lippincott, Williams and Wilkins; 2000. [Google Scholar]